Abstract
Bacteriophages, or simply phages, are viruses infecting bacteria. With an estimated 1031 particles in the biosphere, phages outnumber bacteria by a factor of at least 10 and not surprisingly, they influence the evolution of most bacterial species, sometimes in unexpected ways. “Temperate” phages have the ability to integrate into the chromosome of their host upon infection, where they can reside as “quiescent” prophages until conditions favor their reactivation. Lysogenic conversion resulting from the integration of prophages encoding powerful toxins is probably the most determinant contribution of prophages to the evolution of pathogenic bacteria. We currently grasp only a small fraction of the total phage diversity. Phage biologists keep unraveling novel mechanisms developed by phages to parasitize their host. The purpose of this review is to give an overview of some of the various ways by which prophages change the lifestyle and boost virulence of some of the most dangerous bacterial pathogens.
Keywords: bacteriophage, prophage, lysogenic conversion, evolution, toxins, biofilm, sporulation, virulence, bacterial fitness, Clostridium
Introduction
Viruses that infect bacteria are called bacteriophages, or simply phages. They represent the most abundant biological form in the biosphere with an estimated 1031 phages, and they outnumber bacteria by a factor of about 10 to 1.1 Oceans cover about 70% of the Earth’s surface and represent the main viral reservoir: each milliliter of sea water contains ~107–108 phages and ~1023 phage infections occur every second.2,3 The mammalian gut is also densely populated with 1013–1014 bacteria per gram of fecal matter, and thus represents an incredible playground for phages. The human gut can contain up to 108 viral particles per gram of feces and most of these viruses are bacteriophages.4,5 Escherichia coli phages dominate, with 103–104 phages per gram feces in humans and up to 107 in cows and pigs,6,7 but phages infecting other important commensal bacteria like Bacteroides fragilis and Salmonella spp. have been isolated as well.8 Not surprisingly, phages constitute major players shaping bacterial communities in most if not all ecosystems and they are intimately associated with virulence and evolution of several important bacterial pathogens.9,10 The purpose of this review is to give an overview of some of the different ways by which prophages can modulate virulence of their host.
Lysogeny is Common in the Bacterial World
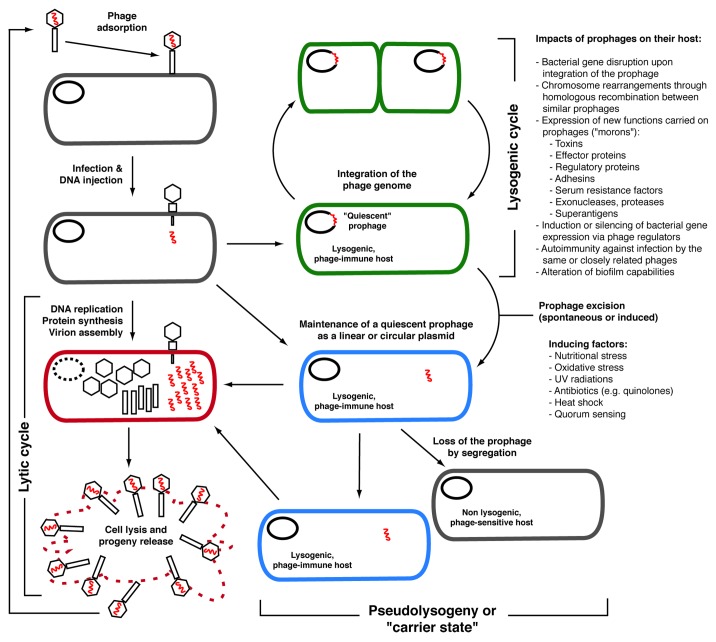
Generally speaking, phages can be classified into two main categories based on their lifecycle: strictly lytic phages (or virulent) and temperate phages, although some phages also adopt a pseudotemperate lifestyle, i.e., they generally do not integrate the chromosome of their host and remain as linear or circular plasmids (Fig. 1).11 Virulent phages can only replicate through a lytic cycle, which is characterized by the lysis of the infected bacterial host at the end of the phage replication cycle. RNA phages such as the E. coli phages MS2 and Qβ are part of this group, but most of the work on lytic phages has involved tailed, double-stranded DNA phages, which are by far the most frequently isolated phages (~96% of the phages described in the literature).12 Typically, the lytic cycle of DNA phages is characterized by the adsorption of the phage to a specific bacterial cell surface receptor, followed by the injection of the phage DNA into the bacterial cytoplasm, transcription of phage genes, and replication of the phage genome. Viral proteins are then synthesized, assembled into capsids (heads) and tails (in the case of tailed phages) and the viral genome is packaged into the capsids to form virions. At the end of the lytic cycle the phage holin, a small protein that forms oligomers, creates pores within the cytoplasmic membrane.13 This enables the phage-encoded endolysin (often called “lysin”) to gain access to the peptidoglycan layer that it hydrolyzes. This results in cell lysis and the release of hundreds to thousands of new infectious particles into the surrounding environment that will be able to re-infect susceptible bacteria nearby and the cycle will start over again. Temperate phages have the same lytic capacity as virulent phages but in addition, they can opt for a non-lytic lifestyle.11 Within minutes after phage DNA injection into the bacterial cytoplasm, and depending on the metabolic state of the bacterial cell, the phage can “choose” to initiate a lytic cycle or to integrate its DNA into the bacterial chromosome of its host to become a prophage. If the latter option is chosen, a specific phage repressor (CI in phage λ) inhibits transcription of most of the phage genes, including those required for the lytic cycle, and the prophage becomes quiescent. Its DNA is replicated concomitantly with the bacterial chromosome during cell division, and this cycle can last for an infinite period of time. A bacterial cell carrying one or more prophages is called a lysogen and generally, it is “immune” toward lytic or lysogenic infection by other phages of the same group.11
Figure 1. Different lifestyles adopted by phages. True virulent phages will only follow the lytic cycle for their replication and will lead to the lysis of the host cell at the end of the cycle. Temperate phages have the choice to replicate through the lytic cycle like virulent phages, or they can opt for the lysogenic cycle. In most cases of lysogeny, the phage integrates its genome into the host bacterial chromosome and remains quiescent. The prophage DNA is replicated along with the bacterial chromosome and is transmitted to daughter cells. Lysogeny can sometimes have significant impacts on the host (lysogenic conversion).9 Under certain conditions, including various stresses causing DNA damages, the prophage is excised and initiates a lytic cycle. Some phages also adopt a pseudotemperate lifestyle, i.e., they generally do not integrate the chromosome of their host and replicate as linear or circular plasmids within the cytoplasm. Sometimes, the phage genome is lost during cell division and segregation, which is characterized by instability of the lysogens carrying these phages. Note that chronic infections by non-lytic phages (e.g., filamentous phages such as V. cholerae CTXϕ) are not represented on this figure.147
The capacity of temperate phages to establish a stable relationship with their host (i.e., lysogens) can have profound repercussions on the lifestyle, fitness and virulence of the lysogens.9,10,14,15 With next generation sequencing technologies, an increasing number of complete and draft bacterial genome sequences are now becoming available in public databases. As a direct consequence of that, genome mining and comparative genomics have revealed the pervasiveness of prophages in most bacterial genomes. Prophages constitute one of the main sources of genetic diversity and strain variation associated with the virulence of many bacterial pathogens including E. coli,16,17 Streptococcus pyogenes,15,18,19 Salmonella enterica,20-23 and Staphylococcus aureus.24-26 Many phages associated with virulent strains encode powerful extracellular toxins, effector proteins participating in invasion, various enzymes such as superoxide dismutase, staphylokinase, phospholipase, DNase, proteins affecting resistance to serum and altering antigenicity, superantigens, adhesion factors, proteinases, and mitogenic factors (for reviews describing these factors, see refs. 9 and 27). The phenomenon of lysogenic conversion, whereby a phage converts a non-virulent bacterial strain into a virulent one, has been linked with the emergence of new virulent and epidemic clones such as the E. coli O157:H7 strain that acquired two Shiga toxin-encoding prophages (Sp5 and Sp15)16,28 and Vibrio cholerae that acquired the filamentous phage CTXϕ encoding the cholera toxin.29,30 Furthermore, prophage induction and mobility, which is often increased by the action of antibiotics, can shape bacterial communities,31,32 and favor the dissemination of antibiotic resistance genes33 and other mobile genetic elements such as pathogenicity islands in S. aureus,34,35 thereby promoting bacterial evolution.9,10 With the massive amount of genomic data available in public repositories, one of the great challenges in phage biology will be to link the presence of prophages in bacterial chromosomes to evident bacterial phenotypes. This task is more complex when prophages do not encode known virulence factors based on bioinformatics analyses.
