Abstract
Background
No more than half of patients with neurofibromatosis type 1 (NF1)–associated optic pathway gliomas (OPGs) develop vision loss. Prospectively identifying those who will require therapy remains challenging, because no reliable factors have yet been identified that predict future vision loss. To determine whether brain tissue microstructure is associated with visual acuity loss, we examined diffusion tensor imaging (DTI) and ophthalmologic evaluations in children with NF1-associated OPG.
Methods
We retrospectively reviewed ophthalmology records and concurrent DTI measurements of the optic nerves, tracts, and radiations from 50 children with NF1-associated OPGs. Multivariate linear regression measured the association between fiber trajectory quantity and white matter integrity on visual acuity measured by the logarithm of the minimal angle of resolution (logMAR).
Results
In multivariate analysis, fractional anisotropy (FA) of the optic radiations was associated with visual acuity loss (adjusted coefficient = −6.081 logMAR/FA; P = .006) after adjusting for age, extent of tumor, DTI acquisition type, prior chemotherapy, and fundus examination findings. The association remained after eliminating tumors involving the optic radiations. In an evaluation of 15 subjects with paired ophthalmologic examination and DTI a year apart, initial FA of the optic radiation was associated with a trend toward change in visual acuity a year later (coefficient = −2.652 logMAR/FA; P = .069).
Conclusions
A decrease in FA of the optic radiations is associated with abnormal visual acuity in NF1-associated OPGs and may be predictive of visual acuity loss during the following year.
Keywords: diffusion tensor imaging, neurofibromatosis type 1, neuro-ophthalmology, optic pathway glioma, visual acuity
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder caused by a mutation on chromosome 17q11.2 that affects ∼1 in 3000 children.1 Affected individuals have an error in the tumor suppressor gene encoding neurofibromin and are at increased risk for both benign and malignant tumors. Optic pathway gliomas (OPGs), involving optic nerves, chiasm, or tracts and rarely the optic radiations, develop in 15%–20% of children with neurofibromatosis type 1 (NF1)2,3 and cause vision loss in up to 50%.4
Determining when to treat young children with an NF1-associated OPG is a challenging clinical problem. One of the largest epidemiologic studies to date showed that no specific presenting factor was associated with OPGs that will require treatment.5 OPGs in patients with NF1 are generally indolent, and the most commonly cited indications for treatment are declining vision or radiographic progression.6 However, radiographic progression has never been shown to predict vision loss accurately,6,7 and visual acuity testing is highly dependent on patient cooperation.8 Because the median age of vision loss in patients with NF1-associated OPG is 4.9 years2 and many children with NF1 are affected by attentional disorders,9,10 accurate visual acuity is often difficult to obtain reliably. The ability to diagnose and predict vision loss accurately in children with NF1-associated OPGs would lead to more efficient and effective use of chemotherapy and early interventions.
Vision loss in OPGs is presumably attributable to direct infiltration of or pressure on the visual pathways by tumor. Although tumor extent by conventional magnetic resonance imaging (MRI) does not correlate with vision loss,6,7,11,12 direct measurements of the integrity of optic pathway white matter tracts may better identify vision loss. Diffusion tensor imaging (DTI) allows identification and quantitation of white matter pathways, including optic nerves, tracts, and radiations. DTI uses the Brownian motion of water to probe tissue microstructure. By sensitizing diffusion-weighted imaging in 6 (or more) noncollinear axes, it is possible to detect anisotropic patterns of diffusion and, thus, characterize both the degree and direction of preferred diffusion to assess white matter structure and integrity.
We conducted a retrospective cross-sectional study to evaluate the association between visual acuity and DTI measurements of white matter tract integrity in the visual pathways among individuals with an NF1-associated OPG. We hypothesized that DTI parameters would provide a useful in vivo imaging biomarker to help assess visual acuity and may help predict future vision loss in children with NF1 and an OPG.
