Abstract
Background
The optimal treatment strategy in patients with aggressive B cell central nervous system lymphoma suitable to receive intensive therapy is unknown. The benefit of incorporating rituximab in systemic therapy remains unclear. We performed a retrospective study examining the impact of rituximab in the context of concomitant therapies, including methotrexate, cytarabine, and radiotherapy, in patients treated with curative intent at 4 university teaching hospitals during 1996–2011.
Methods
A retrospective study of CNS lymphoma cases treated at the participating institutions was performed in accordance with institutional ethical guidelines. Patients were included if they received a diagnosis of primary diffuse large B cell lymphoma of the CNS, were HIV negative, and were treated with curative intent.
Results
One hundred twenty patients aged 21–81 years were identified. Rituximab recipients and nonrecipients were similar, except for rituximab recipients being more likely to have received a diagnosis after 2004. The median follow-up of surviving patients was 30 months. The 5-year overall survival was 46%. Univariate analysis revealed age ≤60 years, ECOG performance status ≤1, normal lactate dehydrogenase, diagnosis after 2004, and treatment with cytarabine and rituximab as predictive of favorable overall survival. Multivariate analysis identified age to be an independent predictor of overall survival, with a trend toward improved survival from the other variables that were significant in univariate analyses.
Conclusions
In this retrospective analysis, the addition of rituximab to high-dose methotrexate-based chemotherapy in patients with aggressive B cell CNS lymphoma was associated with improved overall survival. Further studies are underway to prospectively validate these findings.
Keywords: CNS, immunotherapy, lymphoma, rituximab
Introduction
Primary central nervous system lymphoma (PCNSL) represents a rare and biologically distinct subset of aggressive non-Hodgkin lymphoma. The median age at presentation of PCNSL is 60 years, with the most common histological subtype (90%) being diffuse large B cell lymphoma (DLBCL).1 Because of the low incidence, optimal treatment strategies remain uncertain and are currently based on outcomes from retrospective series, small phase 2 studies, and scant phase 3 clinical trials. Since the introduction of high-dose methotrexate (HD-MTX) as the mainstay of therapy for PCNSL, 5-year overall survival rates up to 40% have been reported, representing a significant improvement from the previous dismal outcomes of radiotherapy alone.2–4 The addition of cytarabine has been shown to further improve survival in a prospective randomized setting.5
The contemporary question facing clinicians involved with the treatment of PCNSL is which additional therapies will be most effective at providing a further improvement in survival with minimal toxicity. Adjuvant radiotherapy has been shown to provide further survival benefit, but its use is limited by a propensity for neurotoxicity in older persons and at higher cumulative doses.4,6–8 High-dose chemotherapy with autologous stem cell transplantation (ASCT) is an effective method of dose intensification and has been shown to increase the overall response rate among small numbers of patients in the prospective setting.9–14 However, the extent of neurotoxicity associated with ASCT remains poorly defined, because these studies did not prospectively assess cognitive function.
Immunotherapy currently provides the third option of treatment intensification for PCNSL. Intravenous rituximab treatment unequivocally improves survival outcomes in systemic DLBCL and reduces the rate of CNS relapse in high-risk disease, despite low CNS drug levels.15 It has been shown to be well tolerated when added to HD-MTX–based therapy for PCNSL without any apparent increase in neurotoxicity or infection, but whether it impacts favorably on the outcome of PCNSL is controversial.6,15–19 To address the question of whether rituximab may be of benefit, we examined the impact of rituximab in the context of concurrent methotrexate, cytarabine, and radiotherapy in patients treated with curative intent at 4 university teaching hospitals during 1996–2011.