Several prophages integrate into transfer RNA genes (tRNA), and in most cases the prophage carries a duplication of a portion of the disrupted gene, thereby reconstituting the coding sequence upon integration.36 A number of prophages also encode their own tRNA genes that can complement the disrupted tRNA sequence. For example, the Lactobacillus johnsonii phage Lj965 encodes 4 tRNA genes.37 It is important to mention however that prophage integration can sometimes lead to a loss of function and a negative lysogenic conversion phenotype. Good examples of that are the L54a and phi13 prophages that integrate into the chromosome of S. aureus and cause the inactivation of a lipase and a β toxin gene, respectively.38,39 Other examples of gene inactivation upon prophage integration can be found in the literature, although in many cases the functional consequences on the host have not been investigated. One such example is the integration of the ϕC2 prophage into the C. difficile chromosome, which disrupts a transcriptional regulator of the GntR family. A possible consequence of this inactivation event could be deregulation of a mannose-specific phosphotransferase system, although experimental evidence of that is not available.40
Role of Prophages in the Pathogenesis of Bacterial Infections
Bacteria have to adapt to multiple growth environments and conditions in order to survive. Some bacterial pathogens can transit between very diverse ecological niches throughout their life cycle. For example, aquatic ecosystems are the natural reservoir for V. cholerae, but this bacterium can cause severe and lethal diarrhea in humans as well.41 Bacillus anthracis is able to survive as dormant spores in soil, but can also replicate as vegetative cells in earthworms, and can cause the deadly anthrax disease in animals and humans.42 To be able to thrive under these very diverse conditions, bacteria have to express different sets of genes and modulate the expression of their genes in a coordinated manner depending on the environmental conditions. The pathogenesis of bacterial infections is often complex and generally involves several stages where bacteria need to express specific genes in a timely fashion. For example one of the very first steps in many bacterial infections is attachment and adhesion to the appropriate cell target, which is often promoted by biofilm formation.43 This close contact is necessary for several gram-negative bacteria like S. enterica and Pseudomonas aeruginosa to inject effector proteins through type II and III secretion systems (TTSS) and manipulate the host cell.44,45 The expression of extracellular toxins is also a hallmark of several pathogens.46 Some strictly anaerobic intestinal pathogens like Clostridium perfringens and Clostridium difficile are unable to survive for prolonged periods of time in the presence of oxygen. Bacteria that can reside in soil for years like B. anthracis must also be ready to face very unfavorable growth conditions. Therefore, spore formation by these pathogens is intrinsically associated with their survival and dissemination in the environment and in host-to-host transmission. For example, highly resistant spores of C. difficile can contaminate hospital settings, leading to nosocomial transmission of highly virulent strains that cause outbreaks.47 Moreover, pathogens that colonize the mammalian gut must compete for nutrients with trillions of bacteria, in addition to all associated phages that can reach several orders of magnitude.48,49 In such hostile environments, any metabolic advantage or resistance mechanism provided by prophages has its importance and can make the bacterial host cell more competitive.
“Morons”, Phage Evolution and Lysogenic Conversion
In most instances, virulence genes carried within prophages are organized as discrete autonomous genetic elements called “morons”, which are flanked on one side by a σ70-like promoter region and on the opposite side by a factor-independent transcriptional terminator. This type of organization minimizes interference with adjacent prophage genes, while allowing optimal expression of the virulence gene(s) during the lysogenic cycle where the prophage is latent and most of its genes are repressed.50,51 This structure also favors the easy transfer of morons as a functional cassette from one phage to another through genetic recombination, without problems linked to regulation of gene expression. Comparative phage genomics generally enables the quick identification of morons: an extra gene or a group of genes generally interrupts a DNA region that is contiguous in other closely relate phage genomes.50 In addition, morons often have a significantly different G + C content, which is a hallmark of a recent acquisition through horizontal gene transfer.52 Phage evolution is in part driven by horizontal exchange of functional modules between more or less related phages, thus explaining the extensive genome mosaicism among related phages.53,54 Many bacteria harbor more than one prophage in their chromosome, and the higher the degree of DNA homology between these prophages, the higher the probability that a genetic recombination event will occur. These events may lead to large chromosome rearrangements such as inversions and deletions.9,10 Homologous recombination is not restricted to endogenous prophages. In fact, virulent phages can also recombine with integrated prophages. These events occur at a low frequency, but under strong selective pressure, such as in the presence of phage resistance mechanisms, they can be observed relatively easily.55-58 A very interesting case was described by Labrie and Moineau in which mutants of the virulent phage ul36 were successively isolated upon infection of two derivatives of the same Lactococcus lactis strain carrying two different abortive phage infection (Abi) systems, AbiK and AbiT.57 Abi systems are potent antiphage mechanisms expressed by several bacterial species including E. coli, Bacillus subtilis, Bacillus licheniformis, Shigella dysenteriae, S. pyogenes, V. cholerae, and L. lactis (for a review see ref. 59). Upon infection by a sensitive phage, the Abi mechanism is activated, leading to abortion of the phage infection process and death of the infected cell, thereby reducing the viral progeny and limiting the propagation of a phage in a given bacterial population.59 In their study, Labrie and Moineau showed that infection of a L. lactis strain expressing the AbiK system with the virulent phage ul36 led to the isolation of AbiK-resistant phage mutants (ul36.k1) at a frequency of 10−7. One of these mutants, ul36.k1, was then used to infect the same L. lactis strain, but expressing a different Abi system, AbiT. Again, phage mutants were obtained at a frequency of 10−8 and these double phage mutants were fully resistant to both Abi mechanisms. Upon sequencing of the mutant phage genomes, the ul36.k1t1 mutant was found to have exchanged 79% of its genome compared with the initial ul36 genome.57 Unexpectedly, it was found that the genomic sequences were exchanged with endogenous prophages present in the L. lactis host used in these experiments.
Virulent phages have received increasing attention over the last decade because they represent a promising therapeutic alternative (“phage therapy”) to fight multidrug-resistant bacterial pathogens.60-63 For such applications, virulent phages are regarded as safe “professional killers” and are preferred over temperate phages because the only thing they can do upon infection of a susceptible host is to kill it by lysis.64 However, the elegant experiments described by Labrie and Moineau underline the great capacity of phages to interact with endogenous prophages in order to rapidly adapt to different hosts. Similar recombination events, although they would probably occur at low frequencies, could possibly occur as well if virulent phages are to be used on a large scale for therapeutic applications against pathogenic bacteria. This could eventually lead to the acquisition of virulence factors by homologous recombination between a virulent therapeutic phage and endogenous prophages from the targeted host, or the conversion of a virulent phage into a temperate phage upon acquisition of a lysogeny module or spontaneous mutation. The probability of these events occurring is unknown but it is possible that they could be subjected to selective pressures similar to those seen with antibiotics.65
The Role of Antibiotics in Phage Mobility, Horizontal Gene Transfer, and Evolution
