Abstract
The Candida genus expresses virulence factors that, when combined with immunosuppression and other risk factors, can cause different manifestations of oral candidiasis. The treatment of mucosal infections caused by Candida and the elucidation of the disease process have proven challenging. Therefore, the study of experimentally induced oral candidiasis in rats and mice is useful to clarify the etiopathology of this condition, improve diagnosis, and search for new therapeutic options because the disease process in these animals is similar to that of human candidiasis lesions. Here, we describe and discuss new studies involving rat and mouse models of oral candidiasis with respect to methods for inducing experimental infection, methods for evaluating the development of experimental candidiasis, and new treatment strategies for oral candidiasis.
Keywords: Candida albicans, oral candidiasis, murine model, oral cavity
Introduction
Oral candidiasis is caused by Candida yeasts, which are present in the oral cavities of approximately 50% of healthy individuals. Candida albicans is the most virulent and prevalent species, followed by the non-albicans species C. tropicalis, C. glabrata, C. parapsilosis, C. krusei, C. dubliniensis, and others.1-7 C. albicans colonizes the oral surface and can cause damage through the expression of its virulence factors, including adherence to host cells, morphological transition, hydrophobicity and secretion of hydrolytic enzymes.8-10 A major virulence factor of C. albicans is its ability to adapt to a variety of different habitats and the consequent formation of surface-attached microbial communities known as biofilms.11 Initially, the biofilm is established by the adherence of yeast to host cells or abiotic surfaces, e.g., prostheses. These yeast form colonies, produce germ tubes and filaments, and secret extracellular polymeric substance (EPS) that contribute to the three-dimensional structure of the biofilm.12,13 The organization of the biofilm protects the C. albicans cells from host immune mechanisms and antifungal agents.12,14,15
C. albicans causes disease mainly in individuals with local or systemic risk factors,16 including denture use,17 steroid inhaler use,18,19 reduced salivary flow,20 a high-sugar diet,21 extreme age or youth,22 endocrine disorders,23 nutritional deficiencies,24 receipt of broad-spectrum antibiotics,25 and immunosuppression.5,7,26 In fact, oropharyngeal candidiasis (OPC) is the most frequent opportunistic infection encountered in HIV positive individuals. The disease occurs in up to 90% of patients during the course of HIV infection.27,28
Candida infections of the oral mucosa manifest in various guises and are subdivided into pseudomembranous, erythematous, and hyperplastic. The last form is chronic, whereas the first two are classified as acute lesions. Hyperplastic candidiasis is the least common of the triad of major clinical variants. Pseudomembranous candidiasis or thrush, which is characterized by white patches on the surface of the buccal mucosa, tongue, and the soft palate, occurs in patients using corticosteroids topically or by aerosol, in HIV-positive patients, and in other types of immunocompromised patients. Erythematous candidiasis is characterized by localized erythema of the oral mucosa that commonly occurs on the tongue and the palate and is associated with broad-spectrum antibiotics, corticosteroids, and HIV infection. In the tongue dorsum, erythematous candidiasis presents depapillated areas caused by the loss of filiform papillae. In addition to the three major variants of oral candidiasis, there are other clinical manifestations that are called “Candida-associated lesions”, since the yeast is not the sole etiologic agent. These lesions include denture stomatitis, angular cheilitis, and median rhomboid glossitis.29-34
The recognition that C. albicans is an important pathogen, especially in immunocompromised patients, has led to the development of a suitable experimental model that supplies a standard and easy tool to control and manipulate the assays required to obtain knowledge of the disease process.35,36 Thus, various animal models have been used to explore the host–Candida interaction during oral infections to assess the etiopathology, diagnose and manage the disease, and test therapeutic approaches.37-43 Therefore, the purpose of this review is to discuss new studies in the literature that describe experimental oral candidiasis in rats and mice and to compare different methods for the induction, development and treatment of oral candidiasis.
Structure of the Tongue Dorsum of Rats and Mice (Normal Aspects)
Since the tongue dorsum is the primary habitat of the yeast in the oral cavity of healthy and immunocompromised individuals,44 most studies of experimental oral candidiasis in rodent models were performed on the tongue dorsum. Therefore, the general morphological features observed in the tongue dorsum of rats and mice are described below.
The rat or mouse tongue is a muscular organ coated with keratinized stratified squamous epithelium that forms an upper region, the dorsum, and a lower region, the ventral. Under the epithelium, the tongue is formed of conjunctive tissue, which is rich in blood vessels, and striated muscle, in which the fibers are grouped in bundles in three planes. Among the muscle fibers of the posterior region, there are serous and mucous salivary glands.
The tongue dorsum presents a buccal section, which extends from the tip to the vallate papilla, and a pharyngeal section, which extends from the vallate papilla to the most posterior area of the tongue dorsum. Similar to the human tongue dorsum, the buccal section of these rat and mouse models is formed by several projections, namely filiform and fungiform papillae (Fig. 1). In rats and mice, filiform papillae are classified as simple conic papillae (located on the anterior two thirds of the tongue dorsum), true papillae (located on the intermolar tubercle), and giant papillae (located between the simple conic papillae and the true papillae). Among the simple conic papillae, there are a lower number of fungiform papillae, which are short and broad with a rounded base that contains the taste corpuscle in the epithelium of the superior surface (Fig. 2).
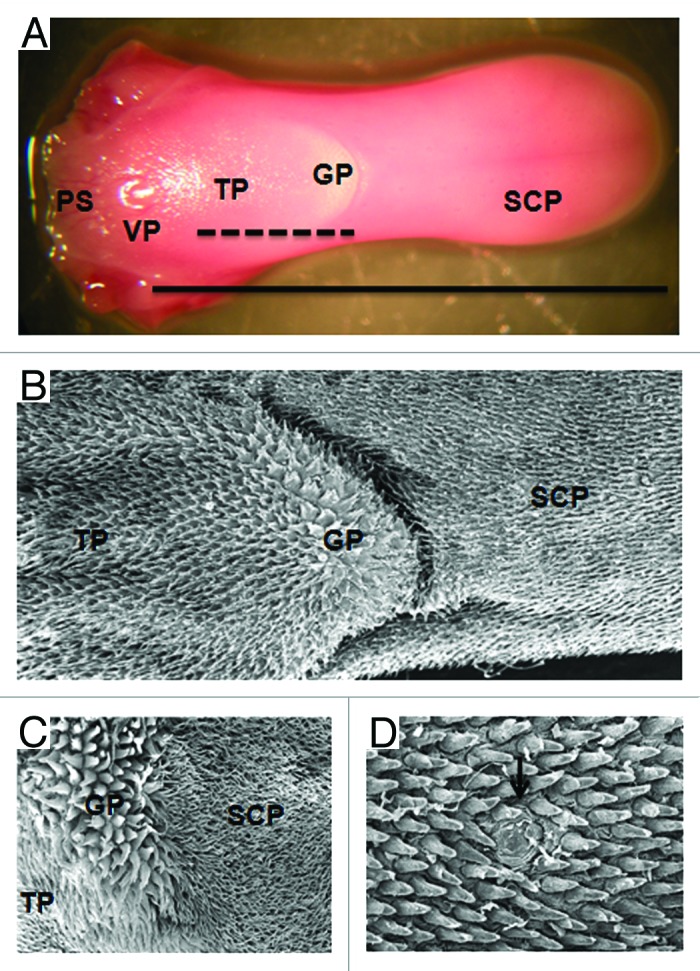
Figure 1. Morphological features of the murine tongue dorsum. (A) Macroscopic aspect of the mouse tongue dorsum. The vallate papilla (VP) shared the pharyngeal section (PS) from the buccal (___). In the buccal section, we can see the area of simple conic papillae (SCP), true papillae (TP), giant papillae area (GP) and the intermolar tubercle (---). (B) Distribution of the simple conic papillae (SCP), giant papillae (GP) and true papillae (TP) on the tongue dorsum of the mouse, SEM: 35×. (C) Distribution of the SCP, GP and TP on the tongue dorsum of the rat, SEM: 75X. (D) Fungiform papilla (↓) among the simple conic papillae of the mouse, SEM: 200×.