Materials and Methods
A retrospective study of cases of primary DLBCL of the CNS treated at the participating institutions in Victoria, Australia, during 1996–2011 was performed in accordance with institutional ethical guidelines. Cases were identified from collaboration among anatomical pathology, hematology, neurosurgery, radiology, radiotherapy, and pharmacy departments, including pathology records, pharmacy databases of rituximab and intravenous methotrexate use, and several existing PCNSL databases from the various departments. This multidisciplinary approach ensured comprehensive case identification. Patients were included if they were HIV negative and had a first presentation of biopsy-proven CD20+ primary DLBCL affecting the CNS.20 Patients were excluded if they were HIV positive (n = 2), treated with palliative intent from diagnosis because of frailty and perceived inability to tolerate cytotoxic therapy or radiotherapy (n = 27), or had a histological diagnosis of intravascular large B cell lymphoma (n = 4), insufficient follow-up information (n = 1), or died within 24 h after surgery (n = 1). The date of final analysis was December 15, 2011.
Data were obtained from hospital medical records and laboratory and radiology information systems and included patient demographic characteristics; ECOG performance status (PS); histological diagnosis; radiological findings; staging investigations, including bone marrow biopsy, serum lactate dehydrogenase (LDH), and cerebrospinal fluid (CSF) cytology and protein; treatment intent (curative or palliative); therapy received; toxicities; and outcomes. HD-MTX was defined as ≥2 g/m2 per cycle. The primary outcome assessed was overall survival (OS), measured from the date of diagnosis until death or censoring. Progression-free survival was not included as an end point because of the variability of subjective radiological interpretation and lack of standardized reporting criteria. The variables examined for impact on survival were age, sex, ECOG PS, serum LDH, location of lesions (deep vs superficial location and solitary vs multiple lesions), CSF cytology (involved with disease vs not involved), and treatment received (methotrexate dose of 8 g/m2 per cycle vs 2–3.5 g/m2, cytarabine, rituximab, radiotherapy, and intrathecal methotrexate). Deep lesions were defined as those affecting the periventricular regions, basal ganglia, brainstem, or cerebellum. To detect for bias resulting from global improvements in survival, including improvements in earlier detection, diagnostics, and supportive care over time, we also assessed the impact of the date of diagnosis (1996–2004 vs 2005–2011). This arbitrary date was assessed, because it approximated the median time point of diagnosis for the patient cohort. Statistical analysis was performed using SPSS statistical software with 2-tailed Fisher's exact test for contingency tables and Kaplan-Meier survival curves with comparison using log-rank (Mantel-Cox) test. Multivariate analysis was performed using Cox regression.
Results
One hundred twenty patients with primary DLBCL of the CNS met the criteria for inclusion in the study. Patient and disease demographic characteristics are shown in Table 1. The baseline characteristics of rituximab recipients and nonrecipients are shown in Table 2. Rituximab recipients were more likely to have received a diagnosis after 2004 (P < .0001) and less likely to have received intrathecal methotrexate (P = .011). There were no other significant differences between rituximab recipients and nonrecipients. Calculation of the IPI-CNS was not included, because only 37 patients had complete data available for this.
Table 1.
Patient and disease demographic data
| Parameter | Proportion (%) |
|---|---|
| Patient demographic characteristic | |
| Age (median/range) years | 65 (21–81) |
| Age >60 | 63 |
| Female | 52 |
| ECOG PS >1 | 33 |
| Elevated serum LDH | 41 |
| Deep lesions | 59 |
| Multiple lesions (>1) | 50 |
| CSF cytology positive | 15 |
| Diagnosis after 2004 | 50 |
| Treatment demographic characteristic | |
| HD-MTX | 95 |
| Cytarabine | 30 |
| Rituximab | 15 |
| Radiotherapy | 35 |
| Intrathecal methotrexate | 31 |
Table 2.