The maintenance of lysogeny depends on a fine balance between phage and host factors. Typically, a phage repressor (CI in phage λ) binds to phage operator sequences and represses early promoters controlling the expression of genes involved in the lytic cycle. Prophage induction generally requires proteolytic cleavage and displacement of the phage repressor, which most of the time occurs upon activation of the RecA-dependent SOS response following DNA damage.66 Prophage repression is not totally efficient and depending on the phage and the host, some level of spontaneous prophage induction can occur. DNA-damaging agents such as UV light, mitomycin C, and antibiotics, in particular quinolones, are often used to force quiescent prophages to switch to a lytic cycle in laboratory conditions (Fig. 1).67 However, in “real life” situations, antibiotic-induced prophage activation can have multiple consequences. For example, the expression and secretion of Shiga toxins rely on prophage induction and ciprofloxacin treatment of mice infected by Shiga-encoding E. coli O157:H7 can aggravate the infection.68,69 Similarly, the temperate phage ϕsc1 from Streptococcus canis was found to encode a gene called scm for S. canis mitogen factor. In vitro experiments demonstrated that scm was able to elicit an inflammatory response similar to the one induced by superantigens, although to a lesser extent. Fluoroquinolones were found to induce transcription of the scm gene by 58-fold and it was concluded that the higher incidence of canine streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis (NF) observed after the introduction in canine veterinary medicine of enrofloxacin (a fluoroquinolone antibiotic) might be caused by prophage induction.70 Interestingly, the scm gene is encoded in the middle of a putative tail minor structural gene, in a different coding frame, thus underlining the importance of scrutinizing phage genomic sequences to find potential virulence genes.70 Prophage induction can also lead to transduction, i.e., the phage-mediated transfer of genetic material between bacterial cells. For example, the staphylococcal pathogenicity islands SaPI1 and SaPIBov1 are excised and circularized, encapsidated in phage-like particles and transferred at high frequency upon activation of the SOS response and induction of prophages such as 80α, ϕ11, ϕ13, and ϕ147.35,71,72 Because SaPIs from S. aureus encode various virulence factors, transduction of SaPIs represents an important mechanism driving bacterial evolution in this species.34,73
Prophages Altering Production and Secretion of Toxins
The phage-mediated transfer of extracellular toxin genes is the classical type of lysogenic conversion. For example, phage CEβ encodes one of the most powerful toxins ever described, the botulinum neurotoxin type C1.74 Some bacterial toxins rely on a specialized secretion apparatus to be exported to the extracellular milieu. For instance, the release of cholera toxin and the enterotoxin A produced by V. cholerae and P. aeruginosa, respectively, rely on a type II secretion system.75 Other toxins however, rely on bacterial cell lysis to be released, like all clostridial neurotoxins produced by Clostridium botulinum and Clostridium tetani.76 The Shiga toxins encoded by E. coli prophages also require cell lysis.77,78 The Shiga toxins carried on the E. coli phages H-19B and 933W are expressed from a late phage promoter (PR’) located downstream of the antiterminator protein Q and upstream of the lysis cassette of this lambdoid phage.79 Upon prophage induction and initiation of the lytic cycle, the PR’ promoter initiates transcription of the late genes, including the lysis cassette and the Shiga toxins. Subsequent phage-induced cell lysis enables the release of toxins into the extracellular environment.80 Transcription of the stx2 gene is mainly initiated at the PR’ phage promoter and thus, Stx2 production is greatly dependent and influenced by prophage induction.68,81 Lysogenic conversion involving other phage-encoded toxins has been well described in other pathogens, including Corynebacterium diphtheriae producing the diphteria toxin, which is encoded on phage β,82 S. pyogenes that produces several phage-encoded toxins (A, A1, A3, C, I, H, M, L, K),83-86 and S. aureus that encodes enterotoxins (A and P), exfoliative toxin A, and Panton-Valentine leukocidin, all carried on prophages.87-90
In many cases however, phages do not code for toxin genes and hence, they are incapable of directly converting their host into toxin producers. Nevertheless, recent studies in C. difficile have shown that prophages can indirectly modulate toxin production in an unexpected way. C. difficile is an emerging gram-positive nosocomial pathogen causing opportunistic intestinal infections in humans and animals. The toxigenic potential of C. difficile is mainly due to the expression of two large exotoxins, TcdA and TcdB.91 These toxins are encoded on a ~19.6 kb pathogenicity locus called the PaLoc and are under the positive regulation of the alternative sigma factor TcdR (Fig. 2).92 TcdC is an anti-sigma factor also encoded on the PaLoc and which interferes with the RNA polymerase/TcdR holoenzyme, thereby repressing toxin gene transcription.93,94 Under normal laboratory conditions, TcdC represses transcription of the toxin genes during logarithmic growth. Upon entry into stationary phase, the expression of TcdC decreases, thus relieving the inhibition of TcdR. Transcription of tcdA and tcdB is then allowed and toxins are produced. Various signals have been identified that participate in the control of toxin gene expression, like the presence of carbon sources, various amino acids and temperature,95-99 and the master regulator CodY plays a central role in this regulation.100 In 2005, Goh et al. reported an increased toxin production in lysogens of C. difficile carrying the temperate phages ϕC2, ϕC6, and ϕC8.101 Likewise, the temperate phage ϕCD38-2 was shown to stimulate the production of TcdA and TcdB toxins about 2-fold once introduced as a prophage into epidemic strains of C. difficile.102 In the latter case, the level of mRNA from all 5 PaLoc genes was 2- to 3-fold higher in the lysogens carrying the prophage and the level of expression was even greater in some lysogens.102 The molecular mechanism by which these phages affect toxin gene expression remains unknown, but in a similar study, Govind et al. demonstrated that RepR, a transcriptional regulator encoded by the temperate phage ϕCD119, could directly repress toxin gene transcription in C. difficile.103 In lysogens carrying ϕCD119, the repR gene is transcribed and the gene product binds to its own promoter region. However, RepR also binds DNA regions downstream of the tcdR promoter thereby blocking TcdR expression. In the absence of TcdR, transcription of tcdA and tcdB is not possible (Fig. 2).103
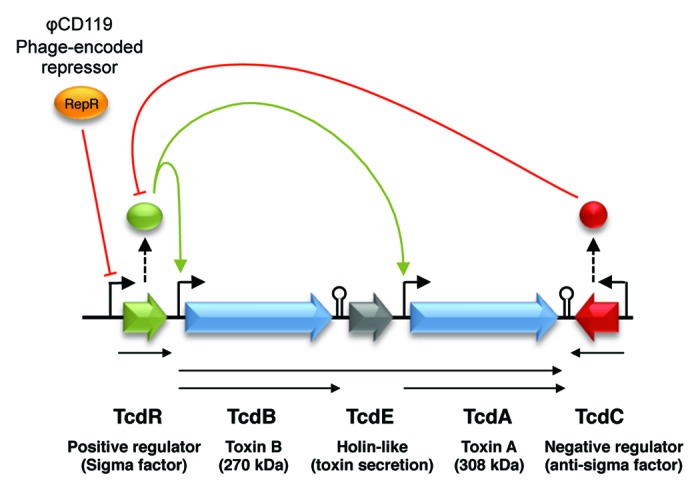
Figure 2. Organization of the pathogenicity locus (PaLoc) in C. difficile. Arrowheads indicate a positive effect on the target and blunt ended lines indicate repression of the target. Phage ϕCD119 expresses a repressor that binds to the promoter region of TcdR, thereby repressing transcription of tcdR and hence, transcription of the toxin genes.103
The similarity between PaLoc genes in C. difficile and phage genes, as well as the possibility for a phage protein like RepR to bind regulatory regions from the PaLoc underlines the existence of some level of crosstalk between prophages and bacterial genes in C. difficile. Prophages have co-evolved with their bacterial hosts for millions of years. As a consequence of this proximity, some prophages have been seamlessly integrated within the regulatory network of their host. A good example of this is the phage-encoded SopE effector protein for which secretion is dependent on the invasion-associated type III secretion system (T3SS-1) of the Salmonella pathogenicity island (SPI-1).104 SopE is a potent inducer of intestinal inflammation and activates several innate immune signaling pathways.105 Salmonella cells lysogenized with phage SopEϕ are more invasive because they produce the membrane-associated effector protein SopE, which functions as a guanine nucleotide exchange factor activating Rac1 and Cdc42 causing membrane ruffling and promoting Salmonella invasion.106,107 The interesting feature of SopE is that its expression and secretion are controlled in trans by the T3SS-encoded factors InvB, InvF, and SicA. The InvF/SicA complex binds the sopE promoter region within the SopEϕ prophage to allow its transcription, and the InvB chaperone enables secretion of SopE.104,108 This example shows that regulatory circuits of prophages within their hosts can be complex and exquisitely interconnected.