Methods
Patient Selection
Individuals were identified for this retrospective cross-sectional cohort from a preexisting clinical database of patients with NF1 at the Children's Hospital of Philadelphia. Eligible participants included those with NF1 and an OPG confirmed by MRI. Subjects with a neuro-ophthalmic or ophthalmic evaluation and MRI evaluable for DTI within 3 months of each other from January 2009 through May 2012 were included in the study. DTI has been performed at the Children's Hospital of Philadelphia since 2009 as part of standard clinical MRI for patients with known or suspected brain tumors. If multiple pairs of neuro-ophthalmology/ophthalmology-MRI evaluations were available, the most recent time point was used. Longitudinal analysis was performed on subjects who had a second paired DTI and ophthalmology evaluation during the previous 9–15 months. Exclusion criteria included other factors that might impact visual acuity or DTI measurements, including a history of radiation therapy to the brain, surgery or biopsy to the OPG, or a neurologic or ophthalmologic disease other than OPG (including amblyopia, cataract, retinopathy of prematurity, or increased intracranial pressure with a ventricular shunt). Institutional review board approval with waiver of consent was obtained before study initiation.
Ophthalmology Evaluation
A pediatric neuro-ophthalmologist or pediatric ophthalmologist evaluated all subjects in the cohort. Best corrected visual acuity was assessed using an age-appropriate testing method (Teller, Lea, HOTV, Snellen) and converted to the logarithm of the minimal angle of resolution (logMAR) to create a linear scale of visual acuity. Similar to prior studies,13 counting fingers was assigned a logMAR of 1.6, hand movements a logMAR of 2, light perception a logMAR of 2.5, and no light perception a logMAR of 3. Vision deficit was defined as the difference between visual acuity and age-matched norms,14 and abnormal visual acuity was defined as a logMAR vision of ≥0.2 (correlating to ∼2-line decrease in visual acuity on the Snellen chart). In per-subject analyses, the worse eye determined visual acuity. Visual field deficits were defined as any noncentral scotomata detected reliably with either confrontation testing or automated perimetry examination (Humphrey). Optic discs were assessed for pallor and edema. Both visual field testing and optic disc appearance were categorized as either normal or abnormal.
MRI Evaluation
The presence of an OPG was confirmed on routine brain MRI in all subjects. Because tumors involving posterior structures rarely occur without anterior involvement,6 tumor location was categorized by its most posterior involvement: optic nerve, chiasm, optic tracts, or optic radiations. All MR examinations were performed at 3T on either a Trio (n = 45 exams), Skyra (n = 3 exams), or Verio (n = 17 exams) scanner (Siemens; Erlangen, Germany). Diffusion MR was acquired with an echo planar pulse sequence with 128 × 128 matrix, in-plane voxel size of 2 × 2 mm, diffusion weighting of b = 1000 s/mm2, and full brain coverage with no gap between slices. Sixteen examinations were acquired with 20 diffusion gradient directions and 2 mm slice thickness, and 49 examinations were acquired with 30 diffusion gradient directions and 2.5 mm slice thickness. On the Trio, TE was 91–93 ms, with TR of 7.3–11.6 s and bandwidth of 1395 Hz/pixel. On the Skyra, TE was 84 ms, with TR of 9.4–9.6 s and bandwidth of 1565 Hz/pixel. On the Verio, TE was 91–104 ms, with TR of 9.4–14 s and bandwidth of 1395 Hz/pixels. Differences in TR in this range are unlikely to significantly affect diffusion-weighted signal intensity.
Postprocessing DTI analysis was performed using DTI-Studio, version 3.0.1 (Johns Hopkins University; Baltimore, MD). Deterministic streamline fiber tracking with the fiber assignment by continuous fiber tracking algorithm was used with minimum fractional anisotropy (FA) value of 0.15 and maximum turning angle of 70°.15 To trace fibers in the optic nerves, a region of interest was placed in the posterior half of the optic nerve, identified on the echo planar images with no diffusion weighting (b = 0 s/mm2). Fibers found in this area and coursing toward the chiasm were included as optic nerve. Optic tracts were identified as fibers coursing between 2 regions of interest: immediately posterior to the optic chiasm and anterior to the lateral geniculate body. Optic radiations were also measured between 2 regions of interest. An initial region of interest was placed posterior to Meyer's loop at the level of the splenium of the corpus callosum. A second region of interest was placed immediately anterior to tract branching before the calcarine cortex. Fibers that coursed anterior to the first region of interest or across the corpus callosum were excluded from analysis. White matter tracts and their associated regions of interest can be seen in Fig. 1.
Fig. 1.
