Abstract
Methods used to deter biofouling of underwater structures and marine vessels present a serious environmental issue and are both problematic and costly for government and commercial marine vessels worldwide. Current antifouling methods include compounds that are toxic to aquatic wildlife and marine ecosystems. Dihydrooroidin (DHO) was shown to completely inhibit Halomonas pacifica biofilms at 100 μM in a static biofilm inhibition assay giving precedence for the inhibition of other marine-biofilm-forming organisms. Herein we present DHO as an effective paint-based, non-cytotoxic, antifouling agent against marine biofouling processes in a marine mesocosm.
Keywords: Antifouling, Biofilms, Natural products
1. Introduction
Biofouling describes the natural process of organic matter accumulation on submerged materials (Fusetani, 2004). This process is often initiated by microfouling, which is driven by marine bacterial biofilms. Ultimately, microfouling precedes macrofouling, which is the accumulation of invertebrates (i.e., barnacles) on submerged objects such as fishing apparatus or ship hulls (Flemming et al., 1996). Additionally, bacterial biofilms have been directly correlated in the biofouling process, causing biocorrosion of submerged surfaces (Flemming et al., 1996; Fusetani, 2004). It has been documented that if a biofilm does not form on the hull of a ship, invertebrate aggregation will not readily occur (Beech et al., 2005).
The impact of biofouling in marine habitats drives a number of the significant problems that our society faces in both water pollution and the emission of greenhouse gases. Most notably, accumulation of biomasses on ship hulls increases drag, which directly correlates with increased fuel consumption (Chambers et al., 2006). Thus increased fuel consumption due to biofouling has become an important topic in recent years as global warming pushes academia and industry to find alternative approaches for lowering greenhouse gas emissions.
The first methods used to control biofouling relied on copper or lead sheathing of wooden ships over 4000 years ago; more modern methods have been based upon the addition of tributyl tin (TBT) to paints used on ships and other equipment that is in constant contact with the oceanic environment (Champ and Lowenstein, 1987; Omae, 2003). This and other frequently used chemicals (organomercury, arsenic, and lead) are extremely toxic to marine life (Tang and Cooney, 1998; Omae, 2003). TBT causes severe reproductive damage to marine life due to an alkyl tin radical metabolite of TBT, and it has been shown that triorganotins are remarkably stable in dark, sedimentary places (i.e., sea beds), thus prolonging their negative ecological effects on the marine ecosystem (Omae, 2003). TBT-containing paints are now being replaced by copper-based paints; however, these are still toxic to marine life and remain far from an environmentally safe means to regulate the fouling process.
Research in finding alternate avenues for controlling biofouling while circumventing the toxic properties of the aforementioned metal-based methods includes the application of natural product antifouling agents. Work in this area has yielded several natural products from both marine and terrestrial sources. Studies have been performed investigating the ability of capsaicin and zosteric acid as paint additives to prevent biofouling (Xu et al., 2005). There have also been reports of nanoparticle-based coatings to prevent fouling (Anyaogu et al., 2008).
A variety of marine natural products are antifouling agents and some have been shown to inhibit the formation of marine-based bacterial biofilms (Yamada et al., 1997). The limiting issues with the use of marine natural products are two-fold. First, the act of harvesting the material from natural sources is ecologically damaging and does not yield adequate quantities of material for commercial applications. Second, the complex architecture of many marine natural products makes their syntheses impractical for commercial use.
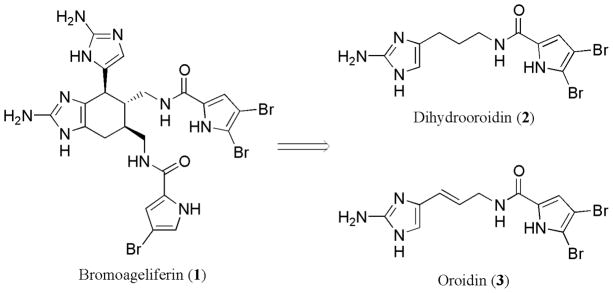
Oroidin 3 is a marine natural product isolated from sponges of the Agelasidae family and is considered a biogenic precursor to bromoageliferin 1 (Fig. 1) (Forenza et al., 1971; Braekman et al., 1992). One of the biological activities of these nitrogen-dense molecules is to serve as anti-feeding deterrents for the sponges in their native warm shallow water habitats (Braekman et al., 1992; Chanas et al., 1997; Assmann et al., 2000). Both bromoageliferin and oroidin were also shown to have the additional, but biologically unrelated, activity of inhibiting the formation of biofilms by the marine α-proteobacterium Rhodospirillum salexigens (Yamada et al., 1997). Oroidin has also been documented to possess activity in a limited number of studies involving bacterial attachment and colonization (Kelly et al., 2003, 2005).