“Phage-Like” Genes Involved In Toxin Secretion
The fact that a phage protein can bind regulatory regions of the C. difficile PaLoc supports the hypothesis that the PaLoc evolved from an ancient prophage. Indeed, one very interesting characteristic of the PaLoc is the functional similarity of one of its gene product, TcdE, with phage holins.109 Because of its potential cell-permeabilizing activity, TcdE was proposed to participate in toxin secretion.109 This hypothesis was later confirmed experimentally in the study by Govind et al. who observed a dramatic reduction in extracellular toxin accumulation in a mutant C. difficile strain 630 carrying an inactivated tcdE gene. In addition, the C. difficile tcdE gene was able to complement an E. coli λ lysogen encoding a non-functional holin gene.109,110 Although there is yet no proof that phage holins participate in toxin secretion in C. difficile, this possibility cannot be ruled out considering the functional similarities between holins and TcdE.109 Lysis-associated genes are normally expressed at the end of the phage lytic cycle, thus suggesting that prophage induction could be linked to toxin release in C. difficile, as for Shiga toxins in E. coli. Spontaneous prophage induction of phage ϕCD38-2 did not contribute significantly to toxin release in vitro,102 but the situation might be different with other phages, or during a real infection, especially if antibiotics that stimulate prophage induction are present.67
Recently, Salmonella enterica serovar Typhi was found to encode a homolog of phage muramidases that control secretion of the typhoid toxin. S. Typhi is an exclusive human pathogen causing the life-threatening typhoid fever, due to the expression of the cytolethal distending toxin (CDT). This toxin is composed of three subunits encoded by the cdtA, cdtB, and cdtC genes. CdtA and CdtC form the heterologous protein subunit B necessary for the delivery into mammalian cells of subunit A, the active part of the toxin composed of CdtB.111 Several bacterial species produce the CDT toxin, including E. coli, Shigella dysenteriae, and S. Typhi, but in the latter pathogen, orthologs of the cdtA and cdtC genes are absent. The consequence is that S. Typhi must be internalized within mammalian cells in order to deliver the CDT toxin.111 A gene encoding an N-acetyl-β-d-muramidase similar to a phage endolysin has been identified within the islet encoding the cdtB gene in S. Typhi. Deletion of this gene prevented delivery of the toxin upon infection of epithelial cells, so the gene product was named TtsA for typhoid toxin secretion A.112 Although TtsA is not encoded within a phage genome in S. Typhi, a number of other muramidases of the same family were identified in the context of complete or incomplete prophages. As observed with many phage lysis cassettes, a gene encoding a holin is often located next to the muramidase, but not in S. Typhi. This has led to the suggestion that a holin encoded elsewhere in the genome of S. Typhi probably fulfils the task of permeabilizing the membrane in order for TtsA to reach the peptidoglycan layer. Thus, TtsA might represent the recent adaptation of a phage-associated enzyme carrying out a different function, which might also be the case for TcdE encoded in the PaLoc of C. difficile.110,112
The Contribution of Prophages to Biofilm Formation
The importance for microorganisms to build complex, surface-attached microbial communities termed biofilms is now well established.113,114 Usually characterized by a complex environment composed of exopolysaccharides (EPS), proteins, and DNA, biofilms have been tightly linked to bacterial persistence and dissemination in particular niches.115 The mechanisms and ecological consequences of biofilm formation and dispersal have been studied in detail (for a recent review see ref. 116) and temperate phages have been shown to drastically influence the dynamics of biofilm formation and dispersal by a number of important pathogens such as P. aeruginosa,117 Streptococcus pneumoniae,118 B. anthracis,42 and E. coli.119
B. anthracis causes anthrax, which is a deadly zoonotic disease. It is generally assumed that once B. anthracis has killed its host, the bacterium remains in the carcass and the surrounding environment in the form of highly resistant and inert spores until another host ingests the spores and the infection cycle starts again.42 An elegant series of experiments by Schuch and Fischetti42 demonstrated the importance of temperate phages in altering multiple phenotypes in B. anthracis, including biofilm formation, thus readdressing the “spore dogma” around B. anthracis. Temperate phages infecting B. anthracis were isolated from various environmental samples (e.g., phage Wip1, Wip2, Frp1, Slp1, and Bcp1) or induced from bacteria from the soil, fern rhizosphere, or the gut of the earthworm Eisenia fetida (e.g., phage Wβ, Wip4, Wip5, and Frp2). The role of these phages on B. anthracis lifestyle has been assessed in the prophage-free ΔSterne strain into which either of these phages was introduced as a prophage. Among multiple phenotypes, biofilm formation was deeply influenced by lysogenization. As demonstrated in laboratory conditions, the ΔSterne strain did not produce biofilm after 3 mo. However, lysogenization of ΔSterne with phages Bcp1, Wip1, Wip4, and Frp2 resulted in formation of a robust, complex and viable biofilm as witnessed by microscopy observations and bacterial counts. A ΔSterne/Wip2 lysogen was isolated from the 3-mo-old biofilm and the Wip2 prophage was cured from the strain. The cured ΔSterne strain had completely lost the ability to form viable biofilms but reintroduction of Wip2 reversed the phenotype. Using expression libraries of Bcp1 and Wip4, a phage-encoded RNA polymerase sigma factor was identified in each phage genome and when cloned and expressed on a plasmid, the genes induced EPS production and biofilm production similar to that observed with ΔSterne lysogens carrying the entire prophages. Insertional inactivation of the sigma factor in a ΔSterne/Wip4 lysogen completely abolished biofilm formation. This study clearly demonstrated that phage-encoded RNA polymerase sigma factors can act in trans to specifically induce the expression of bacterial genes required for biofilm formation by B. anthracis.42
P. aeruginosa is well known for its capacity to produce biofilm and to colonize water lines and various abiotic surfaces. This important gram-negative pathogen often colonizes the lungs of cystic fibrosis (CF) patients. P. aeruginosa is also capable of forming small colony variants (SCVs) and since these variants grow slowly, they are generally resistant to drug therapies, which makes CF patients more vulnerable to chronic infection by P. aeruginosa.120 Interestingly, the most highly activated genes in P. aeruginosa PAO1 biofilms are those within a filamentous prophage, Pf4,121 and accordingly, free phages have been isolated from biofilm effluents from strain PAO1 and other clinical isolates. Rice et al. showed that complete deletion of the filamentous phage Pf4 from P. aeruginosa PAO1 prevented the typical lysis and death of bacteria at the late stages of biofilm development.117 The occurrence of SCVs at late stages of biofilm development generally correlates with the conversion of Pf4 into a superinfective form, but SCVs were not detected in biofilm effluents of the PAO1 strain cured of Pf4. Using a nasal aspiration mouse model of acute pneumonia, Rice et al. demonstrated that mice infected with P. aeruginosa PAO1 lacking the Pf4 prophage survived significantly longer than mice infected with the wild-type strain. Therefore, their study confirmed the important role of filamentous prophages in modulating biofilm dispersal, in the formation of drug-resistant SCVs, and in the virulence of P. aeruginosa.117
Extracellular DNA (eDNA) appears to be an important constituent of robust biofilms in many bacterial species including Staphylococcus epidermidis,122 V. cholerae,123 Bordetella sp.,124 and S. pneumoniae.118 In the latter study, Carrolo et al. demonstrated that the presence of eDNA in S. pneumoniae biofilms was the result of bacterial lysis caused by the synergic action of the host-encoded autolysin LytA and a phage-encoded lysin Sv1 encoded on the temperate phage SV1. Total biofilm production was compared between S. pneumoniae R36A and the R36AP lysogen carrying phage SV1, and strain R36AP produced denser and more robust biofilm than its non-lysogenic counterpart. This difference seemed to rely on the activity of the phage lysin Sv1, since inactivation of the corresponding gene resulted in lower biofilm production. Inactivation of the bacterial autolysin LytA had a similar effect, but the capacity of S. pneumoniae to produce biofilm was completely abolished in a double mutant lacking both LytA and Sv1.118 The expression of the phage lysin Sv1 is expected to occur at the end of the lytic cycle, which suggested that the Sv1-mediated cell lysis and release of eDNA could in fact be the result of spontaneous induction of the SV1 prophage. Accordingly, free phage particles were detected during biofilm formation and the highest titers were observed at the peak of biofilm formation. Taken together, these results strongly suggest that eDNA is essential for proper biofilm formation by S. pneumoniae, which is mainly resulting from cell lysis of a fraction of the bacterial population either by autolysis (mediated by LytA) or upon spontaneous prophage induction.
Cryptic or “defective” prophages are sometimes considered harmless because it is generally assumed that they are quiescent and that most of their genes should be repressed. Moreover, since they have lost their capacity to produce genuine infectious particles, they should not pose any problem to the host. Wang et al.119 underlined the importance of such cryptic prophages in modulating biofilm formation in E. coli. The E. coli K-12 strain has 9 cryptic prophages in its chromosome, comprising 3.6% of the total genomic DNA. Precise removal of all nine cryptic prophages yielded a cured E. coli strain termed Δ9. When assessed for biofilm formation in minimal medium, the cured strain Δ9 had almost completely lost the ability to form biofilm. Additionally, single-phage mutants within the same E. coli K-12 strain have been constructed in order to assess the contribution of each individual phage to the observed phenotype. The phage remnants e14, rac, and CP4-44 were found to have the greatest impact on biofilm formation since curing E. coli K-12 from any of these remnants impaired biofilm production.119 Furthermore, single gene deletions were created to identify specific genes within e14 and rac that were directly responsible for the observed phenotype. The genes identified were intE (integrase) and ymfD (putative SAM-dependent methyl transferase) for e14 and intR (integrase), stfR (putative tail-fiber), ydaS and ydaW (putative transcriptional regulators), and ydaF (unknown function) for rac. The exact mechanism(s) by which these genes affect biofilm formation remains to be elucidated. Interestingly, the susceptibility of E. coli toward quinolones and β-lactams was significantly increased in the Δ9 strain cured of its prophage remnants. Single prophage deletions showed that CP4-6, rac, and Qin were responsible for the lower susceptibility to these antibiotics. The authors showed that rac and Qin encoded DicB and KilR respectively, and whose expression correlated with an inhibition of cell division and reduced susceptibility to quinolones and β-lactams. The study by Wang et al. clearly showed that the presence of defective prophages can contribute significantly to important bacterial phenotypes such as biofilm formation and drug-resistance.119
Phages that Modulate Sporulation
A number of bacterial species sporulate in order to survive under nutrient limitation or harsh environmental conditions. The endospore is a metabolically dormant and inert form of the bacterial cell, which can withstand extremes of temperature, ionizing radiation, and desiccation. Spores are also resistant to many chemical agents, including antibiotics and several disinfectants.47,125 Estimates of the longevity of bacterial spores range from thousands to millions of years.125 Sporulation is a multistage process involving a cascade of gene activation and repression that has been studied extensively in B. subtilis, which is to date one of the best models for studying the molecular mechanism of sporulation.126,127 In practical terms, bacterial spores formed by pathogenic bacteria can be of serious concern in food safety and human health. Important human and animal pathogens produce spores that cause food poisoning (Bacillus cereus and C. botulinum), wound infections (C. perfringens and C. tetani) and intestinal diarrhea and colitis (C. difficile).128,129 Spores from highly pathogenic bacteria such as B. anthracis that cause anthrax also represent potential biological weapons.130 In hospitals and long-term care facilities, spores of C. difficile are frequently responsible for outbreaks due to the nosocomial transmission of spores that are difficult to eradicate from surfaces and equipments and that can be transmitted via the hands of healthcare workers and visitors.47
Several spore-converting phages have been isolated in B. subtilis,131,132 Bacillus pumilus,133,134 Bacillus thuringiensis,135 and B. cereus.136 These phages were isolated based on their capacity to enhance the rate or extent of sporulation of wild-type cells or their ability to complement different mutant strains defective in sporulation. For example, lysogenization of B. subtilis with phages PMB12 and SP10 convert sporulation-negative strains into sporulating cells.131-133,137 PMB12 was found to suppress the stage 0 sporulation mutations spoCM-1, spo0J93, and spo0K141, suggesting that this phage influenced the sporulation process at an early stage,132,137 and at least 3 genes from PMB12 (scn genes) seemed to be required to induce sporulation.137 A subsequent study showed that lysogens of B. subtilis strain 3-13 carrying either of PMB12 or SP10 were able to sporulate in the presence of enough glucose to inhibit sporulation and the expression of α-amylase in the wild-type parental strain.132 Therefore, PMB12 and SP10 relieve the catabolite repression-associated inhibition of sporulation and α-amylase expression in B. subtilis.