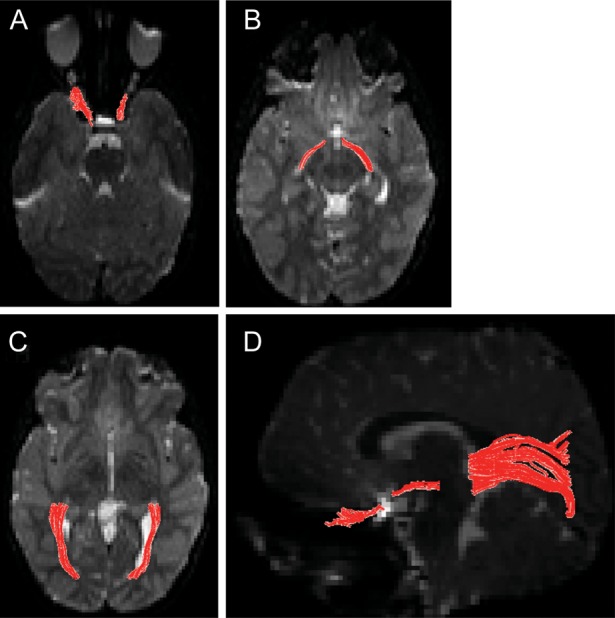
Transverse sections seen on mean image of all B0s demonstrating location of regions of interest for optic nerves (A), tracts (B), and radiations (C). (D) All 3 optic pathway tracts are superimposed on sagittal images.
White matter tracts were assessed for number of fiber trajectories, tract volume, tract density (number of trajectories/tract volume), FA, mean diffusivity (MD), and tensor eigenvalues (λ1, λ2, and λ3). Radial diffusivity (RD) describes diffusion perpendicular to axons and is the mean of the second and third eigenvalues. Because the optic nerve contributes to vision in a single eye, correlation of DTI measurements of individual optic nerves to visual acuity was performed on a per-eye basis. In contrast, because optic tracts and radiations contribute to visual acuity in both eyes, DTI measurements of these white matter tracts were averaged from both sides in a per-subject comparison of vision loss and white matter characteristics. Mean FA, RD, MD, and eigenvalues were calculated from weighted averages, based on the number of fiber trajectories passing through each voxel.
Statistical Analyses
Summary statistics were constructed using frequencies and proportions for categorical data elements and means and medians for continuous variables. One-way analysis of variance and Student's t test were used to compare proportions and means between groups with and without normal visual acuity. A P value of < .05 was considered to be statistically significant. Bivariate analysis was conducted to determine the association between potential covariates and abnormal visual acuity. A P value of < .20 was used to identify covariates to be analyzed in a multivariable model. A linear regression model compared visual acuity deficit with identified covariates, which were eliminated from the multivariate model if the P value of their association with visual acuity was > .05 and if their elimination from the model did not change the coefficient estimates of other covariates by >15%. Because of their presumed association with both DTI measurements and vision deficits, age at ophthalmology evaluation, tumor location, and DTI acquisition parameter type (20 directions with 2 mm slice thickness vs. 30 directions with 2.5 mm slice thickness) were included in the model a priori.
Results
Fifty-six potentially eligible subjects with NF1 and an OPG were identified. Six subjects were excluded because of inadequate MR imaging (motion degraded or incorrect DTI acquisition). Of the 50 remaining subjects, 91 eyes were evaluable: 8 optic nerves were eliminated from the analysis because of inadequate MR imaging, and 1 patient had undergone a prior enucleation.
Twenty-six subjects had abnormal visual acuity in one or both eyes. The mean logMAR of those categorized with normal visual acuity was 0.08 (±0.07), compared with 0.72 (±0.82) in those with abnormal visual acuity (P < .001). Demographic characteristics and ophthalmology results of the cohort are shown in Table 1. The median interval between ophthalmology evaluation and MRI was 31 days (range, 0–91 days) and did not differ between the groups (P = .82). Individuals with abnormal visual acuity were more likely to have abnormal optic discs (P = .002), but there were no other significant differences between the 2 groups.
Table 1.