Fig. 1.
Marine-based bacterial biofilm modulators.
Our research group has been interested in the design of small molecules, which are structurally inspired by both bromoageliferin and oroidin that inhibit and disperse bacterial biofilms through a non-microbicidal mechanism (which we refer to as anti-biofilm activity). The underlying motivation for the design of molecules with this activity is two-fold. First, by preventing bacterial attachment, we would mitigate processes (such as microfouling) that are underpinned by the formation of a bacterial biofilm. Second, evolution of bacterial resistance to molecules that inhibit and disperse bacterial biofilms through non-microbicidal mechanisms would also be mitigated due to lack of selection pressure. In this vein, we recently reported the synthesis and anti-biofilm activity of a 50-compound library of small molecules that were based upon oroidin (Richards et al., 2008). One of the lead compounds from this study that had anti-biofilm activity was dihydrooroidin (DHO) 2. Although the mechanism by which these compounds inhibit and disperse bacterial biofilms is not known and under active investigation, we posited that the anti- biofilm activity of these compounds would directly translate to antifouling activity in a marine environment. This hypothesis was based upon the current picture of biofouling development that microfouling (i.e., biofilm development) drives macrofouling (Beech et al., 2005).
To this end, this report examines the ability of DHO to inhibit the formation of Halomonas pacifica biofilms in vitro. H. pacifica was specifically chosen because previous studies have established that this bacterium is involved in the microfouling process (Bakker et al., 2003; Ista et al., 2004). Based upon this inhibition result, we investigated, and subsequently demonstrated, that DHO would suppress biofouling when mixed with commercially available marine paint (absent of additional antifoulants) and placed in an oceanic marine mesocosm. Finally, we provide preliminary toxicity screening results with two mammalian cell lines that indicate DHO is devoid of cytotoxicity. The objective of this work was to validate DHO’s potential to act as a non-toxic, antifouling additive to marine paint.
2. Materials and methods
2.1. Synthesis and biological evaluation of oroidin and DHO
Oroidin and dihydrooroidin were synthesized as previously reported (Richards et al., 2008). Their purity was confirmed to be ≥ 95% by 1H NMR, 13C NMR and HRMS analysis. For the general static biofilm inhibition assay, stock solutions in biological grade DMSO (100 mM and 10 mM) of oroidin and DHO were prepared and stored at room temperature.
2.2. General static biofilm inhibition assay protocol for Halomonas pacifica
Halomonas pacifica (ATCC 27122) was purchased from ATCC. H. pacifica has frequently been employed in studies involving fouling processes (Bakker et al., 2003; Ista et al., 2004). An overnight culture (grown in Luria-Bertani [LB]) of the wild type strain was subcultured at an OD600 of 0.01 into LB along with oroidin or DHO (at 100 μM, respectively) to be tested for biofilm inhibition. Samples were then aliquoted (100 μL) into the wells of a 96-well PVC microtiter plate (O’Toole and Kolter, 1998). The microtiter dishes were covered and sealed before incubation under stationary conditions at 37°C for 24 hours. After that time, the medium was discarded and the plates thoroughly washed with water. The wells were then treated with a 0.1% aqueous solution of crystal violet (100 μL) and allowed to stand at ambient temperature for 30 minutes. Following another thorough washing with water the remaining stain was solubilized with 200 μL of 95% ethanol. Biofilm inhibition was quantified by measuring the OD540 for each well by transferring 125 μL of the ethanol solution into a fresh polystyrene microtiter dish for analysis.