A few studies have addressed the role of temperate phages in sporulation of C. perfringens. In 1977, Stewart and Johnson reported that curing C. perfringens strain S9 from its integrated prophage ϕS9 resulted in a 1-log reduction in the maximum number of spores. The time that was necessary to reach the maximum number of spores was also increased to 9–10 h in the cured strain S9CR instead of 3 h in the S9 lysogen.138 Reintroduction of ϕS9 into the cured strain restored sporulation to levels close to the parental lysogen. These results suggested that ϕS9 was able to stimulate sporulation in C. perfringens. However, the genome sequence of ϕS9 was published recently and the capacity of the phage to influence sporulation of C. perfringens was reassessed.138,139 The sporulation-promoting phenotype initially reported by Stewart and Johnson could not be reproduced and the sporulation kinetics and maximum number of spores observed in the lysogen and the cured strain were similar, although similar experimental conditions were used.139 In 2002, the genome sequence of ϕ3626, another temperate phage infecting C. perfringens, was published.140 The presence of a sporulation-associated sigma factor (ORF32) in the genome of ϕ3626 suggested that reprogramming of the RNA polymerase toward sporulation genes could possibly enable the prophage to promote sporulation of its host as reported for ϕS9, but the experimental demonstration of this has not yet been reported.138,140
The alternative sigma factor σK is the last sigma factor to be activated during the sporulation cascade.127 In B. subtilis, the full-length sigK is encoded by two genes, spoIVCB and spoIIIC, interrupted by a 48-kb intervening region.141 This DNA fragment, called skinBs in B. subtilis for sigK intervening element, is precisely excised from the chromosome of the mother cell compartment of sporulating cells by a recombination event between two 5-bp repeated sequences, thereby enabling expression of sigK in this specific compartment.142 A 14-kb skinCd element interrupting the sigK gene was also identified in the genome of C. difficile strain 630.143 Creation of a merodiploid CD196 strain carrying both interrupted and uninterrupted copies of sigK led to a defective sporulation phenotype as opposed to wild-type CD196 cells that sporulated efficiently. This result led to the conclusion that interruption of sigK and timely excision of the skinCd element were required for full sporulation of C. difficile.144 The observation that strains CD37 and ATCC9689, two natural isolates of C. difficile lacking a skinCd, were defective in sporulation further supported the above conclusion.144 The skinBs and skinCd elements contain phage-related genes, which suggests that the skin elements might represent cryptic remnants of ancient prophages.141,143 In agreement with that hypothesis, the temperate phage ϕS63 was found to integrate within σK in C. perfringens, thus representing the first example of a skin element in this species, and also the first example of a functional prophage interrupting σK.139 However, contrary to C. difficile where interruption of σK is required for efficient sporulation, interruption of σK in B. subtilis, and probably C. perfringens as well, is not essential because in the two latter species, σK is expressed as an inactive pro-σK that must be processed by a membrane-associated metalloprotease to release the active σK.126,139
But what could be the advantage for a phage to promote sporulation, knowing that spores are refractory to phage infection? A possible reason could be that phage genomes trapped inside the spores have a selective advantage over free viral particles, due to the protection provided by the spore under extreme conditions. For typical prophages that integrate into the genome of their host, promoting sporulation might not be a critical feature because every single lysogen carries one copy of the phage genome. However, a common feature of spore-converting phages, including phage SP10 in B. subtilis, is their pseudotemperate nature, i.e., prophages do not integrate into the genome of their host but remain as extrachromosomal DNA during lysogeny (Fig. 1).145 This leads to a characteristic instability of the prophage genome in these lysogens and a fraction of the bacterial population eventually loses the phage during cell division.145-147 A number of pseudotemperate phages have evolved in such a way that their genome is efficiently partitioned in the growing forespore along with the bacterial chromosome, thus escaping a certain death by remaining trapped inside the endospore. Even the strictly lytic phage ϕ29 is able to exploit this survival strategy. When sporulation of B. subtilis initiates, the lytic cycle of ϕ29 is repressed, the phage genome is partitioned inside the spore where it remains quiescent until favorable growth conditions are met to allow spore germination.148 If a pseudolysogenic bacterial species is unable to sporulate efficiently, survival of pseudotemperate phages might be seriously compromised because only a fraction of the spores will contain the trapped phage genome, as opposed to all spores derived from true lysogens. Hence, these pseudotemperate phages manage to increase the frequency of sporulation of their host possibly to increase the chances that a sufficient number of phage genomes will get trapped inside spores in order to sustain the phage population.131,132
On the other hand, preventing sporulation could also provide some advantage to the bacterial host, as observed for temperate phages infecting B. anthracis.42 A number of temperate phages active on B. anthracis have been isolated from diverse environments and the role of these phages on the host lifestyle of B. anthracis has been assessed in lysogens of the ΔSterne strain carrying either of these phages. Findings by Schuch and Fischetti revealed that some of the prophages were able to profoundly alter the sporulating capacity of the bacterium in broth cultures.42 For example, after 1 d of incubation at 37 °C under good aeration, the ΔSterne strain produced about 5 × 108 spores/ml whereas lysogens carrying Wip4, Wip5, or Frp1 prophages did not sporulate (<10 spores/ml). Blocking of B. anthracis sporulation by some prophages (e.g., Wip1 and Wip4) also coincided with strong biofilm formation, resulting in lysogens surviving in soil for several months whereas the non-lysogenic strain ΔSterne did not survive under these conditions.42 On the other hand, after 5 d of incubation at 24 °C under poor aeration, the ΔSterne strain did not sporulate (<10 spores/ml), but lysogens carrying phages Wβ, Wip1, Wip2, or Frp2 produced between 105 and 106 spores/ml. Modification of the sporulation phenotype required the expression of an alternative RNA polymerase-associated sigma factor encoded by the phage. The expression of the phage locus encoding this gene (locus bcp25,26 in phage Bcp1 and wip48,49 in phage Wip4) gave the same phenotype as the complete prophage.42 These experiments demonstrated that some prophages efficiently block sporulation of B. anthracis, therefore enabling the bacterium to replicate in the form of vegetative cells in an alternative host, for instance the gut of the earthworm E. fetida that serves as a natural reservoir for the active amplification of this pathogen. Long-term survival of B. anthracis vegetative cells within biofilms in the soil could also favor survival and mobility of this group of phages that seem to have adopted a pseudotemperate lifestyle.42
Concluding Remarks and Future Perspectives
Prophages can modify the lifestyle, fitness, virulence, and evolution of their bacterial host in numerous ways. Lysogenic conversion involving potent toxins is probably the most determinant contribution of prophages known to date, but as we study phage–host interactions in different bacterial species, we find novel ways by which prophages can influence their host. We currently grasp only a small fraction of the total phage diversity, both in terms of genetic content, but also in terms of novel molecular mechanisms enabling modulation of bacterial phenotypes and lifestyles, including virulence. The growing number of whole bacterial genomic sequences released in public databases will provide great opportunities to seek novel prophage sequences. However, all this sequence data will be of limited use if the underlying phage biology is not understood and this is why it will be important to continue basic phage research in the post-genomic era. There is a current trend toward understanding phage–host interactions and bacterial–host interactions on a global scale. For example, the gut microbiota is now recognized as a key element in human health. Dysbiosis, which occurs when the “normal” microbiota is altered, for example by antibiotic treatments, is associated with opportunistic bacterial pathogens such as C. difficile, probably being one of the most striking cases. But dysbiosis of the gut microbiota is also associated with important chronic disorders, such as obesity, type 2 diabetes, and inflammatory bowel diseases (Crohn disease and ulcerative colitis).149 The human gut contains large amounts of free viral particles, most of them being bacteriophages probably released after spontaneous prophage induction of lysogenic gut bacteria.5 This is not surprising since phages outnumber bacteria by a factor of at least 10 and the gut is colonized with trillions of bacteria with 60–70% of them being lysogenic.150 However, although the influence of phages on bacterial populations from aquatic environments and other ecological niches has been studied in more detail,2 the influence of bacteriophages in shaping the human gut microbiota has been addressed in only a few studies (for a review see ref. 32) and this will certainly represent one of the major challenges over the next era of phage research.