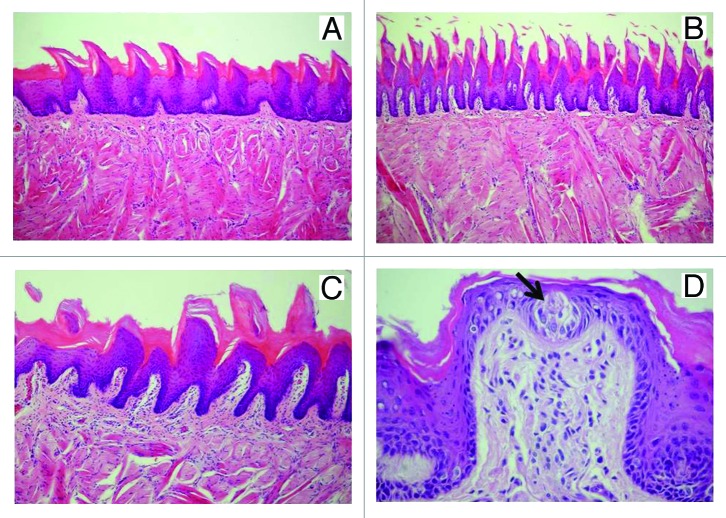
Figure 2. Sagittal cut of the tongue dorsum of the rat. (A) The simple conic papillae are short and distant from each other with many interpapillar surfaces, HE: 200×. (B) The true papillae are more elongated and closer to each other, with few interpapillar surfaces, HE: 200×. (C) The giant papillae are larger, are present in fewer places and are more visible than the other types of papillae, 200×. (D) Fungiform papilla with taste corpuscle (↓), HE: 400×.
Models of Experimental Oral Candidiasis in Rats
There are two varieties of rats, Sprague-Dawley and Wistar, that are commonly used to study experimental oral Candida infections. Rats have the advantages of a low maintenance cost, ease of breeding and handling, ready availability, and a sufficiently sized oral cavity that easily permits inoculation and sample collection.35,36 Moreover, the tongues of the rats are easily colonized by C. albicans, eliciting disease conditions such as erythematous candidiasis and median rhomboid glossitis.36,45 This model is useful for studying long-term candidal colonization and chronic infection.35 For the establishment of persistent oral candidiasis in rats, C. albicans infection has been induced by exclusion of the protective effects of normal salivary flow, alteration or manipulation of the oral cavity by the administration of antibiotics and treatment with immunosuppressive agents.35,46
It has already been established that in humans alterations in salivary glands caused by Sjögren syndrome, radiotherapy, advanced age, and some medications lead to a reduction in salivary flow and xerostomia, favoring the development of oral candidiasis by decreasing the mechanical removal and action of antimicrobial peptides present in saliva.47-49 This condition has been used by various researchers to induce oral candidiasis in rat models.38,47-50 Green et al.50 studied C. albicans’ expression of ALS genes (encoding large cell-surface glycoproteins that function in the process of adhesion to host surfaces) in the hyposalivatory Sprague-Dawley rat model after surgical removal of the salivary glands. At 5 d post-infection, disease progression produced more severe lesions in a defined hierarchy of lesion severity (tongue > mandible > buccal mucosa > palatal and pharyngeal mucosa) and a greater number of different ALS transcripts, such as ALS1–5 and ALS9. The expression of the ALS gene in the experimental candidiasis in rats were compared with clinical specimens collected from HIV-positive patients, demonstrating a similarity in expression patterns between the rat model and the human clinical specimens. This study suggests that the hyposalivatory rat model can be useful for studying gene expression in oral candidiasis.
Immunosuppression and administration of broad-spectrum antibiotics are also important risk factors for developing of oral candidiasis in humans.29,30 Thus, these predisposing factors have been widely used to induce oral candidiasis in rats.45,51,52 Chami et al.53 compared the effect of substances from a vegetal source (carvacrol and eugenol) to nystatin on experimental oral candidiasis induced in Wistar rats that were immunosuppressed with dexamethasone and treated with tetracycline in the drinking water. Microbiological analysis showed that treatment with carvacrol, eugenol, and nystatin reduced 94.5%, 76.9%, and 91.54% of the CFUs of C. albicans, respectively, compared with a control group (infected and untreated). Histologically, the control group showed extensive colonization of the epithelium of the tongue dorsum by numerous hyphae. For the group treated with carvacrol, no C. albicans were observed in the folds of the tongue mucosa. However, under eugenol and nystatin treatment, hyphae were found in some folds of the tongue mucosa. The authors reported that this model of experimental oral candidiasis in rats was a simple and highly reproducible method of studying the efficacy of antifungal agents.
Junqueira et al.52 induced oral candidiasis in Wistar rats while treating the animals with tetracycline only in the drinking water. A C. albicans suspension was dripped into the mouth and spread on the tongue with a swab that was previously soaked in the suspension. This study verified that the majority of the rats no longer showed C. albicans colonization after 8 d of infection, suggesting that during the development of experimental candidiasis, the yeast and hyphae are eliminated from the organism by the host’s immune system. However, all animals showed typical candidiasis lesions, which were characterized by numerous epithelial lesions, including epithelial hyperplasia, disorganization of the basal layer, exocytosis, spongiosis, and loss of filiform papillae. After the establishment of candidiasis lesions, the rats were treated with photodynamic therapy (PDT) using methylene blue and a laser. Subsequently, the action of PDT on oral candidiasis was evaluated by the quantification of the epithelial lesions and by the intensity of inflammatory infiltrate in the conjunctive tissue. The rats treated with photodynamic therapy exhibited fewer epithelial alterations and had a lower inflammatory response than the untreated rats, suggesting that PDT was effective for the treatment of oral candidiasis. Furthermore, the experimental oral candidiasis in rats was a useful model to assess the effects of in vivo PDT.