Demographic data on rituximab recipients and nonrecipients
| Parameter | Proportion (%) |
P valuea | |
|---|---|---|---|
| Rituximab (n = 18) | No rituximab (n = 99) | ||
| Patient demographic characteristic | |||
| Age (median/range) years | 65 (29–78) | 65 (21–81) | |
| Age >60 | 61 | 63 | 1.00 |
| Female | 56 | 49 | .799 |
| ECOG PS >1 | 22 | 34 | .416 |
| Elevated serum LDH | 17 | 45 | .110 |
| Deep lesions | 72 | 55 | .207 |
| Multiple lesions (>1) | 50 | 50 | 1.00 |
| CSF cytology positive | 25 | 13 | .593 |
| Diagnosis after 2004 | 94 | 41 | <.0001 |
| Treatment demographic characteristic | |||
| Cytarabine | 47 | 27 | .150 |
| Radiotherapy | 28 | 37 | .441 |
| Intrathecal methotrexate | 6 | 36 | .011 |
aP value of the 2-tailed Fisher's exact test for difference between rituximab recipients and nonrecipients.
Planned front-line chemotherapy regimens included single agent HD-MTX alone (n = 74), HD-MTX and cytarabine (n = 34), other combination chemotherapy with HD-MTX (n = 5) and combination chemotherapy without HD-MTX (n = 6). Treatment data were unavailable for one patient who had been transferred to another institution for treatment. The dosing regimen for HD-MTX was available for 108 patients, of which 54 (50%) were administered treatment according to protocols for 8 g/m2 per cycle and the remainder were given 2–3.5 g/m2 per cycle. HD-MTX was administered as solitary therapy (without other immunochemotherapy or radiotherapy) to 45 patients. No patients received consolidation with high-dose chemotherapy and ASCT in first remission.
Cytarabine use (4 doses at 2–3 g/m2) was distributed evenly during 1996–2011, with 15 (43%) of the 35 recipients receiving a diagnosis after 2004. Memorial Sloan-Kettering Cancer Center (MSKCC) protocols for CNS lymphoma were used for 43 patients. In 35 patients, these comprised 5 cycles of HD-MTX (3.5 g/m2), procarbazine (100 mg/m2/day), and vincristine (1.4 mg/m2), with or without adjuvant radiotherapy before planned cytarabine consolidation (2 courses, each comprising 3 g/m2/day for 2 days); in 8 patients, these consisted of 5–7 cycles of rituximab (500 mg/m2), methotrexate (3.5 g/m2), vincristine (1.4 mg/m2), and procarbazine (100 mg/m2/day) for 7 days with odd-numbered cycles, followed by consolidation radiotherapy stratified according to disease status, then cytarabine, in accordance with the previous protocol.3,6 Planned cytarabine was not received by 14 of these patients.
Adjuvant front-line therapies included intrathecal methotrexate (n = 37), radiotherapy (n = 41; cumulative dose, 15–45 Gy), and rituximab (n = 18). Data on rituximab use were unavailable for 3 patients (excluded from the rituximab analysis). The pattern of use of cytarabine or rituximab was not uniform in relation to other therapies received or the dose of HD-MTX. The variation in inclusion of rituximab was attributable to differences in institutional policies and clinician preference. Of the 18 rituximab recipients, 17 (94%) received a diagnosis after 2004. Rituximab was administered at a dose of 500 mg/m2 for 2–7 cycles in conjunction with 8 g/m2 HD-MTX–based protocols (n = 9), MSKCC CNS lymphoma protocol (n = 8), and 3.5 g/m2 HD-MTX with temozolomide (n = 1).6
By the time of censoring, 57 patients had disease progression, of whom 26 were still alive. Data on salvage therapy were available for 31 patients and included radiotherapy without HD-MTX–based chemotherapy (n = 14; 6 alive with a median postsalvage follow-up of 9 months and range of 1–12 months), radiotherapy with HD-MTX–based chemotherapy (n = 3; all alive with a postsalvage follow-up range of 4–44 months), HD-MTX–based chemotherapy without radiotherapy (n = 9; 6 alive with a median postsalvage follow-up of 17 months and range of 4–48 months), and HD-MTX–based chemotherapy, followed by consolidation ASCT with BEAM conditioning (n = 1; alive at 24 months follow-up). One patient with nodal extra-CNS relapse of DLBCL was successfully treated with 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone and was still alive with ongoing complete response (CR) 9 months later. Another patient with peripheral neurolymphomatosis as the only site of relapse was successfully treated with intrathecal methotrexate and was still alive in CR 61 months later. Two patients progressed and died within 2 months of attempted salvage with topotecan and temozolomide monotherapy, respectively.