Demographic characteristics of 50 children with NF1-associated OPG.
| Characteristic | Normal Visual Acuity (n = 24) | Abnormal Visual Acuity (n = 26) | P value |
|---|---|---|---|
| Male | 10 (41.7%) | 15 (57.7%) | .26 |
| Age (mean) at exam (years) | 7.7 ± 4.3 | 8.9 ± 3.7 | .28 |
| Mean visual deficit (logMAR) | 0.08 ± 0.07 | 0.72 ± 0.82 | < .001 |
| Interval between ophthalmology evaluation and MRI (days) | 30 ± 34 | 32 ± 28 | .82 |
| 30 directional DTI | 19 (79.2%) | 18 (69.2%) | .42 |
| Furthest posterior involvement | |||
| Nerve | 10 (41.6%) | 6 (23.1%) | .14 |
| Chiasm | 8 (33.3%) | 5 (19.2%) | |
| Tracts | 5 (20.8%) | 12 (46.1%) | |
| Radiations | 1 (4.2%) | 3 (11.5%) | |
| Abnormal Visual Fields | 1 (4.4%) | 4 (19.1%) | .13 |
| Abnormal Visual Discs | 6 (27.3%) | 18 (72%) | .002 |
| Received Chemotherapy | 8 (33.3%) | 13 (50.0%) | .23 |
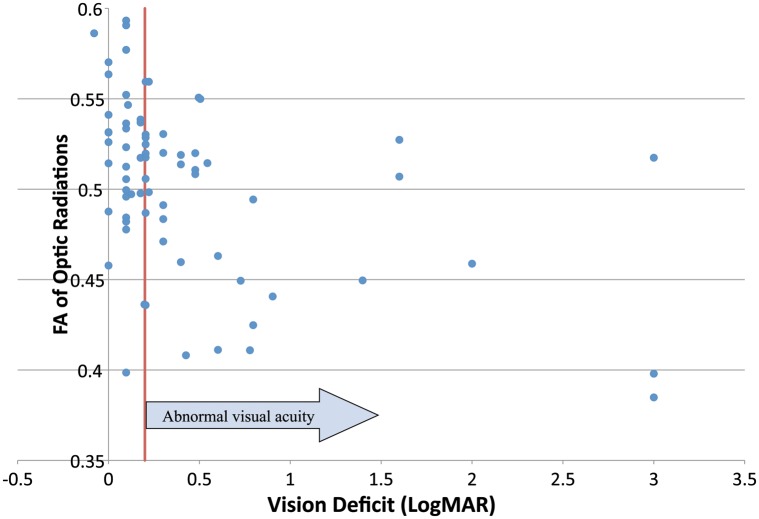
There were no significant differences in diffusion tensor imaging parameters in the optic nerves (Table 2) or optic tracts (Table 3). In contrast, significant differences were seen in measures of fiber integrity (FA, RD, MD, and minor eigenvalues [λ2 and λ3]) but not fiber quantity (number of trajectories, fiber volume, and fiber density) in optic radiations between those with normal and those with abnormal acuity (Table 3). Because FA, RD, and MD of the optic radiations were collinear in multivariate analysis, mean FA was further investigated as having the strongest association with visual acuity. Fig. 2 is a scatter plot of visual acuity deficit and FA of the optic radiations. To determine the sensitivity of FA in identifying abnormal visual acuity at various cutoffs, the FA of the optic radiations was divided into quartiles (Table 4). In the lowest quartile (FA < 0.482), 77% of subjects had visual acuity loss, compared with 15% of those in the highest quartile (FA > 0.530), representing a significant difference in the proportion with visual acuity loss across all quartiles (P = .014).
Table 2.
Univariate analysis of DTI of the optic nerve on visual acuity.
| DTI Measure | Normal Visual Acuity (n = 53) | Abnormal Visual Acuity (n = 38) | P value |
|---|---|---|---|
| Fibers | 17.952 | 24.478 | .32 |
| Tract Volume (mm3) | 431.524 | 573.913 | .36 |
| Fiber Density (fiber trajectories/mm3) | 0.303 | 0.295 | .81 |
| Fractional Anisotropy | 0.402 | 0.396 | .82 |
| Radial Diffusivity (mm2/s) | 0.00120 | 0.00114 | .45 |
| Mean Diffusivity (mm2/s) | 0.00154 | 0.00145 | .41 |
| λ1 (mm2/s) | 0.00218 | 0.00207 | .39 |
| λ2 (mm2/s) | 0.00138 | 0.00131 | .50 |
| λ3 (mm2/s) | 0.00104 | 0.00096 | .40 |
Table 3.