In addition to the studies relating to genes expression and new antifungal countermeasures, such as substances from a vegetal source and PDT, rat models have also been used to study the influence of physiological factors on oral candidiasis. The influence of ovarian hormone on the development of oral candidiasis was demonstrated in the literature by Junqueira et al.39 These authors inoculated the oral cavity of ovariectomized Wistar rats and sham-ovariectomized rats with C. albicans suspensions. The ovariectomized rats presented fewer occurrences of candidiasis than sham-ovariectomized rats (33% vs. 75%) and exhibited a faster recovery time (22 d vs. 67 d), suggesting that ovarian hormones have a significant influence on oral candidiasis.
Since psychological stress has been found to suppress cell-mediated immune responses that are important for limiting the proliferation of C. albicans, Núnez et al.54 evaluated the effects of stress on oral candidiasis in rats exposed to a repeated auditory stressor. The results showed that stress exacerbated C. albicans infection in the tongue dorsum of the rats. Significant increases in C. albicans counts, the percentage of the tongue’s surface covered with clinical lesions, the percentage of abnormal papillae, and the colonization of the epithelium by hyphae were found in stressed rats compared with the nonstressed rats.
Another advantage of oral candidiasis model in rats is that this model allows the placement of a prosthetic device in the palate to mimic human Candida denture stomatitis, the most common form of oral candidiasis in the elderly population denture wearers. Unlike mice, rats have a larger oral cavity that facilitates denture placement. Previous models of denture stomatitis in rats were pioneered in the 1970s and 1980s. However, research since then has been scarce, presumably due to the HIV epidemic and the concomitant rise in the incidence of oropharyngeal candidiasis.55 Recently, new studies using rat models for denture stomatits were developed with a focus on Candida biofilm formation.55,56 Nett et al.56 induced denture stomatitis in Sprague-Dawley rats by using acrylic denture material applied over a palate around the cheek teeth (8 by 10 mm), immunosuppression with cortisone, and subsequently administering C. albicans (108 cells/mL) on the palate. The analysis of the images revealed a biofilm composed of adherent cells, yeasts, hyphae, host cells encased in extracellular matrix, and associated bacteria. The mucosal histopathology was consistent with that of acute human denture stomatitis, as it exhibited fungal invasion and neutrophil infiltration. In this study, the authors demonstrated that the oral denture model in rats can be very useful for testing the impact of gene disruption on biofilm formation, studying anti-infectives agents, examining the biology of mixed Candida-oral bacterial flora biofilm infections, and characterizing the host immunologic response to the disease process.
Models of Experimental Oral Candidiasis in Mice
The major advantage of using mice instead of rats for experimental oral candidiasis is that the immunobiology of the healthy mouse mucosa has also been fairly well characterized, enabling the suitable evaluation of the humoral and cellular immune response in oral candidiasis.35,45,46 Additionally, mice are widely available, easy to handle and to inoculate, and inexpensive to maintain.35,46 The availability and ease of production of genetically modified animals is another advantage of mice.46,57 The development of transgenic and knockout mice with targeted immune defects has prompted a more closely focused assessment of the role of specific components of the immune response to C. albicans.58 According to Samaranayake and Samaranayake,35 the variants of the mouse model include gnotobiotes, athymic (nu/nu), euthymic (nu/+), beige (bg/bg), black (bg/+), beige athymic (bg/bg nu/nu), beige euthymic (bg/bg nu/+), severe combined immune deficiency (SCID), and murine acquired immune deficiency syndrome (MAIDS). Nevertheless, the mouse model has the disadvantage of a small oral cavity that interferes with the examination of the mucosa by the naked eye.
As in rat models, mice models require the use of immunosuppressive agents or other predisposing factors to establish persistent infection since these animals are not naturally colonized by C. albicans.35,38,46,59-61 The effect of xerostomia in sialoadenectomized mice prolonged oral colonization by C. albicans to 75 d post-infection vs. 30 d of colonization for the control animals.38 With 1 or 4 inoculations with a C. albicans suspension, the last procedure increased the permanence of C. albicans in the oral cavity of sialoadenectomized mice and produced lesions in the tongue that contained pseudohyphae inside the epithelium, acanthosis, and neutrophilic infiltrate forming intraepithelial microabscesses, with a higher prevalence of lesions in the true and simple conic papillae.59 This model favored the permanence of C. albicans in the oral cavity, and higher frequencies of yeast inoculation influenced the presence and extension of candidiasis lesions.
Rhaman et al.60 reported a new murine model of oral candidiasis using estrogen administration. BALB/c mice received a weekly intramuscular and subcutaneous 5 µg dose of estrogen, of which the intramuscular administration was more powerful for inducing oral colonization. After 2 and 3 weeks of C. albicans infection, fungal cells and hyphae were detected in the keratinized and upper prickle-cells layer of the epithelium at varying sites, namely, the tongue dorsum, the buccal mucosa, the mouth floor, the palate, and the lingual alveolar mucosa. By week 5, fungal cells and hyphae were still clearly present in the oral epithelium. In this study, the authors also evaluated immunization for C. albicans. The animals were immunized intranasally on two consecutive days with 5 μg purified Sap2 (secreted aspartyl proteinase 2) that is a putative virulence factor of C. albicans thought to contribute to human mucosal infections). On days 14 and 15 after the second immunization, mice were inoculated orally with C. albicans and exhibited a significant reduction of the fungal burden compared with the control group. According to the authors, this model of mucosal colonization can be used to assess potential vaccine candidates and permits the detailed analysis of host-fungal interactions during the natural state of Candida colonization.
Although predisposing factors, such as xerostomia and estrogen administration, have been used for inducing oral candidiasis in mice, most studies have been performed in immunosuppressed mice models.37,41,43,62-68 Takakura et al.37 developed a murine model for experimental candidiasis that combines immunosuppression and treatment with tetracycline for successful disease progression, allowing the study of fungal pathogenesis and new therapeutic options. These authors observed symptoms characteristic of oral thrush in the oral cavities of mice treated with prednisolone at a concentration of 100 mg/kg body weight and 0.83 mg/mL tetracycline in the drinking water 1 d prior to infection. At 3 and 7 d post-infection, 105–106 CFU were recovered from each mouse, and whitish, curd-like patches were observed on most of the tongues. Animals with symptoms of pseudomembranous candidiasis showed extensive colonization on the epithelium of the dorsal surface by numerous hyphae, as well as the severe destruction of the epithelial layers. Furthermore, this model was useful for verifying the therapeutic activity of fluconazole and amphotericin B, both of which reduced C. albicans and cured the lesions.