Acknowledgments
LCF is member of the Etienne-Le Bel clinical research center of the CHUS and the holder of a junior 2 salary award from the Fonds de la Recherche du Québec—Santé (FRQS).
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/24498
References
- 1.Brüssow H, Hendrix RW. Phage genomics: small is beautiful. Cell. 2002;108:13–6. doi: 10.1016/S0092-8674(01)00637-7. [DOI] [PubMed] [Google Scholar]
- 2.Suttle CA. Marine viruses--major players in the global ecosystem. Nat Rev Microbiol. 2007;5:801–12. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 3.Suttle CA. Viruses in the sea. Nature. 2005;437:356–61. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 4.Breitbart M, Haynes M, Kelley S, Angly F, Edwards RA, Felts B, et al. Viral diversity and dynamics in an infant gut. Res Microbiol. 2008;159:367–73. doi: 10.1016/j.resmic.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, et al. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220–3. doi: 10.1128/JB.185.20.6220-6223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chibani-Chennoufi S, Bruttin A, Dillmann ML, Brüssow H. Phage-host interaction: an ecological perspective. J Bacteriol. 2004;186:3677–86. doi: 10.1128/JB.186.12.3677-3686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibani-Chennoufi S, Sidoti J, Bruttin A, Dillmann ML, Kutter E, Qadri F, et al. Isolation of Escherichia coli bacteriophages from the stool of pediatric diarrhea patients in Bangladesh. J Bacteriol. 2004;186:8287–94. doi: 10.1128/JB.186.24.8287-8294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon TS, Dhillon EK, Chau HC, Li WK, Tsang AH. Studies on bacteriophage distribution: virulent and temperate bacteriophage content of mammalian feces. Appl Environ Microbiol. 1976;32:68–74. doi: 10.1128/aem.32.1.68-74.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brüssow H, Canchaya C, Hardt W-D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canchaya C, Fournous G, Brüssow H. The impact of prophages on bacterial chromosomes. Mol Microbiol. 2004;53:9–18. doi: 10.1111/j.1365-2958.2004.04113.x. [DOI] [PubMed] [Google Scholar]
- 11.Guttman B, Raya R, Kutter E, Kutter E, Sulakvelidze A. Basic phage biology. In: Kutter E, Sulakvelidze A, eds. Bacteriophages: biology and applications. Boca Raton: CRC Press, 2005;29–66. [Google Scholar]
- 12.Ackermann H-W, Prangishvili D. Prokaryote viruses studied by electron microscopy. Arch Virol. 2012;157:1843–9. doi: 10.1007/s00705-012-1383-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- 14.Casjens S. Prophages and bacterial genomics: what have we learned so far? Mol Microbiol. 2003;49:277–300. doi: 10.1046/j.1365-2958.2003.03580.x. [DOI] [PubMed] [Google Scholar]
- 15.Banks DJ, Beres SB, Musser JM. The fundamental contribution of phages to GAS evolution, genome diversification and strain emergence. Trends Microbiol. 2002;10:515–21. doi: 10.1016/S0966-842X(02)02461-7. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi M, Kurokawa K, Hayashi T. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends Microbiol. 2001;9:481–5. doi: 10.1016/S0966-842X(01)02173-4. [DOI] [PubMed] [Google Scholar]
- 17.Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998;352:1207–12. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 18.Aziz RK, Edwards RA, Taylor WW, Low DE, McGeer A, Kotb M. Mosaic prophages with horizontally acquired genes account for the emergence and diversification of the globally disseminated M1T1 clone of Streptococcus pyogenes. J Bacteriol. 2005;187:3311–8. doi: 10.1128/JB.187.10.3311-3318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary PP, LaPenta D, Vessela R, Lam H, Cue D. A globally disseminated M1 subclone of group A streptococci differs from other subclones by 70 kilobases of prophage DNA and capacity for high-frequency intracellular invasion. Infect Immun. 1998;66:5592–7. doi: 10.1128/iai.66.11.5592-5597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke FJ, Wain J, Fookes M, Ivens A, Thomson N, Brown DJ, et al. Prophage sequences defining hot spots of genome variation in Salmonella enterica serovar Typhimurium can be used to discriminate between field isolates. J Clin Microbiol. 2007;45:2590–8. doi: 10.1128/JCM.00729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermans APHM, Abee T, Zwietering MH, Aarts HJM. Identification of novel Salmonella enterica serovar Typhimurium DT104-specific prophage and nonprophage chromosomal sequences among serovar Typhimurium isolates by genomic subtractive hybridization. Appl Environ Microbiol. 2005;71:4979–85. doi: 10.1128/AEM.71.9.4979-4985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson N, Baker S, Pickard D, Fookes M, Anjum M, Hamlin N, et al. The role of prophage-like elements in the diversity of Salmonella enterica serovars. J Mol Biol. 2004;339:279–300. doi: 10.1016/j.jmb.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa-Bossi N, Uzzau S, Maloriol D, Bossi L. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol Microbiol. 2001;39:260–71. doi: 10.1046/j.1365-2958.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- 24.Rahimi F, Bouzari M, Katouli M, Pourshafie MR. Prophage and antibiotic resistance profiles of methicillin-resistant Staphylococcus aureus strains in Iran. Arch Virol. 2012;157:1807–11. doi: 10.1007/s00705-012-1361-4. [DOI] [PubMed] [Google Scholar]
- 25.Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, et al. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J Bacteriol. 2009;191:3462–8. doi: 10.1128/JB.01804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae T, Baba T, Hiramatsu K, Schneewind O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol. 2006;62:1035–47. doi: 10.1111/j.1365-2958.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 27.Boyd EF. Bacteriophage-encoded bacterial virulence factors and phage-pathogenicity island interactions. Adv Virus Res. 2012;82:91–118. doi: 10.1016/B978-0-12-394621-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–4. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 30.Boyd EF, Heilpern AJ, Waldor MK. Molecular analyses of a putative CTXphi precursor and evidence for independent acquisition of distinct CTX(phi)s by toxigenic Vibrio cholerae. J Bacteriol. 2000;182:5530–8. doi: 10.1128/JB.182.19.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duerkop BA, Clements CV, Rollins D, Rodrigues JLM, Hooper LV. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci U S A. 2012;109:17621–6. doi: 10.1073/pnas.1206136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills S, Shanahan F, Stanton C, Hill C, Coffey A, Ross RP. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013;4:4–16. doi: 10.4161/gmic.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, LeJeune JT. Transduction of bla(CMY-2), tet(A), and tet(B) from Salmonella enterica subspecies enterica serovar Heidelberg to S. Typhimurium. Vet Microbiol. 2008;129:418–25. doi: 10.1016/j.vetmic.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009;323:139–41. doi: 10.1126/science.1164783. [DOI] [PubMed] [Google Scholar]
- 35.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penadés JR. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol. 2005;56:836–44. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 36.Campbell AM. Chromosomal insertion sites for phages and plasmids. J Bacteriol. 1992;174:7495–9. doi: 10.1128/jb.174.23.7495-7499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ventura M, Canchaya C, Pridmore D, Berger B, Brüssow H. Integration and distribution of Lactobacillus johnsonii prophages. J Bacteriol. 2003;185:4603–8. doi: 10.1128/JB.185.15.4603-4608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman D, Knights J, Russell R, Shanley D, Birkbeck TH, Dougan G, et al. Insertional inactivation of the Staphylococcus aureus β-toxin by bacteriophage phi 13 occurs by site- and orientation-specific integration of the phi 13 genome. Mol Microbiol. 1991;5:933–9. doi: 10.1111/j.1365-2958.1991.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee CY, Iandolo JJ. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986;166:385–91. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh S, Ong PF, Song KP, Riley TV, Chang BJ. The complete genome sequence of Clostridium difficile phage phiC2 and comparisons to phiCD119 and inducible prophages of CD630. Microbiology. 2007;153:676–85. doi: 10.1099/mic.0.2006/002436-0. [DOI] [PubMed] [Google Scholar]
- 41.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–14. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuch R, Fischetti VA. The secret life of the anthrax agent Bacillus anthracis: bacteriophage-mediated ecological adaptations. PLoS One. 2009;4:e6532. doi: 10.1371/journal.pone.0006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 44.Müller AJ, Kaiser P, Dittmar KEJ, Weber TC, Haueter S, Endt K, et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe. 2012;11:19–32. doi: 10.1016/j.chom.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–65. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet. 2000;356:1324. doi: 10.1016/S0140-6736(00)02819-1. [DOI] [PubMed] [Google Scholar]