Univariate analysis of DTI of the optic tracts and radiations on visual acuity.
| DTI measure | Normal visual acuity (n = 24) | Abnormal visual acuity (n = 26) | P value |
|---|---|---|---|
| Optic Tracts | |||
| Fiber trajectories | 10.229 | 9.000 | .63 |
| Tract volume (mm3) | 380.125 | 393.500 | .78 |
| Fiber trajectories/mm3 | 0.369 | 0.379 | .86 |
| Fractional anisotropy | 0.432 | 0.432 | .99 |
| Radial diffusivity (mm2/s) | 0.000881 | 0.000853 | .37 |
| Mean diffusivity (mm2/s) | 0.00118 | 0.00114 | .24 |
| λ1 (mm2/s) | 0.00178 | 0.00172 | .23 |
| λ2 (mm2/s) | 0.00100 | 0.000962 | .24 |
| λ3 (mm2/s) | 0.000756 | 0.000743 | .67 |
| Optic radiations | |||
| Fiber trajectories | 311.208 | 301.942 | .87 |
| Tract volume (mm3) | 5731.583 | 5582.885 | .78 |
| Fiber trajectories/mm3 | 1.326 | 1.238 | .50 |
| Fractional anisotropy | 0.523 | 0.482 | < .01 |
| Radial diffusivity (mm2/s) | 0.000631 | 0.000691 | < .01 |
| Mean diffusivity (mm2/s) | 0.000932 | 0.000975 | .02 |
| λ1 (mm2/s) | 0.00153 | 0.00153 | .81 |
| λ2 (mm2/s) | 0.000747 | 0.000805 | .01 |
| λ3 (mm2/s) | 0.000516 | 0.000577 | < .01 |
Fig. 2.
Fractional anisotropy of the optic radiations versus visual acuity deficit.
Table 4.
Frequency of abnormal visual acuity based on optic radiation FA quartile.
| FA quartile | Normal Visual Acuity | Abnormal Visual Acuity | P value |
|---|---|---|---|
| Quartile 1 (≤0.482) | 3 (23.1%) | 10 (76.9%) | .014 |
| Quartile 2 (>0.482, ≤0.510) | 5 (41.7%) | 7 (58.3%) | |
| Quartile 3 (>0.510, ≤0.530) | 5 (41.7%) | 7 (58.3%) | |
| Quartile 4 (>0.530) | 11 (84.6%) | 2 (15.4%) |
To evaluate whether low optic radiation FA values were secondary to direct disruption by tumors involving the radiations, tumors involving the optic radiations were excluded in a subanalysis. Among subjects without optic radiation tumor involvement, the FA of the optic radiations was significantly lower in those with abnormal visual acuity than in those with normal visual acuity (0.488 vs. 0.528; P < 0.001).
A multivariate linear regression analysis evaluated the effect of covariates on the degree of visual acuity deficit. Because age and extent of OPG involvement were assumed to be associated with both DTI measurements and visual acuity, these were a priori included in the model. Table 5 lists the unadjusted and adjusted linear regression analysis of FA of the optic radiations and important covariates on visual acuity. Lower FA scores in the optic radiations and older age were associated with worse visual acuity. This association remains after excluding individuals with OPGs involving the optic radiations (adjusted coefficient for FA of the optic radiations, −6.733; P = .003) (data not shown).
Table 5.
Multivariate linear regression of FA of the optic radiation on visual acuity loss
| Variable | Unadjusted Coefficient | Adjusted Coefficient | 95% Confidence Interval | P value |
|---|---|---|---|---|
| Fractional Anisotropy | −5.586a | −6.081 | –10.322 to –1.841 | .006 |
| Age | 0.0581b | 0.0577 | 0.0143 to 0.1011 | .010 |
| Furthest Involvement | ||||
| Optic nerve | Reference | Reference | ||
| Chiasm | 0.334 | 0.227 | −0.233 to 0.686 | .326 |
| Optic tract | 0.381 | 0.165 | −0.264 to 0.594 | .442 |
| Optic radiation | 0.466 | −0.0144 | −0.806 to 0.777 | .971 |
| 30 directional DTI | 0.190 | 0.105 | −0.307 to 0.518 | .610 |
| Abnormal visual fields | 0.246 | Not significant | ||
| Abnormal optic discs | 0.594a | Not significant | ||
| Received Chemotherapy | 0.288 | Not significant | ||
a p < .01, bp < .05.