This murine model of experimental candidiasis, developed by Takakura et al.,37 inspired many other studies, which are described in Table 1.37,41,43,62-68 The advantage of this model is the development of thrush-type oral candidiasis that mimics the natural infection in humans and is useful for both symptomatological and mycological evaluation of the responsiveness to antifungal treatments. Therefore, several treatments for oral candidiasis were tested in this model including: salivary factors,62,63 quorum-sensing molecules,64 photodynamic therapy,43,66 natural plant extracts,41,67 and inhibitors of efflux pump.68
Table 1. Studies of experimental candidiasis using the methodology developed by Takakura et al. (2003)26 for establishment of a mice model with local symptoms characteristic of oral thrush that mimics the natural infection in humans.
| Study | Agents tested for the control of oral candidiasis | Results |
|---|---|---|
| Takakura et al.62 (2004) |
Bovine lactoferrin |
Lactoferrin, that is an iron-binding glycoprotein, present in saliva and milk, showed a direct antifungal activity and induced the enhancement of Th1-type cytokine production in the mice model. Therefore, lactoferrin from cows’ milk could be used as a dietary supplement with action for antifungal treatment. |
| Kamagata-Kyoura et al.63 (2004) |
Human saliva |
The administration of human saliva inhibited the colonization of the oral cavity by C. albicans in mice and the subsequent onset of oral candidiasis. The human salivary factors, such as lactoferrin, transferrin, histatin, cystatin, and lysozyme, should be studied as new therapeutic agents against oral candidiasis. |
| Hisajima et al.68 (2008) |
Farnesol |
Farnesol, known as a quorum-sensing molecule for C. albicans, showed a protective effect against oral candidiasis in a dose-dependent manner. Farnesol suppressed mycelial growth of C. albicans on the surface of tongues, but did not prevent the change of CFU of C. albicans cells, suggesting that farnesol has very characteristic roles in protection against mucosal candidiasis. |
| Mima et al.66 (2010) |
PDT mediated by Photogen® and LED |
PDT promoted significant reduction in the viability of C. albicans biofilm, but there was no significant reduction in macroscopic or microscopic lesions, indicating that further in vivo studies are still necessary to investigate the parameters of PDT required for complete inactivation of C. albicans. |
| Taguchi et al.41 (2010) |
Spices and herbs |
The cassia (Cinnamomum cassia) preparation significantly reduced the lesions on the tongue and the hyphae and inflammatory infiltrate in the epithelium. On the other hand, preparations of lemongrass (Cymbopogon citratus) and green tea (Camellia sinensis) reduced neither symptoms nor the viable C. albicans cells. These finds suggested that cassia may possibly be a candidate for a prophylactic or therapeutic tool against oral Candida infection. |
| Hayama et al.68 (2012) |
Association of inhibitor of Cdr1p efflux pump with azoles |
High-level azole resistance is often caused by the overexpression of C. albicans efflux pump Cdr1p. The co-application of the d-octapeptide derivate RC21v3, an inhibitor of efflux pump Cdr1p, potentiated the therapeutic performance of fluconazole for mice infected with a fluconazole-resistant C. albicans clinical isolate. These results indicated that RC21v3 in combination with azoles has potential as a therapy against azole-resistant oral candidiasis. |
| Ninomiya et al.67 (2012) |
Tea tree oil (TTO) (Essential oil of M. alternifolia) |
TTO and its major component, terpinen-4-ol, caused a decrease in the symptom of oral candidiasis of tongues and in the viable Candida cell number in the oral cavity of mice infected by azole-resistant C. albicans. These results suggested that TTO and terpinen-4-ol may have the potential of therapeutic ability for oral candidiasis. |
| Costa et al.43 (2012) |
PDT mediated by erythrosine and green LED | PDT showed a significant reduction of viable C. albicans cells without damaging adjacent tissues, but there was no difference among the groups in macroscopic and microscopic analysis. |
In addition to the murine model developed by Takakura et al.,37 other models of experimental candidiasis in immunosuppressed mice have been published in the literature and are described below. Teichert et al.69 induced oral candidiasis in beige nude mice with severe combined immunodeficiency disease (SCID) by swabbing the oral cavity with C. albicans 3 times a week for a period of 4 weeks. The animals were treated with photodynamic therapy using methylene blue and diode laser light. The therapy exhibited antifungal activity, decreasing the number of fungal cells and increasing the inflammatory infiltrate and neutrophilic exocytosis, thereby demonstrating the capability of the SCID model to generate an immune response despite the absence of T cells and B cells. Solis and Filler61 described a simple method to induce oropharyngeal candidiasis by injecting cortisone acetate (220 mg/kg) subcutaneously before infection with C. albicans. The mice were inoculated sublingually for 75 min with a swab saturated with the C. albicans suspension. On day 5 after inoculation, 105–106 CFU were recovered from the oral tissue, and the histopathology showed characteristics of oropharyngeal candidiasis.
In summary, multiple experimental models of oral candidiasis using immunosuppressed mice have been developed and, regardless to the type of immunosuppression used, the animals developed typical lesion of pseudomembranous candidiasis that is seen in humans. These models have become important tools to study the virulence of C. albicans mutants, the host response to mucosal infection, and the efficacy of new antifungal agents.
Methods for Analysisof Experimental Oral Candidiasis
To estimate colonization by C. albicans in the rat or mouse oral cavity, CFU counting is the most useful method. Samples from the entire oral cavity or only from a specific site, e.g., the surface of the tongue, are collected with the aid of a swab for posterior CFU counting onto Sabouraud dextrose agar (SDA) with antibiotics. This method assesses whether the animals were colonized by C. albicans and the antifungal effects of the therapeutic modality tested.37,42,43,60,69 Additionally, the tongue and other oral tissue can be removed from the oral cavity, homogenized in saline solution, and later plated on SDA.61 After plating, the Petri dishes are incubated at 35–37 °C for 24 or 48 h.