2.3. Challenge tank
The mesocosm design is based on standard literature precedence with slight modifications (Lauth et al., 1996; Pennington et al., 2004, 2007). Each tank contains seawater, four to six sediment trays, and various estuarine species including but not limited to Spartina alterniflora, grass shrimp (Palaemonetes pugio), sheepshead minnows (Cyprinodon variegatus), and hard clams (Mercenaria mercenaria). The systems are subjected to natural (ambient) light and temperature regimes for the coastal region of South Carolina.
The seawater for all experiments was collected from Cherry Point boat landing on Wadmalaw Island, SC (32° 35.876′ N, 080° 10.973′ W). The seawater was collected in 250-gal fractions (1250 gal total) and homogenized by mixing for 24–48 hours. Homogenization was conducted to uniform salinity, temperature, aeration, and microorganism distribution. After homogenization, the seawater was diluted to 20 parts per thousand with deionized water. Depending on the exact experimental design, 300 to 350 l of 20 ppt seawater is added to each system. A semidiurnal tidal cycle is set for each tank using a pump switched by a multi-event timer. The system was set up 2–3 months prior to dosing for equilibration and acclimation.
Intertidal sediment was collected from Leadenwah Creek (32° 38.848′ N, 080° 13.283′ W), sieved with a 3-mm sieve, and homogenized. It was then dispensed into sediment trays (7.5 l ~ 49 kg of sediment per tray). Four to six sediment trays were placed randomly into the systems and placed at various elevations to simulate tidal flat elevation.
Estuarine organisms are generally collected from the same site as the sediment with the exception of Spartina, sheepshead minnows, and hard clams, which were obtained from commercial vendors. Since seawater is collected whole, the systems contain natural planktonic community assemblages. Likewise, the sediments contain the natural benthos that will pass through a 3-mm sieve.
The challenge tank experiment began by mixing DHO into a generic marine-based paint absent of any antifouling agents to a concentration of 1 mM (~ 400 mg l−1). Vigorous mixing of the paint and DHO was performed to affect dispersion of DHO into the paint. Two PVC plastic boards were used as the designated surfaces for the experiment. Surface abrasions were introduced into both boards prior to paint application to ensure better adherence. These surfaces were then submerged and held stationary in a challenge tank to determine the effect that DHO had upon fouling in a simulated marine environment. The surfaces were placed at a depth of 6–8 inches and kept at this depth during tidal surges.
2.4. Quantification of biofouling
All quantification of biomass accumulation was done through pixel analysis as previously described (Stafslien et al., 2006). Briefly, all digital images of the boards were converted to grayscale tagged imaged file formats (tif) using Adobe Photoshop. The tif images were then imported into ImageQuant. Pixel analysis was then performed to determine the abundance of fouling organisms over the paint strips.
2.5. Toxicity
For cytotoxicity screening, both GH4C1 rat pituitary cells and N2A mouse neuroblastoma cells were chosen. Cell viability is assessed through a MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) dye based colorimetric assay. After an 18-hour incubation with DHO at concentrations up to 600 μM, MTT dye is added. Within 4 hours, living cells take up the dye in the mitochondria where they metabolize the MTT into a blue formosan. The cells are then lysed with SDS and HCl. Dark blue crystals are solubilized, yielding a purple color. Wells exhibiting cell death due to the presence of toxin remain yellow.
3. Results and discussion
DHO is a synthetic analogue of oroidin (Olofson et al., 1998). To assess the activity of DHO in comparison to oroidin for potential antifouling applications, both compounds were initially screened in a 96-well format using the standard crystal violet reporter assay (O’Toole and Kolter, 1998) to assess each compound’s ability to inhibit the formation of H. pacifica biofilms. Results indicated that oroidin and DHO were able to completely inhibit the formation of H. pacifica biofilms at 100 μM as evident by the ethanol solubilized crystal violet stain (Fig. 2). Follow-up analysis of the growth curves of H. pacifica grown in the absence or presence of DHO or oroidin (data not shown) showed no difference in the planktonic growth rates, indicating that we were inhibiting biofilm development through a non-microbicidal mechanism. While oroidin was shown to be just as active against H. pacifica in the biofilm inhibition assays, DHO is a more attractive candidate due to its ability to be chemically synthesized on large scales (Olofson et al., 1998).