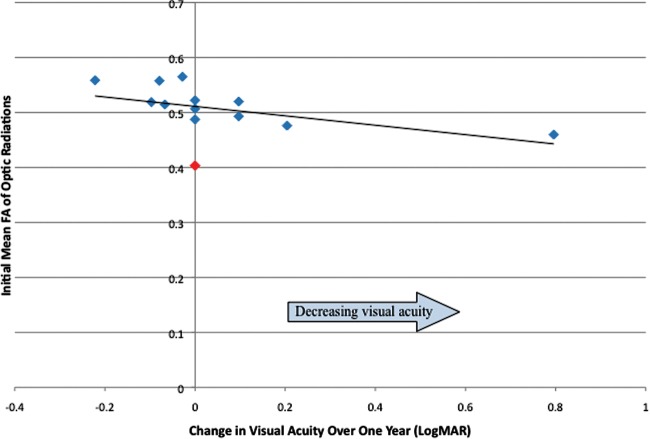
Seventeen potentially eligible subjects had 2 sets of paired ophthalmology/MRI evaluations ∼1 year apart. MRI or ophthalmologic evaluation was inadequate in 2; therefore, longitudinal analysis was performed in 15 subjects. Four of these subjects had received chemotherapy before the initial evaluation, and 2 were receiving chemotherapy during the 1-year follow-up period. There was no association between receiving chemotherapy and change in visual acuity (1-way analysis of variance, F = 0.68; P = .42). There was a trend associating initial FA of the optic radiations and visual acuity change a year later in univariate analysis (coefficient = −2.652; P = .069). Among those followed up longitudinally, at initial evaluation, no subjects had perfect visual acuity and one subject had maximal vision deficit (no light perception). Eliminating the latter subject, who could not demonstrate progressive vision loss, resulted in a significant association between initial FA of the optic radiations and visual acuity change in univariate analysis (coefficient = −5.624; P = .003) and a trend toward significance in multivariate analysis after adjusting for age, tumor location, and DTI acquisition type (adjusted coefficient = −2.525; P = .061) (data not shown). A scatterplot of initial FA of the optic radiations and visual acuity change over the following year is shown in Fig. 3.
Fig. 3.
Fractional anisotropy of the optic radiations and change in visual acuity over the following year. The data point in red represents a subject with maximal visual deficit (no light perception) on initial visual acuity testing.
Discussion
This study demonstrates that white matter tract integrity, measured by FA of the optic radiations but not of the optic nerves or tracts, is associated with visual acuity loss in NF1-associated OPGs. Previous experiments in a mouse model of NF1 have shown that gliomatous optic nerves have an increase in RD, compared with optic nerves from wild-type mice, and that the difference increases as mice and tumors age.16 Intrinsic optic nerve pathology (including OPGs) in humans has also been associated with decreased FA,17 and 9 children with NF1 (5 with OPGs) were shown to have decreased FA of the optic nerve and radiations, compared with healthy controls.18 However, neither study focused on a homogeneous population of OPGs nor compared DTI measures with functional outcomes, such as vision loss. In a small study of 10 children with OPGs (4 with NF1), Lober et al demonstrated a decrease in the number of visual fiber trajectories in all subjects and found no association with visual acuity.19 Similarly, our study found no significant difference in fiber quantitation (number of trajectories, tract volume, or trajectory density) between subjects with and without visual acuity loss. However, measurements of white matter integrity and microstructure (FA, RD, MD) were different between those with normal and abnormal visual acuity. The association between FA and visual acuity on a continuous logMAR scale remains after adjusting for age, extent of tumor involvement, DTI acquisition parameters, previous chemotherapy, and fundus examination findings. These findings suggest that the gross anatomy reflected by the number and density of fiber trajectories may be less important than measures of microstructural integrity.