Acute oral candidiasis can present as pseudomembranous or erythematous forms. These manifestations cause macroscopic lesions (Fig. 3) that can be quantitatively analyzed. Takakura et al.37 evaluated macroscopic lesions of pseudomembranous candidiasis by scoring lesions from 0 to 4 according to the extension and severity of whitish, curd-like patches on the tongue surface. The authors also described the appearance of lesions consisting of white patches on the tongue dorsum. This methodology has been used by many others authors to analyze the development of disease during their experiments.41,43,62,63,67,68
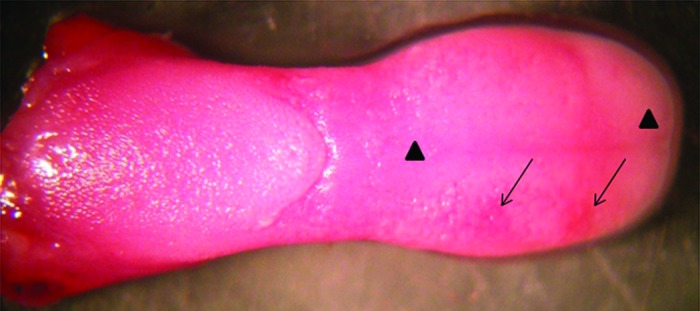
Figure 3. Macroscopic analysis of a mouse tongue with oral candidiasis presenting whitish regions (▲) and papillary atrophy (↓).
After euthanasia of the animals, the tissues are removed and prepared for histopathological study. The sections are stained with periodic acid-Schiff (PAS) reagent, which allows for the verification of the extent of fungal filamentation and the depth of tissue penetration.61 Some authors proposed a quantification of the amount of yeasts/hyphae by attributing scores to histological fields in the anteroposterior direction (Fig. 4).39 Through hematoxylin-eosin (HE) staining, many epithelial alterations and the presence of inflammatory cells are observed and can be quantified according to the methodology of Junqueira et al.,52 such as epithelial hyperplasia, disorganization of the basal cell layer, exocytosis, spongiosis, loss of filiform papillae, hyperkeratosis, development of intraepithelial microabscesses, and extension of the inflammatory infiltrate (Fig. 5). Histopathological data are important because from a histopathological and diagnostic point of view, most of the lesions described in animal models faithfully reproduce human candidal lesions.35
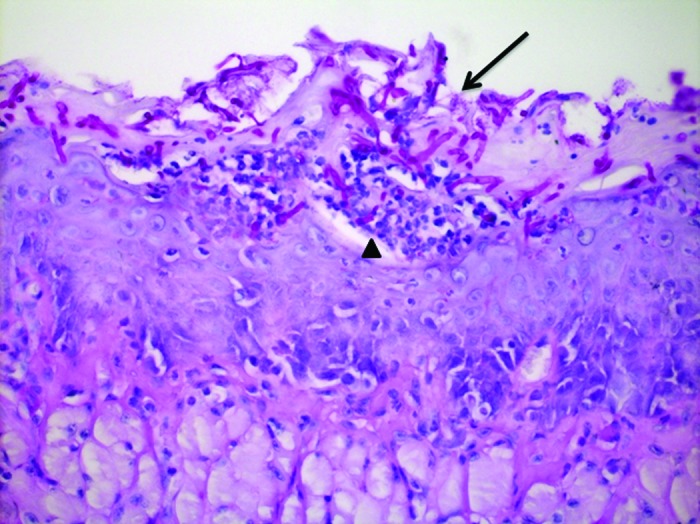
Figure 4. Sagittal section of mouse tongue dorsum showing yeasts and hyphae in keratin (↓) and intraepithelial microabscesses (▲). PAS: 400×.
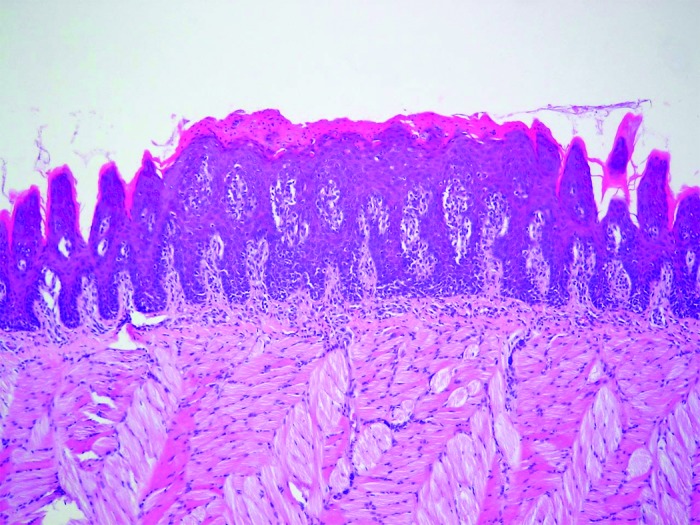
Figure 5. Sagittal section of rat tongue dorsum showing tissue lesion characterized by epithelial hyperplasia, disorganized basal layer, exocytosis, spongiosis, loss of filiform papillae, and hyperparakeratosis. HE: 100×.
In addition to the histopathological study, the tissue, which is usually the tongue dorsum, can be analyzed by scanning electron microscopy (SEM) and laser confocal scanning microscopy (LCSM). In the SEM analysis, alterations and fungal elements are observed on tongue dorsum tissue from rats and mice infected with C. albicans. Analysis reveals hyphae penetrating perpendicularly into the tissue on the anterior surface of the papillae, desquamation and degradation of tissues, atrophy and destruction of the filiform papillae, and increased interpapillar surfaces (Fig. 6A and B).39
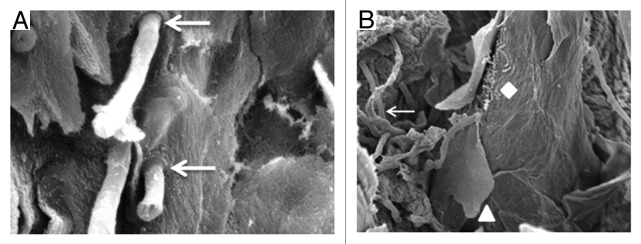
Figure 6. (A) SEM of the tongue dorsum of the mouse. The presence of hyphae penetrating perpendicularly into the tissue (↓) is verified. SEM: 3 700×. (B) SEM of the tongue dorsum of the rat. Hyphae (↓), keratin desquamation (▲), and bacteria (♦) can be observed. SEM: 1200×.
The use of LCSM to analyze the oral candidiasis in mouse model was recently proposed by Dongari-Bagtzoglou et al.70 Oral candidiasis was induced in mice immunosuppressed by inoculation of a GFP-expressing strain of C. albicans. Confocal images followed by 3D reconstruction of C. albicans biofilms formed on the dorsum tongue revealed microanatomical epithelial variations of the lingual papillae, including “valleys”, higher “elevations” of stacked fungal cells, and dark areas suggestive of extracellular matrix. The presence of β-glucan was immunoaccessible throughout the biofilm mass and on the surfaces of both yeast and hyphal organisms. Neutrophils were visualized as forming “nests” within the biofilm mass. The biofilm formed on the tongue was a mixed biofilm composed of C. albicans and various bacteria that were identified as Enterococcus spp., Lactobacillus spp., and Staphylococcus spp., while some bacteria were observed invading the epithelium together with C. albicans. Therefore, LCSM analysis provided data about the architecture of the living tongue biofilm, epithelial microanatomical variations and the distribution of fungal cells in the structure of the biofilm, and gave an idea of the dispersion of the extracellular matrix. In addition, this study provides new insights related to potential synergistic relationship between C. albicans and the oral bacterial flora during the development of oral candidiasis.