Fig. 2.
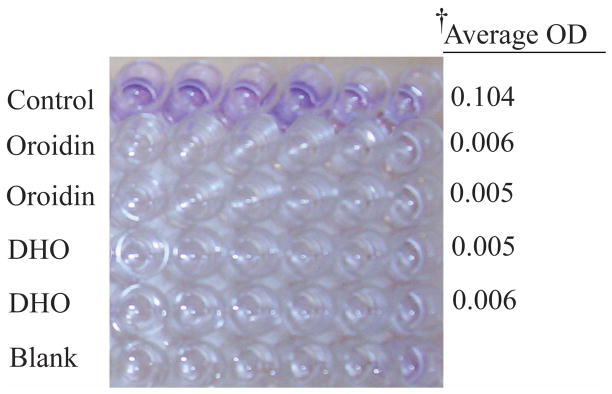
Anti-biofilm activity of oroidin and DHO at 100 μM. Lanes with either oroidin or DHO show complete biofilm inhibition as compared to the control lanes. †Average O.D. at 540 nm with blank subtracted.
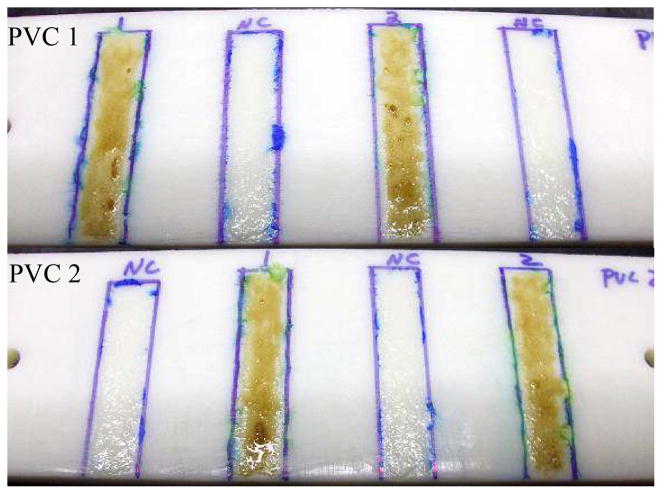
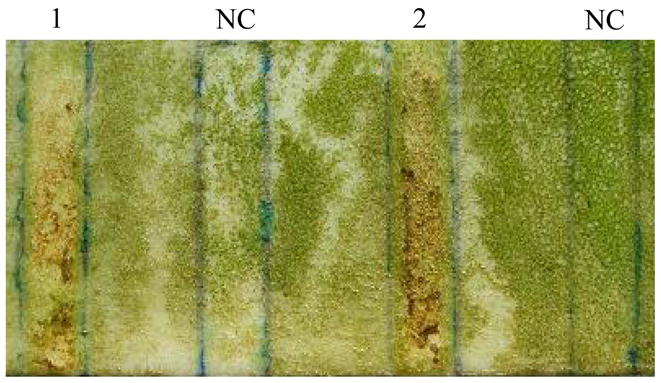
After determining that DHO had anti-biofilm properties against H. pacifica, it was then dissolved in generic marine-based paint absent of any antifouling agents to a concentration of 1 mM (~ 400 mg l−1). Strips of control paint (NC: no compound) and paint containing DHO (lanes 1 and 2) were then applied in a duplicate fashion to two PVC boards (Fig. 3). The plates were then submerged into the challenge tank at a depth of 6–8 inches and kept at this depth throughout tidal surges. After one week in the tank the boards were pulled out and examined (Fig. 4). Visual inspection of the control and experimental paint strips on each board indicated that both were relatively free of fouling, and we were unable to qualitatively determine fouling difference between the paint strips by this method. Quantification of the biofouling rate, however, revealed that the paint alone accumulated 25% more biomass coverage than paint containing DHO. Each board was again submerged (under identical conditions as above) and then pulled after an additional two weeks sitting stationary in the tank. Upon visual examination, there was a significant qualitative difference between the control and experimental paint in preventing fouling (Fig. 5). Again, quantification of the rate of biofouling indicated 125% more biomass coverage on the paint alone than on the paint containing DHO. This also indicated that the DHO within the paint was still active after three weeks in a marine environment.
