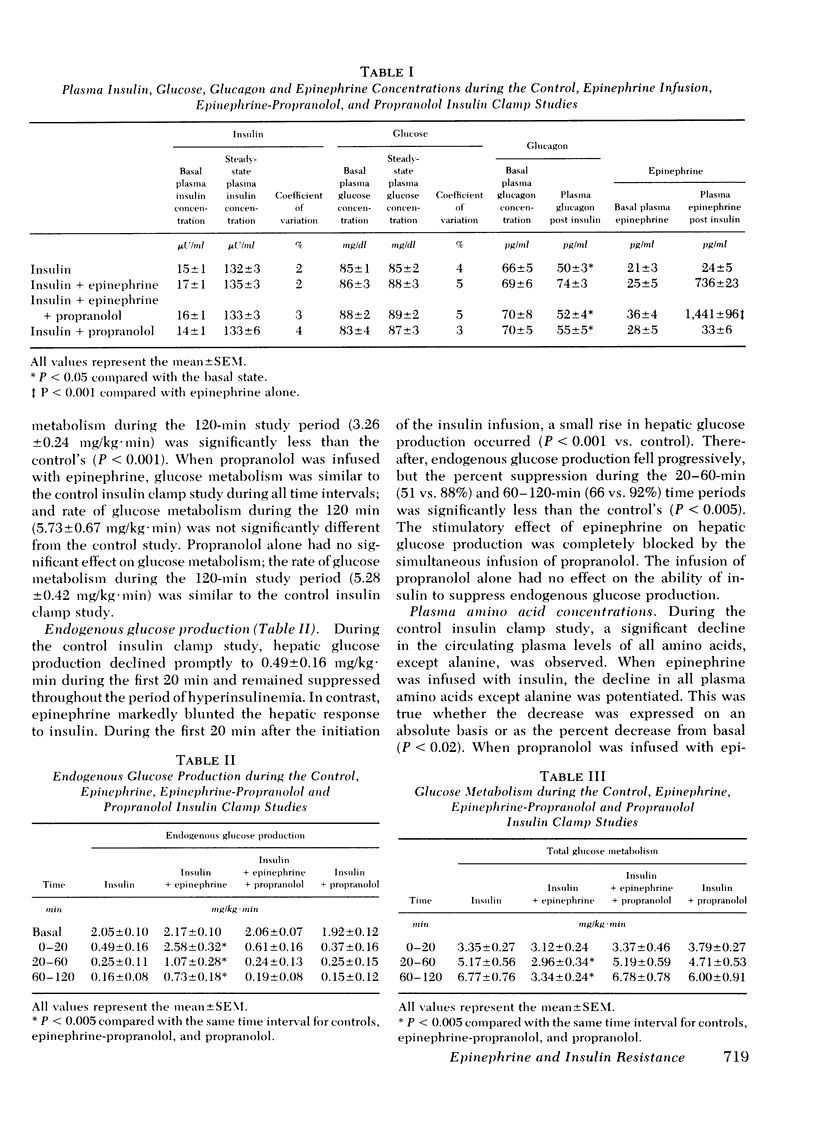
Abstract
Endogenous release of epinephrine after stress as well as exogenous epinephrine infusion are known to result in impaired glucose tolerance. Previous studies of man and animals have demonstrated that this effect of epinephrine results from inhibition of insulin secretion and augmentation of hepatic glucose production. However, the effect of epinephrine on tissue sensitivity to insulin, and the relative contributions of peripheral vs. hepatic resistance to impaired insulin action, have not been defined. Nine young normal-weight subjects were studied with the insulin clamp technique. Plasma insulin was raised by ∼100 μU/ml while plasma glucose concentration was maintained at basal levels by a variable glucose infusion. Under these conditions of euglycemia, the amount of glucose metabolized equals the glucose infusion rate and is a measure of tissue sensitivity to insulin. Subjects received four studies: (a) insulin (42.6 mU/m2·min), (b) insulin plus epinephrine (0.05 μg/kg·min), (c) insulin plus epinephrine plus propranolol (1.43 μg/kg·min), and (d) insulin plus propranolol. During insulin administration alone, glucose metabolism averaged 5.49±0.58 mg/kg·min. When epinephrine was infused with insulin, glucose metabolism fell by 41% to 3.26 mg/kg·min (P < 0.001). After insulin alone, hepatic glucose production declined by 92% to 0.16±0.08 mg/kg·min. Addition of epinephrine was associated with a delayed and incomplete suppression of glucose production (P < 0.01) despite plasma insulin levels >100 μU/ml. When propranolol was administered with epinephrine, total glucose metabolism was restored to control values and hepatic glucose production suppressed normally. Propranolol alone had no effect on insulin-mediated glucose metabolism. These results indicate that epinephrine, acting primarily through a β-adrenergic receptor, markedly impairs tissue sensitivity to an increase in plasma insulin levels, and that this effect results from both peripheral and hepatic resistance to the action of insulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E. A., Arky R. A. Role of beta-adrenergic receptors in counterregulation to insulin-induced hypoglycemia. Diabetes. 1968 Mar;17(3):141–146. doi: 10.2337/diab.17.3.141. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Steele R., Rathgeb I., De Bodo R. C. Glucose metabolism and plasma insulin level during epinephrine infusion in the dog. Am J Physiol. 1967 Mar;212(3):677–682. doi: 10.1152/ajplegacy.1967.212.3.677. [DOI] [PubMed] [Google Scholar]