Of note, white matter tract integrity in the optic radiations was associated with visual acuity loss, whereas integrity changes in the optic nerves and tracts were not observed. Small white matter structures, such as optic nerves and tracts, can be difficult to isolate on DTI without partial voluming, and these pathways may be subject to susceptibility artifacts.20,21 The lack of a significant difference in FA of these structures may be attributable to the difficulty in measuring these anterior pathways accurately. On the other hand, the association between optic radiation FA and visual acuity remains significant even in OPGs that spare the optic radiations, suggesting an alternative pathogenesis than direct disruption of the optic radiations. Changes in optic radiation white matter integrity have been reported previously in OPGs limited to the anterior pathway.18,19 Although subclinical (ie, not seen on MRI) tumor invasion is possible, anterograde trans-synaptic degeneration from disruption of visual input19,22 is a more likely mechanism.
The difference in FA of the optic radiations between subjects with normal and abnormal visual acuity (ΔFA of 0.04) represents an almost 10% divergence, with some overlap between groups that may limit the clinical use of DTI in individual cases. This variability may be attributable to MRI factors (differences in DTI parameters and effect of unidentified bright objects) or ophthalmologic factors (subject cooperation with evaluation and variable visual acuity testing method) that should be controlled for in future prospective studies. Variability of FA measurements is likely to be exacerbated by the averaging of DTI measurements across bilateral structures that may only have tumor infiltration on one side. However, it is important to consider both affected and apparently unaffected sides, because MRI appearance does not correlate with visual acuity and may not be sensitive to tumor involvement. Averaging affected and apparently unaffected sides may increase the variability of FA measurements but adds to the overall validity of our results, because including the unaffected structures should bias our results toward the null.
Our study suggests that initial FA of optic radiations may be associated with decline of visual acuity in the following year. This association becomes significant after eliminating subjects whose visual acuity is unable to improve or decline because of ceiling/floor effects. During the 1-year follow-up period, the single subject with improved visual acuity (defined as a decrease in logMAR of ≥0.2) had a baseline FA of the optic radiations in the top quartile. In contrast, the 2 subjects with a decline in visual acuity (increase in logMAR of ≥0.2) had baseline FA values in the bottom quartile. Although this small cohort is unable to support rigorous multivariate analysis, a trend toward a significant association between FA of the optic radiation and change in visual acuity is observed after controlling for important covariates. DTI has previously been shown to predict clinically relevant outcomes in multiple sclerosis, where it is associated with future persistent demyelination and disability in patients.23,24 However, larger prospective studies that can control for subject age, extent of tumor involvement, and testing characteristics are needed to determine whether measures of white matter microstructure can predict visual outcome in children with OPG.
This study has a number of limitations that may limit its generalizability. The cohort is a convenience sample collected from a large tertiary care center and may contain some selection bias toward more symptomatic patients. However, an increased prevalence of at-risk OPGs may more accurately portray the group with risk factors for progression for whom clinicians struggle to make treatment decisions. Although most patients with NF1-associated OPG had concurrent visual acuity and imaging evaluations, only 15 subjects had longitudinal concurrent evaluations during our study period. This small cohort limited the statistical power of longitudinal analyses and may have introduced selection bias in these results. Our findings need to be confirmed in a larger prospective cohort with longer follow-up, and future studies will need to control for current chemotherapy and the presence of unidentified bright objects.
Prior studies have shown that tumor location predicts visual acuity outcome after chemotherapy,6 but the usefulness of tumor location in guiding treatment decisions remains unclear. Studies using visual-evoked potentials25 and positron emission tomography26 have failed to show that these modalities can usefully predict vision loss. Avery et al.27 have shown that the thickness of the retinal nerve fiber layer measured by optical coherence tomography is associated with vision loss in OPGs, but a longitudinal study to determine optical coherence tomography's predictive power is ongoing.
Prospective studies are necessary to validate these results and to identify a threshold FA below which vision loss is highly likely, despite the variability seen in DTI measures. In addition, if larger longitudinal studies confirm that FA can quantify vision change or predict future visual deficits, DTI may provide additional valuable information to the standard ophthalmologic and radiologic evaluation of OPGs. However, longitudinal studies should include subjects receiving chemotherapy to determine whether changes in FA after chemotherapy also correlate with visual outcomes.
In summary, changes in FA of the optic radiations are associated with current visual acuity loss and may be predictive of future visual acuity loss in subjects with NF1-associated OPG. If future prospective studies corroborate our findings, DTI may be useful in identifying children with NF1-associated OPGs in need of early therapeutic intervention to preserve vision.
Funding
This work was supported by the National Institutes of Health (CA076917-15 to P.d.B.).