Fig. 3.
Painted boards used in mesocosm experiment. NC: no compound, lanes 1 and 2: DHO paint.
Fig. 4.

PVC board 2 after 1 week in the challenge tank.
Fig. 5.
PVC board 1 after 3 weeks in the challenge tank.
After we observed a reduced fouling rate for paint containing DHO, we investigated the cytotoxicity of DHO by employing GH4C1 rat pituitary cells and N2A mouse neuroblastoma cells in a cell-based MTT reporter assay. These cell lines were extensively utilized for evaluating Pfiesteria poisoning (Burkholder et al., 2005) and have been utilized for evaluating toxicity of marine natural products (Van Dolah et al., 1994). DHO was shown to be non-cytotoxic at concentrations up to 600 μM (the highest concentration studied). This is in contrast to bromoageliferin (which served as the structural inspiration for this work), which is known to be cytotoxic and activate Ca2+ channels (Bickmeyer, 2005). Thus, by preliminary screening, DHO appears free from any cytotoxic side effects.
The lack of cytotoxicity coupled with the preliminary antifouling results of the mesocosm challenge tank argues that this class of marine-based natural product analogues may have the potential to be used as antifouling agents without being an environmental threat. Additional antifouling experiments and biofilm inhibition assays with other bacteria known to participate in the fouling process are currently underway with structures homologous to DHO, and our findings will be reported in due course.
Acknowledgments
The authors would like to thank the following sources for funding: NCSU, GlaxoSmithKline, Burroughs-Wellcome, the NIH (GM55769), and the Kenan Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anyaogu KC, Federov AV, Neckers DC. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir. 2008;24:4340–4346. doi: 10.1021/la800102f. [DOI] [PubMed] [Google Scholar]
- Assmann M, Lichte E, Pawlik JR, Kock M. Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Marine Ecology-Progress Series. 2000;207:255–262. [Google Scholar]
- Bakker DP, Klijnstra JW, Busscher HJ, Van Der Mei HC. The effect of dissolved organic carbon on bacterial adhesion to conditioning films adsorbed on glass from natural seawater collected during different seasons. Biofouling. 2003;19:391–397. doi: 10.1080/08927010310001634898. [DOI] [PubMed] [Google Scholar]
- Beech IB, Sunner JA, Hiraoka K. Microbe-surface interactions in biofouling and biocorrosion processes. International Journal of Microbiology. 2005;8:157–168. [PubMed] [Google Scholar]
- Bickmeyer U. Bromoageliferin and dibromoageliferin, secondary metabolites from the marine sponge Agelas conifera, inhibit voltage-operated, but not store-operated calcium entry in PC12 cells. Toxicon. 2005;45:627–632. doi: 10.1016/j.toxicon.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Braekman JC, Dazole D, Stoller C, Vansoest RWM. Chemotaxonomy of Agelas (Porifera, Demospongiae) Biochemical Systematics and Ecology. 1992;20:417–431. [Google Scholar]
- Burkholder JM, Gordon AS, Moeller PD, Maclaw J, Coyne KJ, Lewitus AJ, Ramsdell JS, Marshall HG, Deamer NJ, Cary SC, Kempton JW, Morton SL, Rublee PA. Demonstration of toxicity to fish and to mammalian cells by Pfiesteria species: Comparison of assay methods and strains. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3471–3476. doi: 10.1073/pnas.0500168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers LD, Stokes KR, Walsh FC, Wood RJK. Modern approaches to marine antifouling coatings. Surface & Coatings Technology. 2006;201:3642–3652. [Google Scholar]
- Champ MA, Lowenstein FL. TBT - The Dilemma of High-Technology Antifouling Paints. Oceanus. 1987;30:69–77. [Google Scholar]
- Chanas B, Pawlik JR, Lindel T, Fenical W. Chemical defense of the Caribbean sponge Agelas clathrodes. Journal of Experimental Marine Biology and Ecology. 1997;208:185–196. [Google Scholar]