- 48.Keeney KM, Finlay BB. Enteric pathogen exploitation of the microbiota-generated nutrient environment of the gut. Curr Opin Microbiol. 2011;14:92–8. doi: 10.1016/j.mib.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura M, Sozzi T, Turroni F, Matteuzzi D, van Sinderen D. The impact of bacteriophages on probiotic bacteria and gut microbiota diversity. Genes Nutr. 2010; 10.1007/s12263-010-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, Hendrix RW. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J Mol Biol. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- 51.Hendrix RW, Lawrence JG, Hatfull GF, Casjens S. The origins and ongoing evolution of viruses. Trends Microbiol. 2000;8:504–8. doi: 10.1016/S0966-842X(00)01863-1. [DOI] [PubMed] [Google Scholar]
- 52.Hendrix RW. Bacteriophages: evolution of the majority. Theor Popul Biol. 2002;61:471–80. doi: 10.1006/tpbi.2002.1590. [DOI] [PubMed] [Google Scholar]
- 53.Lucchini S, Desiere F, Brüssow H. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J Virol. 1999;73:8647–56. doi: 10.1128/jvi.73.10.8647-8656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desiere F, Lucchini S, Brüssow H. Evolution of Streptococcus thermophilus bacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology. 1998;241:345–56. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 55.Bouchard JD, Moineau S. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology. 2000;270:65–75. doi: 10.1006/viro.2000.0226. [DOI] [PubMed] [Google Scholar]
- 56.Moineau S, Pandian S, Klaenhammer TR. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl Environ Microbiol. 1994;60:1832–41. doi: 10.1128/aem.60.6.1832-1841.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labrie SJ, Moineau S. Abortive infection mechanisms and prophage sequences significantly influence the genetic makeup of emerging lytic lactococcal phages. J Bacteriol. 2007;189:1482–7. doi: 10.1128/JB.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durmaz E, Klaenhammer TR. Genetic analysis of chromosomal regions of Lactococcus lactis acquired by recombinant lytic phages. Appl Environ Microbiol. 2000;66:895–903. doi: 10.1128/AEM.66.3.895-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chopin M-C, Chopin A, Bidnenko E. Phage abortive infection in lactococci: variations on a theme. Curr Opin Microbiol. 2005;8:473–9. doi: 10.1016/j.mib.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Thiel K. Old dogma, new tricks--21st Century phage therapy. Nat Biotechnol. 2004;22:31–6. doi: 10.1038/nbt0104-31. [DOI] [PubMed] [Google Scholar]
- 61.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 62.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother. 2007;51:2765–73. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care. 2009;18:237–8, 240-3. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 64.Brüssow H. Phage therapy: the Escherichia coli experience. Microbiology. 2005;151:2133–40. doi: 10.1099/mic.0.27849-0. [DOI] [PubMed] [Google Scholar]
- 65.Levin BR, Bull JJ. Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol. 2004;2:166–73. doi: 10.1038/nrmicro822. [DOI] [PubMed] [Google Scholar]
- 66.Waldor MK, Friedman DI. Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol. 2005;8:459–65. doi: 10.1016/j.mib.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Meessen-Pinard M, Sekulovic O, Fortier LC. Evidence of in vivo prophage induction during Clostridium difficile infection. Appl Environ Microbiol. 2012;78:7662–70. doi: 10.1128/AEM.02275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–70. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 69.Kimmitt PT, Harwood CR, Barer MR. Induction of type 2 Shiga toxin synthesis in Escherichia coli O157 by 4-quinolones. Lancet. 1999;353:1588–9. doi: 10.1016/S0140-6736(99)00621-2. [DOI] [PubMed] [Google Scholar]
- 70.Ingrey KT, Ren J, Prescott JF. A fluoroquinolone induces a novel mitogen-encoding bacteriophage in Streptococcus canis. Infect Immun. 2003;71:3028–33. doi: 10.1128/IAI.71.6.3028-3033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–43. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 72.Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, et al. beta-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J Bacteriol. 2006;188:2726–9. doi: 10.1128/JB.188.7.2726-2729.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novick RP, Schlievert P, Ruzin A. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 2001;3:585–94. doi: 10.1016/S1286-4579(01)01414-9. [DOI] [PubMed] [Google Scholar]
- 74.Eklund MW, Poysky FT, Reed SM, Smith CA. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971;172:480–2. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- 75.Scott ME, Sandkvist M. Toxins and Type II Secretion Systems. In: Burns DL, Barbieri JT, Iglewski BH, Rappuoli R, eds. Bacteria protein toxins ASM Press; 2003:81–94. [Google Scholar]
- 76.Rossetto O, Tonello F, Montecucco C. Proteases. In: Burns DL, Barbieri JT, Iglewski BH, Rappuoli R, eds. Bacterial protein toxins ASM Press; 2003:271–282. [Google Scholar]
- 77.Makino K, Yokoyama K, Kubota Y, Yutsudo CH, Kimura S, Kurokawa K, et al. Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet Syst. 1999;74:227–39. doi: 10.1266/ggs.74.227. [DOI] [PubMed] [Google Scholar]
- 78.Yokoyama K, Makino K, Kubota Y, Watanabe M, Kimura S, Yutsudo CH, et al. Complete nucleotide sequence of the prophage VT1-Sakai carrying the Shiga toxin 1 genes of the enterohemorrhagic Escherichia coli O157:H7 strain derived from the Sakai outbreak. Gene. 2000;258:127–39. doi: 10.1016/S0378-1119(00)00416-9. [DOI] [PubMed] [Google Scholar]
- 79.Neely MN, Friedman DI. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–67. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 80.Wagner PL, Livny J, Neely MN, Acheson DWK, Friedman DI, Waldor MK. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol Microbiol. 2002;44:957–70. doi: 10.1046/j.1365-2958.2002.02950.x. [DOI] [PubMed] [Google Scholar]
- 81.Wagner PL, Neely MN, Zhang X, Acheson DW, Waldor MK, Friedman DI. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J Bacteriol. 2001;183:2081–5. doi: 10.1128/JB.183.6.2081-2085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freeman VJ. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951;61:675–88. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakagawa I, Kurokawa K, Yamashita A, Nakata M, Tomiyasu Y, Okahashi N, et al. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 2003;13(6A):1042–55. doi: 10.1101/gr.1096703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A. 2002;99:10078–83. doi: 10.1073/pnas.152298499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weeks CR, Ferretti JJ. The gene for type A streptococcal exotoxin (erythrogenic toxin) is located in bacteriophage T12. Infect Immun. 1984;46:531–6. doi: 10.1128/iai.46.2.531-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goshorn SC, Schlievert PM. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J Bacteriol. 1989;171:3068–73. doi: 10.1128/jb.171.6.3068-3073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–40. doi: 10.1016/S0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 88.Coleman DC, Sullivan DJ, Russell RJ, Arbuthnott JP, Carey BF, Pomeroy HM. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: molecular mechanism of triple conversion. J Gen Microbiol. 1989;135:1679–97. doi: 10.1099/00221287-135-6-1679. [DOI] [PubMed] [Google Scholar]
- 89.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene. 1998;215:57–67. doi: 10.1016/S0378-1119(98)00278-9. [DOI] [PubMed] [Google Scholar]
- 90.Yamaguchi T, Hayashi T, Takami H, Nakasone K, Ohnishi M, Nakayama K, et al. Phage conversion of exfoliative toxin A production in Staphylococcus aureus. Mol Microbiol. 2000;38:694–705. doi: 10.1046/j.1365-2958.2000.02169.x. [DOI] [PubMed] [Google Scholar]
- 91.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 92.Mani N, Dupuy B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc Natl Acad Sci U S A. 2001;98:5844–9. doi: 10.1073/pnas.101126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matamouros S, England P, Dupuy B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol Microbiol. 2007;64:1274–88. doi: 10.1111/j.1365-2958.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- 94.Carter GP, Douce GR, Govind R, Howarth PM, Mackin KE, Spencer J, et al. The anti-sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS Pathog. 2011;7:e1002317. doi: 10.1371/journal.ppat.1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupuy B, Sonenshein AL. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27:107–20. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 96.Mani N, Lyras D, Barroso L, Howarth P, Wilkins T, Rood JI, et al. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J Bacteriol. 2002;184:5971–8. doi: 10.1128/JB.184.21.5971-5978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karlsson S, Dupuy B, Mukherjee K, Norin E, Burman LG, Akerlund T. Expression of Clostridium difficile toxins A and B and their sigma factor TcdD is controlled by temperature. Infect Immun. 2003;71:1784–93. doi: 10.1128/IAI.71.4.1784-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karlsson S, Burman LG, Akerlund T. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology. 1999;145:1683–93. doi: 10.1099/13500872-145-7-1683. [DOI] [PubMed] [Google Scholar]