Conflict of interest statement. Dr. Roberts has been a speaker for Siemens and Elekta and a consultant for Prism Clinical Imaging. Dr. Berman has been a consultant for McGowan Associates. All other authors: no conflicts.
References
- 1.Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005;141(1):71–74. doi: 10.1001/archderm.141.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125:63–66. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- 3.Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114(5):788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 4.Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131:442–445. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- 5.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A(2):95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 6.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shofty B, Ben-Sira L, Freedman S, et al. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57(3):481–485. doi: 10.1002/pbc.22967. [DOI] [PubMed] [Google Scholar]
- 8.Pilling RF, Lloyd IC, Huson S. Utility of optic pathway glioma screening in young children with neurofibromatosis type I: questions generated by a clinical audit. Eye (Lond) 2010;24(10):1603–1605. doi: 10.1038/eye.2010.99. [DOI] [PubMed] [Google Scholar]
- 9.North KN, Riccardi V, Samango-Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48(4):1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 10.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AE, Elder JE, Mackey DA, Waters KD, Ashley DM. Visual improvement despite radiologically stable disease after treatment with carboplatin in children with progressive low-grade optic/thalamic gliomas. J Pediatr Hematol Oncol. 2001;23(9):572–577. doi: 10.1097/00043426-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55(6):1083–1088. doi: 10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 13.Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Hegedus B, Hughes FW, Garbow JR, et al. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. J Neuropathol Exp Neurol. 2009;68(5):542–551. doi: 10.1097/NEN.0b013e3181a3240b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickerson JP, Salmela MB, Koski CJ, Andrews T, Filippi CG. Diffusion tensor imaging of the pediatric optic nerve: intrinsic and extrinsic pathology compared to normal controls. J Magn Reson Imaging. 2010;32(1):76–81. doi: 10.1002/jmri.22228. [DOI] [PubMed] [Google Scholar]
- 18.Filippi CG, Bos A, Nickerson JP, Salmela MB, Koski CJ, Cauley KA. Magnetic resonance diffusion tensor imaging (MRDTI) of the optic nerve and optic radiations at 3T in children with neurofibromatosis type I (NF-1) Pediatr Radiol. 2012;42(2):168–174. doi: 10.1007/s00247-011-2216-y. [DOI] [PubMed] [Google Scholar]
- 19.Lober RM, Guzman R, Cheshier SH, Fredrick DR, Edwards MS, Yeom KW. Application of diffusion tensor tractography in pediatric optic pathway glioma. J Neurosurg Pediatr. 2012;0:0–0. doi: 10.3171/2012.7.PEDS1270. [DOI] [PubMed] [Google Scholar]
- 20.Hickman SJ. Optic nerve imaging in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):42S–45S. doi: 10.1111/j.1552-6569.2007.00136.x. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradov E, Degenhardt A, Smith D, et al. High-resolution anatomic, diffusion tensor, and magnetization transfer magnetic resonance imaging of the optic chiasm at 3T. J Magn Reson Imaging. 2005;22(2):302–306. doi: 10.1002/jmri.20370. [DOI] [PubMed] [Google Scholar]
- 22.Beatty RM, Sadun AA, Smith L, Vonsattel JP, Richardson EP., Jr. Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry. 1982;45(2):143–146. doi: 10.1136/jnnp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naismith RT, Xu J, Tutlam NT, et al. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology. 2010;74(21):1694–1701. doi: 10.1212/WNL.0b013e3181e042c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesaros S, Rocca MA, Pagani E, et al. Thalamic damage predicts the evolution of primary-progressive multiple sclerosis at 5 years. AJNR Am J Neuroradiol. 2011;32(6):1016–1020. doi: 10.3174/ajnr.A2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng Y, North KN. Visual-evoked potentials in the assessment of optic gliomas. Pediatr Neurol. 2001;24:44–48. doi: 10.1016/s0887-8994(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 26.Moharir M, London K, Howman-Giles R, North K. Utility of positron emission tomography for tumour surveillance in children with neurofibromatosis type 1. Eur J Nucl Med Mol Imaging. 2010;37(7):1309–1317. doi: 10.1007/s00259-010-1386-4. [DOI] [PubMed] [Google Scholar]
- 27.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–549 e542. doi: 10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]