Recently, a new bioluminescence mouse model for real-time monitoring of oral candidiasis was described by Mosci et al.71 Experimental oral candidiasis was induced in immunosuppressed mice by inoculation of a C. albicans strain that produces a genetically-engineered cell surface luciferase. At selected days post infection, mice were imaged in the IVIS-200TM Imaging system under s.c. anesthesia. The total photon emission from oral areas within the images of each mouse was quantified with Living ImageR software package. The authors obtained highly reproducible levels of infection, providing tools for longitudinal monitoring of the course of OPC in live animals. The advantages of this novel method compared with the conventional methods for analysis of experimental oral candidiasis include: (1) it gives an accurate estimation of the C. albicans load in the whole oral cavity, including the load of invasive hyphae; (2) it allows the longitudinal monitoring of oral candidiasis in individual animals, providing a unique possibility to determine the progression or resolution of OPC in real-time and avoiding individual variations when the time course of CFU is assessed; (3) it allows the monitoring of C. albicans dissemination from the oral cavity to the gastrointestinal tract; and (4) it decreases the need to sacrifice a large number of animals.
All of the methods described above are very important in analyzing the experimental oral candidiasis in rat or mouse and comparing results from different laboratories. In addition, a monitoring of the body weight of each animal can be performed to estimate the disease severity. The magnitude of weight loss caused by the infection and induction of predisposing factors indicate the time point to conclude the experiment, avoiding unnecessary suffering of rats and mice.61 Another important aspect to consider in the evaluation of experimental oral candidiasis is the use of a sedative agent in the animals for C. albicans inoculation. The inoculation of C. albicans cells in the oral cavity without any anesthetic drug impairs the colonization by Candida and subsequently the development of disease. According to Takakura et al.,37 the degree of severity of the infection is correlated with the length of the sedated period. The sedation avoids the intake of Candida by animals, decreasing the spread for esophagus and stomach, and promoting a better colonization and tissue invasion of C. albicans in the oral cavity.
Conclusions and Future Directions
The study of oral candidiasis in rats and mice constitutes an important tool for the understanding of the pathogenesis of Candida in the oral mucosa and the interference of predisposing factors in the disease process. These animal models have also been very useful in testing different therapeutic modalities for candidiasis lesions in the oral cavity.42,43,66-68 Furthermore, the elucidation of the virulence mechanisms of Candida spp. and of the host–pathogen interaction in experimental animal models can provide important information for the discovery of possible targets of new antifungal agents. For any type of research to be conducted, it is worth noting that the choice of animal model must be guided by the aim of the study and must follow standard protocols to obtain reliable and reproducible results.
In summary, rats and mice models that employ antibiotic therapy and hyposalivatory conditions to induce oral candidiasis develop lesions characterized by areas of papillary atrophy in the tongue dorsum without white patches, such as erythematous candidiasis and median rhomboid glossitis. The animals in these models have an intact immune system and represent what would be expected in individuals treated with broad-spectrum antibiotics or patients with reduced salivary flow caused by Sjögren syndrome, advanced age, or radiotherapy. In the other hand, the immunosuppression models induce candidiasis lesions with white patches that are comparable to the pseudomembrane observed in human thrush and reflect the oral conditions of AIDS patients.50 In addition, rat models have a sufficiently sized oral cavity for placement of a prosthetic device in the palate that induces candidiasis lesions similar to human Candida denture stomatitis. The advantages of using mice for experimental oral candidiasis is that the immunobiology of the healthy mouse mucosa has been well characterized and there are several genetically modified animals available to research.
According to the studies cited in this review, it is evident that the most studies of oral candidiasis in mice and rats were performed with C. albicans. However, other non-C. albicans species have arisen in recent years due to a growing population of immunosuppressed patients, and future studies must be conducted to investigate the interaction of Candida mixed biofilms in the oral cavity and their relation to recurrent episodes of oral candidiasis in these patients. Furthermore, the methods for the analysis of experimental oral candidiasis should be improved because we have new technologies available, such as the real-time monitoring of microbial infections using bioluminescent fungi described recently by Mosci et al.71 This new technology and many more under development will be very useful for the advancement of oral experimental candidiasis.
Acknowledgments
The authors thank the São Paulo Council of Research (FAPESP) for financial support (Grants 2010/18753-7 and 2010/19117-7) and scholarships (Processes 2010/00879-4 and 2011/21346-7).