The median follow-up of surviving patients was 30 months (range, 1–139 months). The 5-year OS was 46%. At the time of censoring, 55 patients had died due to progressive disease (n = 39), toxicity of therapy including infection (n = 3) and perforated viscera (n = 3), probable pulmonary embolus (n = 1), unrelated cause (n = 1), and unknown cause (n = 8). For those due to progressive disease, the median time from diagnosis to death was 7 months (range, 1–104 months).
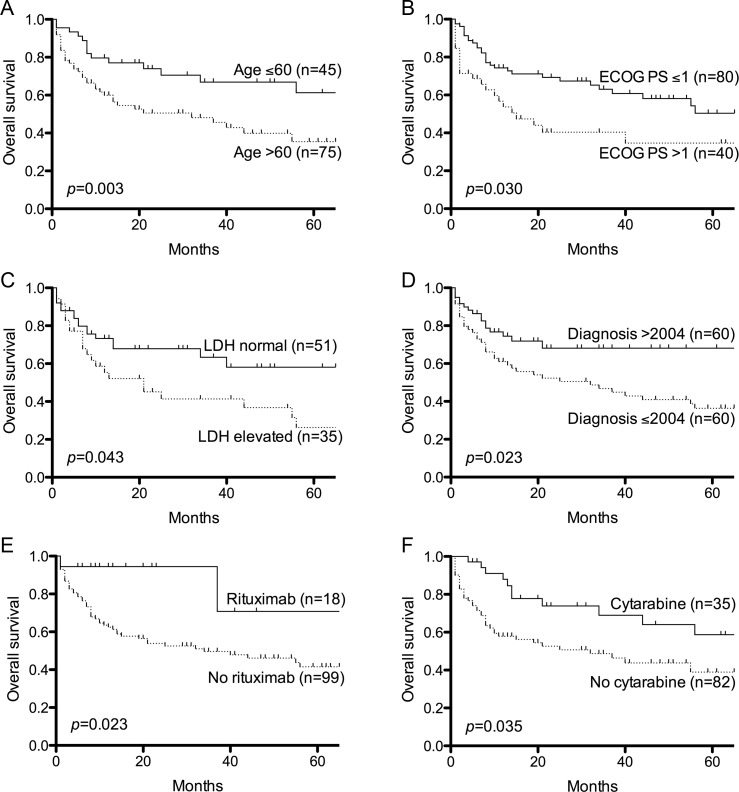
Univariate analysis revealed age >60 years, ECOG PS >1, and elevated LDH as predictive of unfavorable OS, whereas diagnosis after 2004 and treatment with cytarabine and rituximab were predictive of favorable OS (Table 3, Fig. 1). Cox regression analysis identified age >60 years to be an independent predictor of OS, and there was a trend toward improved OS with ECOG PS 0-1, normal LDH, diagnosis after 2004, cytarabine, and rituximab (Table 3). No difference in OS was observed between patients receiving 8 g/m2 vs 2–3.5 g/m2 of HD-MTX (P = .703). Of the 45 patients treated solely with HD-MTX, median survival was 32 months (range, 1–145 months), and 5-year OS was 43%. Twenty-two of 45 patients remained alive with a median follow-up of 36 months (range, 1–136 months). Radiotherapy and intrathecal methotrexate were not associated with improved survival (Table 3).
Table 3.