- Antonis A., Clark M. L., Hodge R. L., Molony M., Pilkington T. R. Receptor mechanisms in the hyperglycaemic response to adrenaline in man. Lancet. 1967 May 27;1(7500):1135–1137. doi: 10.1016/s0140-6736(67)91710-2. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer P. E. Isotope-derivative measurements of plasma norepinephrine and epinephrine in man. Diabetes. 1976 Nov;25(11):1071–1082. doi: 10.2337/diab.25.11.1071. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968 Aug 25;243(16):4189–4196. [PubMed] [Google Scholar]
- Exton J. H., Park C. R. The stimulation of gluconeogenesis from lactate by epinephrine, glucagon, cyclic 3',5'-adenylate in the perfused rat liver. Pharmacol Rev. 1966 Mar;18(1):181–188. [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Tsalikian E., Karam J. H. Studies on the mechanism of epinephrine-induced hyperglycemia in man. Evidence for participation of pancreatic glucagon secretion. Diabetes. 1976 Jan;25(1):65–71. doi: 10.2337/diab.25.1.65. [DOI] [PubMed] [Google Scholar]
- Halter J. B., Pflug A. E., Porte D., Jr Mechanism of plasma catecholamine increases during surgical stress in man. J Clin Endocrinol Metab. 1977 Nov;45(5):936–944. doi: 10.1210/jcem-45-5-936. [DOI] [PubMed] [Google Scholar]
- Kneer N. M., Bosch A. L., Clark M. G., Lardy H. A. Glucose inhibition of epinephrine stimulation of hepatic gluconeogenesis by blockade of the alpha-receptor function. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4523–4527. doi: 10.1073/pnas.71.11.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86–94. doi: 10.1172/JCI105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Felig P. Effect of sequential infusions of glucagon and epinephrine on glucose turnover in the dog. Am J Physiol. 1978 Sep;235(3):E287–E290. doi: 10.1152/ajpendo.1978.235.3.E287. [DOI] [PubMed] [Google Scholar]
- Sherline P., Lynch A., Glinsmann W. H. Cyclic AMP and adrenergic receptor control of rat liver glycogen metabolism. Endocrinology. 1972 Sep;91(3):680–690. doi: 10.1210/endo-91-3-680. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R., DeFronzo R., Wahren J., Felic P. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):348–352. doi: 10.1073/pnas.74.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan I. G., Sawh P. C., Bihler I. Influence of adrenalin on sugar transport in soleus, a red skeletal muscle. Mol Cell Endocrinol. 1978 Feb-Mar;10(1):3–12. doi: 10.1016/0303-7207(78)90054-0. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]