Glossary
Abbreviations:
- EPS
extracellular polymeric substances
- CFU
colony-forming units
- PDT
photodynamic therapy
- SCID
severe combined immune deficiency
- MAIDS
murine acquired immune deficiency syndrome
- LCSM
laser confocal scanning microscopy
- SDA
Sabouraud dextrose agar
- HE
hematoxylin-eosin
- PAS
periodic acid-Schiff
- SEM
scanning electron microscope
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/25199
References
- 1.Gugnani HC, Becker K, Fegeler W, Basu S, Chattopadhya D, Baveja U, et al. Oropharyngeal carriage of Candida species in HIV-infected patients in India. Mycoses. 2003;46:299–306. doi: 10.1046/j.1439-0507.2003.00896.x. [DOI] [PubMed] [Google Scholar]
- 2.Pomarico L, Cerqueira DF, de Araujo Soares RM, de Souza IP, de Araujo Castro GF, Socransky S, et al. Associations among the use of highly active antiretroviral therapy, oral candidiasis, oral Candida species and salivary immunoglobulin A in HIV-infected children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:203–10. doi: 10.1016/j.tripleo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgado ACD, de Jesus Pedro R, Aoki FH, Resende MR, Trabasso P, Colombo AL, et al. Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect. 2009;15:364–71. doi: 10.1111/j.1469-0691.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 4.Jainkittivong A, Lin AL, Johnson DA, Langlais RP, Yeh CK. Salivary secretion, mucin concentrations and Candida carriage in HIV-infected patients. Oral Dis. 2009;15:229–34. doi: 10.1111/j.1601-0825.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 5.Luque AG, Biasoli MS, Tosello ME, Binolfi A, Lupo S, Magaró HM. Oral yeast carriage in HIV-infected and non-infected populations in Rosario, Argentina. Mycoses. 2009;52:53–9. doi: 10.1111/j.1439-0507.2008.01542.x. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro PM, Bacal F, Koga-Ito CY, Junqueira JC, Jorge AOC. Presence of Candida spp. in the oral cavity of heart transplantation patients. J Appl Oral Sci. 2011;19:6–10. doi: 10.1590/S1678-77572011000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junqueira JC, Vilela SFG, Rossoni RD, Barbosa JO, Costa ACBP, Rasteiro VMC, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo. 2012;54:17–24. doi: 10.1590/S0036-46652012000100004. [DOI] [PubMed] [Google Scholar]
- 8.Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–77. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 9.Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, Gupta DK, et al. Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol Immunol Hung. 2007;54:201–35. doi: 10.1556/AMicr.54.2007.3.1. [DOI] [PubMed] [Google Scholar]
- 10.Junqueira JC, Fuchs BB, Muhammed M, Coleman JJ, Suleiman JM, Vilela SF, et al. Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol. 2011;11:247. doi: 10.1186/1471-2180-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva S, Henriques M, Oliveira R, Williams D, Azeredo J. In vitro biofilm activity of non-Candida albicans Candida species. Curr Microbiol. 2010;61:534–40. doi: 10.1007/s00284-010-9649-7. [DOI] [PubMed] [Google Scholar]
- 12.Seneviratne CJ, Jin L, Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14:582–90. doi: 10.1111/j.1601-0825.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35:340–55. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rautemaa R, Ramage G. Oral candidosis--clinical challenges of a biofilm disease. Crit Rev Microbiol. 2011;37:328–36. doi: 10.3109/1040841X.2011.585606. [DOI] [PubMed] [Google Scholar]
- 16.Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol. 2011;3:5771. doi: 10.3402/jom.v3i0.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campisi G, Panzarella V, Matranga D, Calvino F, Pizzo G, Lo Muzio L, et al. Risk factors of oral candidosis: a twofold approach of study by fuzzy logic and traditional statistic. Arch Oral Biol. 2008;53:388–97. doi: 10.1016/j.archoralbio.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Komiyama EY, Ribeiro PM, Junqueira JC, Koga-Ito CY, Jorge AOC. Prevalence of yeasts in the oral cavity of children treated with inhaled corticosteroids. Braz Oral Res. 2004;18:197–201. doi: 10.1590/S1806-83242004000300004. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima C, Matsuse H, Saeki S, Kawano T, Machida I, Kondo Y, et al. Salivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroid. J Asthma. 2005;42:601–4. doi: 10.1080/02770900500216259. [DOI] [PubMed] [Google Scholar]
- 20.Radfar L, Shea Y, Fischer SH, Sankar V, Leakan RA, Baum BJ, et al. Fungal load and candidiasis in Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:283–7. doi: 10.1016/S1079-2104(03)00224-5. [DOI] [PubMed] [Google Scholar]
- 21.Ohman SC, Jontell M. Treatment of angular cheilitis. The significance of microbial analysis, antimicrobial treatment, and interfering factors. Acta Odontol Scand. 1988;46:267–72. doi: 10.3109/00016358809004776. [DOI] [PubMed] [Google Scholar]
- 22.Weerasuriya N, Snape J. Oesophageal candidiasis in elderly patients: risk factors, prevention and management. Drugs Aging. 2008;25:119–30. doi: 10.2165/00002512-200825020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Soysa NS, Samaranayake LP, Ellepola AN. Diabetes mellitus as a contributory factor in oral candidosis. Diabet Med. 2006;23:455–9. doi: 10.1111/j.1464-5491.2005.01701.x. [DOI] [PubMed] [Google Scholar]
- 24.Samaranayake LP. Nutritional factors and oral candidosis. J Oral Pathol. 1986;15:61–5. doi: 10.1111/j.1600-0714.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 25.Soysa NS, Samaranayake LP, Ellepola AN. Antimicrobials as a contributory factor in oral candidosis--a brief overview. Oral Dis. 2008;14:138–43. doi: 10.1111/j.1601-0825.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 26.Egusa H, Soysa NS, Ellepola AN, Yatani H, Samaranayake LP. Oral candidosis in HIV-infected patients. Curr HIV Res. 2008;6:485–99. doi: 10.2174/157016208786501445. [DOI] [PubMed] [Google Scholar]
- 27.Hamza OJ, Matee MI, Moshi MJ, Simon EN, Mugusi F, Mikx FH, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:135. doi: 10.1186/1471-2180-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badiee P, Alborzi A, Davarpanah MA, Shakiba E. Distributions and antifungal susceptibility of Candida species from mucosal sites in HIV positive patients. Arch Iran Med. 2010;13:282–7. [PubMed] [Google Scholar]
- 29.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 30.Farah CS, Lynch N, McCullough MJ. Oral fungal infections: an update for the general practitioner. Aust Dent J. 2010;55(Suppl 1):48–54. doi: 10.1111/j.1834-7819.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA. Candida biofilms and oral candidosis: treatment and prevention. Periodontol 2000. 2011;55:250–65. doi: 10.1111/j.1600-0757.2009.00338.x. [DOI] [PubMed] [Google Scholar]
- 32.Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–60. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 33.Martin R, Wächtler B, Schaller M, Wilson D, Hube B. Host-pathogen interactions and virulence-associated genes during Candida albicans oral infections. Int J Med Microbiol. 2011;301:417–22. doi: 10.1016/j.ijmm.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15:406–12. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junqueira JC. Models hosts for the study of oral candidiasis. Adv Exp Med Biol. 2012;710:95–105. doi: 10.1007/978-1-4419-5638-5_10. [DOI] [PubMed] [Google Scholar]
- 37.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, et al. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol. 2003;47:321–6. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 38.Totti MAG, Santos EB, Almeida OP, Jorge AOC. Implantation and permanency of Candida albicans in the oral cavity of normal sialodenectomized mice after a single inoculation of yeast. Braz J Oral Sci. 2002;1:133–6. [Google Scholar]