Results of univariate and multivariate analyses for overall survival among patients treated with curative intent
| Parameter | Univariate P value | Multivariate P value and hazard ratio |
|
|---|---|---|---|
| Patient demographic characteristic | |||
| Age >60 | .003 | .004 | 2.86 (1.39–5.88) |
| Female | .892 | ||
| ECOG PS >1 | .030 | .079 | 1.79 (.935–3.42) |
| Elevated serum LDH | .043 | .085 | 1.32 (.962–1.82) |
| Deep lesions | .223 | ||
| Multiple lesions (>1) | .069 | ||
| CSF cytology positive | .986 | ||
| Diagnosis after 2004 | .023 | .059 | .521 (.265–1.03) |
| Treatment demographic characteristic | |||
| Cytarabine | .035 | .055 | .735 (.536–1.01) |
| Rituximab | .023 | .064 | .512 (.252–1.04) |
| Radiotherapy | .236 | ||
| Intrathecal methotrexate | .718 | ||
Fig. 1.
Five-year overall survival curves showing parameters that were associated with improved survival. P values for univariate analysis are shown. (A) Age. (B) ECOG PS. (C) Serum LDH. (D) Year of diagnosis. (E) Rituximab treatment. (F) Cytarabine treatment.
The association between rituximab and improved survival was maintained when subset analysis of those who received a diagnosis after 2004 was performed (P = .034). At censoring, 16 of 18 patients treated with rituximab-containing regimens were still alive with a median follow-up of 15 months (range, 1–72 months). Of these, 12 had been restaged with 10 in CR and 2 having achieved a partial response. Both deaths within the group that received rituximab were attributable to perforated viscera complicating corticosteroid therapy, with no deaths directly attributable to disease progression.
Discussion
Although this study is subject to the biases inherent from retrospective analyses, our findings suggest that the addition of rituximab to HD-MTX–based chemotherapy was associated with improved survival in patients with aggressive CD20+ B cell CNS lymphoma. The significance of the association was maintained when subset analysis of patients who received a diagnosis after 2004 was performed. This finding is consistent with the evidence for CSF penetration of rituximab after intravenous administration, possibly aided by disruption of the blood-brain barrier because of parenchymal invasion by tumor and systemic HD-MTX.6,21
Consistent with the reported literature, age, ECOG performance status, and treatment with cytarabine were also associated with OS in our study.5,22,23 It is most likely the small patient numbers that rendered our study underpowered to show statistical significance for all of these variables in multivariate analysis.
Diagnosis after 2004 was also found to be associated with improved survival. The cause for this is likely to be multifactorial, with the more recent cohort benefitting from earlier diagnosis and improved supportive care. Differences in toxicity of therapy are not likely to be contributory, because the 6 deaths reported due to this cause were evenly distributed before and after the end of 2004.
Radiotherapy was not found to be significantly associated with survival in our cohort; however, the number treated was small and its use was not uniform across any age group or chemotherapy regimen. Although our study is underpowered to detect any additional benefit of radiotherapy beyond combination chemotherapy, a possible improvement in survival with rituximab may abrogate that provided by radiotherapy, avoiding the potential for radiotherapy-related dementia. This could possibly relegate radiotherapy to second-line therapy, especially given ongoing controversy regarding its additional benefit in the context of modern HD-MTX–based chemotherapy.7 Furthermore, the favorable adverse effect profile of rituximab may allow its administration to patients who were previously deemed by their treating clinicians as too frail for therapy.
Progression-free survival was not a primary end point of the analysis because of issues with ascertainment of remission. However, our data showed attempts at salvage to be heterogeneous and largely unsuccessful. Despite the low numbers of patients for which salvage therapy was attempted, the most favorable survival appeared to be associated with HD-MTX–based salvage therapy, either alone or with adjuvant salvage radiotherapy. This is consistent with the findings of previous retrospective series that have assessed the role of methotrexate or radiotherapy in relapsed PCNSL.24,25 A very small number of patients received other salvage therapies, such as topotecan or temozolomide, and they fared no better than the small numbers previously reported in the literature.26,27 With a lack of evidence available, our findings would support tailored therapy for those who are unable to access clinical trials, with consideration of HD-MTX re-induction with or without radiotherapy.