- 99.Karlsson S, Burman LG, Akerlund T. Induction of toxins in Clostridium difficile is associated with dramatic changes of its metabolism. Microbiology. 2008;154:3430–6. doi: 10.1099/mic.0.2008/019778-0. [DOI] [PubMed] [Google Scholar]
- 100.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol. 2007;66:206–19. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 101.Goh S, Chang BJ, Riley TV. Effect of phage infection on toxin production by Clostridium difficile. J Med Microbiol. 2005;54:129–35. doi: 10.1099/jmm.0.45821-0. [DOI] [PubMed] [Google Scholar]
- 102.Sekulovic O, Meessen-Pinard M, Fortier LC. Prophage-stimulated toxin production in Clostridium difficile NAP1/027 lysogens. J Bacteriol. 2011;193:2726–34. doi: 10.1128/JB.00787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Govind R, Vediyappan G, Rolfe RD, Dupuy B, Fralick JA. Bacteriophage-mediated toxin gene regulation in Clostridium difficile. J Virol. 2009;83:12037–45. doi: 10.1128/JVI.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ehrbar K, Hardt W-D. Bacteriophage-encoded type III effectors in Salmonella enterica subspecies 1 serovar Typhimurium. Infect Genet Evol. 2005;5:1–9. doi: 10.1016/j.meegid.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Müller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L, et al. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–36. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Mirold S, Rabsch W, Rohde M, Stender S, Tschäpe H, Rüssmann H, et al. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc Natl Acad Sci U S A. 1999;96:9845–50. doi: 10.1073/pnas.96.17.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Humphreys D, Davidson A, Hume PJ, Koronakis V. Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe. 2012;11:129–39. doi: 10.1016/j.chom.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehrbar K, Friebel A, Miller SI, Hardt W-D. Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1. J Bacteriol. 2003;185:6950–67. doi: 10.1128/JB.185.23.6950-6967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan KS, Wee BY, Song KP. Evidence for holin function of tcdE gene in the pathogenicity of Clostridium difficile. J Med Microbiol. 2001;50:613–9. doi: 10.1099/0022-1317-50-7-613. [DOI] [PubMed] [Google Scholar]
- 110.Govind R, Dupuy B. Secretion of Clostridium difficile toxins A and B requires the holin-like protein TcdE. PLoS Pathog. 2012;8:e1002727. doi: 10.1371/journal.ppat.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A. 2004;101:4614–9. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hodak H, Galán JE. A Salmonella Typhi homologue of bacteriophage muramidases controls typhoid toxin secretion. EMBO Rep. 2013;14:95–102. doi: 10.1038/embor.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stanley NR, Lazazzera BA. Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol. 2004;52:917–24. doi: 10.1111/j.1365-2958.2004.04036.x. [DOI] [PubMed] [Google Scholar]
- 114.Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182:2675–9. doi: 10.1128/JB.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 116.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2012;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 117.Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Tay M, et al. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 2009;3:271–82. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carrolo M, Frias MJ, Pinto FR, Melo-Cristino J, Ramirez M. Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS One. 2010;5:e15678. doi: 10.1371/journal.pone.0015678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, et al. Cryptic prophages help bacteria cope with adverse environments. Nat Commun. 2010;1:147–9. doi: 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10:841–51. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 121.Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–4. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 122.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–92. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 123.Seper A, Fengler VHI, Roier S, Wolinski H, Kohlwein SD, Bishop AL, et al. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol Microbiol. 2011;82:1015–37. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Conover MS, Mishra M, Deora R. Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS One. 2011;6:e16861. doi: 10.1371/journal.pone.0016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–72. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Higgins D, Dworkin J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev. 2012;36:131–48. doi: 10.1111/j.1574-6976.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paredes CJ, Alsaker KV, Papoutsakis ET. A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol. 2005;3:969–78. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 128.Mallozzi M, Viswanathan VK, Vedantam G. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol. 2010;5:1109–23. doi: 10.2217/fmb.10.60. [DOI] [PubMed] [Google Scholar]
- 129.Postollec F, Mathot A-G, Bernard M, Divanac’h M-L, Pavan S, Sohier D. Tracking spore-forming bacteria in food: from natural biodiversity to selection by processes. Int J Food Microbiol. 2012;158:1–8. doi: 10.1016/j.ijfoodmicro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–9. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 131.Bramucci MG, Keggins KM, Lovett PS. Bacteriophage PMB12 conversion of the sporulation defect in RNA polymerase mutants of Bacillus subtilis. J Virol. 1977;24:194–200. doi: 10.1128/jvi.24.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silver-Mysliwiec TH, Bramucci MG. Bacteriophage-enhanced sporulation: comparison of spore-converting bacteriophages PMB12 and SP10. J Bacteriol. 1990;172:1948–53. doi: 10.1128/jb.172.4.1948-1953.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bramucci MG, Keggins KM, Lovett PS. Bacteriophage conversion of spore-negative mutants to spore-positive in Bacillus pumilus. J Virol. 1977;22:194–202. doi: 10.1128/jvi.22.1.194-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keggins KM, Nauman RK, Lovett PS. Sporulation-converting bacteriophages for Bacillus pumilus. J Virol. 1978;27:819–22. doi: 10.1128/jvi.27.3.819-822.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perlak FJ, Mendelsohn CL, Thorne CB. Converting bacteriophage for sporulation and crystal formation in Bacillus thuringiensis. J Bacteriol. 1979;140:699–706. doi: 10.1128/jb.140.2.699-706.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boudreaux DP, Srinivasan VR. Bacteriophage-induced sporulation in Bacillus cereus T. J Gen Microbiol. 1981;126:459–62. [Google Scholar]
- 137.Kinney DM, Bramucci MG. Analysis of Bacillus subtilis sporulation with spore-converting bacteriophage PMB12. J Bacteriol. 1981;145:1281–5. doi: 10.1128/jb.145.3.1281-1285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stewart AW, Johnson MG. Increased numbers of heat-resistnat spores produced by two strains of Clostridium perfringens bearing temperate phage s9. J Gen Microbiol. 1977;103:45–50. doi: 10.1099/00221287-103-1-45. [DOI] [PubMed] [Google Scholar]
- 139.Kim K-P, Born Y, Lurz R, Eichenseher F, Zimmer M, Loessner MJ, et al. Inducible Clostridium perfringens bacteriophages ΦS9 and ΦS63: Different genome structures and a fully functional sigK intervening element. Bacteriophage. 2012;2:89–97. doi: 10.4161/bact.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zimmer M, Scherer S, Loessner MJ. Genomic analysis of Clostridium perfringens bacteriophage phi3626, which integrates into guaA and possibly affects sporulation. J Bacteriol. 2002;184:4359–68. doi: 10.1128/JB.184.16.4359-4368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takemaru K, Mizuno M, Sato T, Takeuchi M, Kobayashi Y. Complete nucleotide sequence of a skin element excised by DNA rearrangement during sporulation in Bacillus subtilis. Microbiology. 1995;141:323–7. doi: 10.1099/13500872-141-2-323. [DOI] [PubMed] [Google Scholar]
- 142.Stragier P, Kunkel B, Kroos L, Losick R. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science. 1989;243:507–12. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- 143.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–86. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 144.Haraldsen JD, Sonenshein AL. Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Mol Microbiol. 2003;48:811–21. doi: 10.1046/j.1365-2958.2003.03471.x. [DOI] [PubMed] [Google Scholar]
- 145.Bott K, Strauss B. The carrier state of Bacillus subtilis infected with the transducing bacteriophage SP10. Virology. 1965;25:212–25. doi: 10.1016/0042-6822(65)90200-X. [DOI] [PubMed] [Google Scholar]
- 146.Hemphill HE, Whiteley HR. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975;39:257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–81. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 148.Meijer WJJ, Castilla-Llorente V, Villar L, Murray H, Errington J, Salas M. Molecular basis for the exploitation of spore formation as survival mechanism by virulent phage phi29. EMBO J. 2005;24:3647–57. doi: 10.1038/sj.emboj.7600826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 150.Casjens S, Hendrix R. Bacteriophages and the bacterial genome. In: Higgins NP, ed. The Bacterial Chromosome. Washington DC: ASM Press, 2005:39-52. [Google Scholar]