- 39.Junqueira JC, Colombo CED, Martins JdaS, Koga Ito CY, Carvalho YR, Jorge AOC. Experimental candidosis and recovery of Candida albicans from the oral cavity of ovariectomized rats. Microbiol Immunol. 2005;49:199–207. doi: 10.1111/j.1348-0421.2005.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 40.Ishibashi H, Hisajima T, Hu W, Yamaguchi H, Nishiyama Y, Abe S. A murine model of esophageal candidiasis with local characteristic symptoms. Microbiol Immunol. 2007;51:501–6. doi: 10.1111/j.1348-0421.2007.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 41.Taguchi Y, Takizawa T, Ishibashi H, Sagawa T, Arai R, Inoue S, et al. Therapeutic effects on murine oral candidiasis by oral administration of cassia (Cinnamomum cassia) preparation. Nihon Ishinkin Gakkai Zasshi. 2010;51:13–21. doi: 10.3314/jjmm.51.13. [DOI] [PubMed] [Google Scholar]
- 42.Martins JdaS, Junqueira JC, Faria RL, Santiago NF, Rossoni RD, Colombo CED, et al. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:71–7. doi: 10.1016/j.tripleo.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Costa AC, Campos Rasteiro VM, da Silva Hashimoto ES, Araújo CF, Pereira CA, Junqueira JC, et al. Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:67–74. doi: 10.1016/j.oooo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Miranda TT, Vianna CR, Rodrigues L, Monteiro AS, Rosa CA, Corrêa A., Jr. Diversity and frequency of yeasts from the dorsum of the tongue and necrotic root canals associated with primary apical periodontitis. Int Endod J. 2009;42:839–44. doi: 10.1111/j.1365-2591.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 45.Allen CM. Animal models of oral candidiasis. A review. Oral Surg Oral Med Oral Pathol. 1994;78:216–21. doi: 10.1016/0030-4220(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 46.Naglik JR, Fidel PL, Jr., Odds FC. Animal models of mucosal Candida infection. FEMS Microbiol Lett. 2008;283:129–39. doi: 10.1111/j.1574-6968.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jorge AO, Totti MA, de Almeida OP, Scully C. Oral candidiasis established in the sialoadenectomised rat. J Oral Pathol Med. 1993;22:54–6. doi: 10.1111/j.1600-0714.1993.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Dion MR, Jurasic MM, Gibson G, Jones JA. Xerostomia and salivary hypofunction in vulnerable elders: prevalence and etiology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:52–60. doi: 10.1016/j.oooo.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Desoutter A, Soudain-Pineau M, Munsch F, Mauprivez C, Dufour T, Coeuriot JL. Xerostomia and medication: a cross-sectional study in long-term geriatric wards. J Nutr Health Aging. 2012;16:575–9. doi: 10.1007/s12603-012-0007-2. [DOI] [PubMed] [Google Scholar]
- 50.Green CB, Marretta SM, Cheng G, Faddoul FF, Ehrhart EJ, Hoyer LL. RT-PCR analysis of Candida albicans ALS gene expression in a hyposalivatory rat model of oral candidiasis and in HIV-positive human patients. Med Mycol. 2006;44:103–11. doi: 10.1080/13693780500086527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisker AV, Schiøtt CR, Philipsen HP. Long-term oral candidosis in rats. Acta Pathol Microbiol Immunol Scand B. 1982;90:221–7. doi: 10.1111/j.1699-0463.1982.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 52.Junqueira JC, Martins JdaS, Faria RL, Colombo CED, Jorge AOC. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med Sci. 2009;24:877–84. doi: 10.1007/s10103-009-0673-4. [DOI] [PubMed] [Google Scholar]
- 53.Chami N, Chami F, Bennis S, Trouillas J, Remmal A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Dis. 2004;8:217–26. doi: 10.1590/S1413-86702004000300005. [DOI] [PubMed] [Google Scholar]
- 54.Núñez MJ, Novío S, Suárez JA, Balboa J, Freire-Garabal M. Effects of psychological stress and fluoxetine on development of oral candidiasis in rats. Clin Vaccine Immunol. 2010;17:668–73. doi: 10.1128/CVI.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson CC, Yu A, Lee H, Fidel PL, Jr., Noverr MC. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect Immun. 2012;80:1736–43. doi: 10.1128/IAI.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 2010;78:3650–9. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farah CS, Elahi S, Drysdale K, Pang G, Gotjamanos T, Seymour GJ, et al. Primary role for CD4(+) T lymphocytes in recovery from oropharyngeal candidiasis. Infect Immun. 2002;70:724–31. doi: 10.1128/IAI.70.2.724-731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Repentigny L. Animal models in the analysis of Candida host-pathogen interactions. Curr Opin Microbiol. 2004;7:324–9. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Totti MAG, dos Santos EB, de Almeida OP, Koga-Ito CY, Jorge AOC. Oral candidosis by Candida albicans in normal and xerostomic mice. Braz Oral Res. 2004;18:202–7. doi: 10.1590/S1806-83242004000300005. [DOI] [PubMed] [Google Scholar]
- 60.Rahman D, Mistry M, Thavaraj S, Challacombe SJ, Naglik JR. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007;9:615–22. doi: 10.1016/j.micinf.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–42. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takakura N, Wakabayashi H, Ishibashi H, Yamauchi K, Teraguchi S, Tamura Y, et al. Effect of orally administered bovine lactoferrin on the immune response in the oral candidiasis murine model. J Med Microbiol. 2004;53:495–500. doi: 10.1099/jmm.0.05505-0. [DOI] [PubMed] [Google Scholar]
- 63.Kamagata-Kiyoura Y, Abe S, Yamaguchi H, Nitta T. Protective effects of human saliva on experimental murine oral candidiasis. J Infect Chemother. 2004;10:253–5. doi: 10.1007/s10156-004-0330-6. [DOI] [PubMed] [Google Scholar]
- 64.Hisajima T, Maruyama N, Tanabe Y, Ishibashi H, Yamada T, Makimura K, et al. Protective effects of farnesol against oral candidiasis in mice. Microbiol Immunol. 2008;52:327–33. doi: 10.1111/j.1348-0421.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 65.Yanagi M, Hisajima T, Ishibashi H, Amemiya A, Abe S, Watanabe M. Oral candidiasis deteriorated by local application of a glucocorticoid-containing film in a mouse model. Biol Pharm Bull. 2008;31:278–83. doi: 10.1248/bpb.31.278. [DOI] [PubMed] [Google Scholar]
- 66.Mima EGO, Pavarina AC, Dovigo LN, Vergani CE, Costa CAS, Kurachi C, et al. Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:392–401. doi: 10.1016/j.tripleo.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Ninomiya K, Maruyama N, Inoue S, Ishibashi H, Takizawa T, Oshima H, et al. The essential oil of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol protect mice from experimental oral candidiasis. Biol Pharm Bull. 2012;35:861–5. doi: 10.1248/bpb.35.861. [DOI] [PubMed] [Google Scholar]
- 68.Hayama K, Ishibashi H, Ishijima SA, Niimi K, Tansho S, Ono Y, et al. A D-octapeptide drug efflux pump inhibitor acts synergistically with azoles in a murine oral candidiasis infection model. FEMS Microbiol Lett. 2012;328:130–7. doi: 10.1111/j.1574-6968.2011.02490.x. [DOI] [PubMed] [Google Scholar]
- 69.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:155–60. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 70.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mosci P, Pericolini E, Gabrielli E, Kenno S, Perito S, Bistoni F, et al. A novel bioluminescence mouse model for monitoring oropharyngeal candidiasis in mice. Virulence. 2013;4:250–4. doi: 10.4161/viru.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]