Although the data suggest that rituximab may be of benefit for aggressive B cell CNS lymphoma, we acknowledge the small patient numbers, lack of controls for multiple variables, variability of treatment, and short-term follow-up in this cohort, as well as the potential for other biases to affect the validity of our observations. Definitive proof of the efficacy of rituximab in improving the outcome of CNS DLBCL requires prospective studies, a number of which (NCT01011920, NCT00293475, and NTR2427) are under way.
Funding
The research performed in this study was not funded.
Conflict of interest statement. G.G., A.A., T.L., C.T., A.G., D.R., and S.O. have attended scientific meetings sponsored by Roche. C.T., A.G., D.R., and S.O. have received honoraria from Roche.
Acknowledgments
We thank Hui Gan, Austin Health; Michael McManus, Peter MacCallum Cancer Centre; David Kipp, Royal Melbourne Hospital; and Ron Freilich, Beena Kumar, Ian Simpson, Dee Nandurkar, and Andrew Danks, Monash Medical Centre, for their assistance with the study.
References
- 1.Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H. Pathology with clinical correlations of primary central nervous system non-Hodgkin's lymphoma. The Massachusetts General Hospital experience 1958–1989. Cancer. 1994;74(4):1383–1397. doi: 10.1002/1097-0142(19940815)74:4<1383::aid-cncr2820740432>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Cher L, Glass J, Harsh GR, Hochberg FH. Therapy of primary CNS lymphoma with methotrexate-based chemotherapy and deferred radiotherapy: preliminary results. Neurology. 1996;46(6):1757–1759. doi: 10.1212/wnl.46.6.1757. [DOI] [PubMed] [Google Scholar]
- 3.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNSlymphoma: the next step. J Clin Oncol. 2000;18(17):3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93–10. J Clin Oncol. 2002;20(24):4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 6.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25(30):4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 7.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 8.Omuro A, Taillandier L, Chinot O, et al. Primary CNS lymphoma in patients younger than 60: can whole-brain radiotherapy be deferred? J Neurooncol. 2011;104(1):323–330. doi: 10.1007/s11060-010-0497-x. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T, Forsyth P, Chaudhry A, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31(8):679–685. doi: 10.1038/sj.bmt.1703917. [DOI] [PubMed] [Google Scholar]
- 10.Brevet M, Garidi R, Gruson B, Royer B, Vaida I, Damaj G. First-line autologous stem cell transplantation in primary CNS lymphoma. Eur J Haematol. 2005;75(4):288–292. doi: 10.1111/j.1600-0609.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 11.Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24(24):3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 12.Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38(6):417–420. doi: 10.1038/sj.bmt.1705452. [DOI] [PubMed] [Google Scholar]
- 13.Montemurro M, Kiefer T, Schuler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18(4):665–671. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 14.Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93(1):147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 15.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–1052. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- 16.Fritsch K, Kasenda B, Hader C, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol. 2011;22(9):2080–2085. doi: 10.1093/annonc/mdq712. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol. 2012;109(2):285–291. doi: 10.1007/s11060-012-0891-7. [DOI] [PubMed] [Google Scholar]
- 18.Ruhstaller TW, Amsler U, Cerny T. Rituximab: active treatment of central nervous system involvement by non-Hodgkin's lymphoma? Ann Oncol. 2000;11(3):374–375. doi: 10.1023/a:1008371602708. [DOI] [PubMed] [Google Scholar]
- 19.Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol. 2012;159(1):39–49. doi: 10.1111/j.1365-2141.2012.09247.x. [DOI] [PubMed] [Google Scholar]
- 20.Kluin PM, Deckert M, Ferry JA. Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Lee Harris N, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC Press; 2008. pp. 240–241. [Google Scholar]
- 21.Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009;15(2):189–192. doi: 10.1177/1352458508098268. [DOI] [PubMed] [Google Scholar]
- 22.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 23.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 24.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507–1513. doi: 10.1200/JCO.2005.01.161. [DOI] [PubMed] [Google Scholar]
- 26.Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 27.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]