- Flemming HC, Greibe T, Schaule G. Antifouling strategies in technical systems - A short review. Water Science and Technology. 1996;34:517–524. [Google Scholar]
- Forenza S, Minale L, Riccio R. New Bromo-Pyrrole Derivatives from Sponge Agelas-Oroides. Journal of the Chemical Society-Chemical Communications. 1971:1129–1131. [Google Scholar]
- Fusetani N. Biofouling and antifouling. Natural Product Reports. 2004;21:94–104. doi: 10.1039/b302231p. [DOI] [PubMed] [Google Scholar]
- Ista LK, Callow ME, Finlay JA, Coleman SE, Nolasco AC, Simons RH, Callow JA, Lopez GP. Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Applied Environmental Microbiology. 2004;70:4151–4157. doi: 10.1128/AEM.70.7.4151-4157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SR, Garo E, Jensen PR, Fenical W, Pawlik JR. Effects of Caribbean sponge secondary metabolites on bacterial surface colonization. Aquatic Microbial Ecology. 2005;40:191–203. [Google Scholar]
- Kelly SR, Jensen PR, Henkel TP, Fenical W, Pawlik JR. Effects of Caribbean sponge extracts on bacterial attachment. Aquatic Microbial Ecology. 2003;31:175–182. [Google Scholar]
- Lauth JR, Scott GI, Cherry DS, Buikema AL. A modular estuarine mesocosm. Environmental Toxicology and Chemistry. 1996;15:630–637. [Google Scholar]
- O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Molecular Microbiology. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Olofson A, Yakushijin K, Horne DA. Synthesis of marine sponge alkaloids oroidin, clathrodin, and dispacamides. Preparation and transformation of 2-amino-4,5-dialkoxy-4,5-dihydroimidazolines from 2-aminoimidazoles. Journal of Organic Chemistry. 1998;63:1248–1253. [Google Scholar]
- Omae I. Organotin antifouling paints and their alternatives. Applied Organometallic Chemistry. 2003;17:81–105. [Google Scholar]
- Pennington PL, Delorenzo ME, Key PB, Wirth EF, Fulton MH, Scott GI. The design, construction, operation, and maintenance of the replicated modular estuarine mesocosm. NOAA Technical Memorandum NOS NCCOS. 2007;62:72. [Google Scholar]
- Pennington PL, Delorenzo ME, Lawton JC, Strozier ED, Fulton MH, Scott GI. Modular estuarine mesocosm validation: ecotoxicological assessment of direct effects with the model compound endosulfan. Journal of Experimental Marine Biology and Ecology. 2004;298:369–387. [Google Scholar]
- Richards JJ, Ballard TE, Huigens RW, Melander C. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem. 2008;9:1267–1279. doi: 10.1002/cbic.200700774. [DOI] [PubMed] [Google Scholar]
- Stafslein SJ, Chisholm BJ, Christianson DA, Daniels J, Swain G. High throughput. Materials Research Society Symposium Proceedings. 2006;894:LL04–04.1–7. [Google Scholar]
- Tang RJ, Cooney JJ. Effects of marine paints on microbial biofilm development on three materials. Journal of Industrial Microbiology and Biotechnology. 1998;20:275–280. [Google Scholar]
- Van Dolah FM, Finley EL, Haynes BL, Doucette PD, Ramsdell JS. Development of rapid and sensitive high throughput pharmacologic assays for marine phycotoxins. Natural Toxins. 1994;2:189–196. doi: 10.1002/nt.2620020407. [DOI] [PubMed] [Google Scholar]
- Xu QW, Barrios CA, Cutright T, Newby BMZ. Assessment of antifouling effectiveness of two natural product antifoulants by attachment study with freshwater bacteria. Environmental Science and Pollution Research. 2005;12:278–284. doi: 10.1065/espr2005.04.244. [DOI] [PubMed] [Google Scholar]
- Yamada A, Kitamura H, Yamaguchi K, Fukuzawa S, Kamijima C, Yazawa K, Kuramoto M, Wang GYS, Fujitani Y, Uemura D. Development of chemical substances regulating biofilm formation. Bulletin of the Chemical Society of Japan. 1997;70:3061–3069. [Google Scholar]