Abstract
Antiangiogenic therapy is associated with increased radiographic responses in glioblastomas, but tumors invariably recur. Because tumor-associated macrophages have been shown to mediate escape from antiangiogenic therapy in preclinical models, we examined the role of macrophages in patients with recurrent glioblastoma. We compared autopsy brain specimens from 20 patients with recurrent glioblastoma who received antiangiogenic treatment and chemoradiation with 8 patients who received chemotherapy and/or radiotherapy without antiangiogenic therapy or no treatment. Tumor-associated macrophages were morphologically and phenotypically analyzed using flow cytometry and immunohistochemistry for CD68, CD14, CD163, and CD11b expression. Flow cytometry showed an increase in macrophages in the antiangiogenic-treated patients. Immunohistochemical analysis demonstrated an increase in CD68+ macrophages in the tumor bulk (P < .01) and infiltrative areas (P = .02) in antiangiogenic-treated patients. We also observed an increase in CD11b+ cells in the tumor bulk (P < .01) and an increase in CD163+ macrophages in infiltrative tumor (P = .02). Of note, an increased number of CD11b+ cells in bulk and infiltrative tumors (P = .05 and P = .05, respectively) correlated with poor overall survival among patients who first received antiangiogenic therapy at recurrence. In summary, recurrent glioblastomas showed an increased infiltration in myeloid populations in the tumor bulk and in the infiltrative regions after antiangiogenic therapy. Higher numbers of CD11b+ cells correlated with poor survival among these patients. These data suggest that tumor-associated macrophages may participate in escape from antiangiogenic therapy and may represent a potential biomarker of resistance and a potential therapeutic target in recurrent glioblastoma.
Keywords: antiangiogenic therapy, glioblastoma, myeloid cells, relapse
Despite treatment with surgery, radiation, and chemotherapy, median survival among patients with newly diagnosed glioblastoma (GBM) is only 14.6 months.1 Outcomes for recurrent GBM (rGBM) are even worse, with a median overall survival (OS) of <6 months with conventional salvage therapies.2 In 2009, the US Food and Drug Administration (FDA) approved bevacizumab (Avastin; Genentech/Roche), a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), as a monotherapy for rGBM based on radiographic response rates from phase II studies.3,4 Subsequent trials with bevacizumab and other antiangiogenic agents have yet to demonstrate an unequivocal improvement in OS.5,6 Moreover, GBMs invariably progress after use of antiangiogenic therapies and show a unique progression pattern characterized by increased invasion.7 We previously reported that rGBM progression after treatment with cediranib, a pan-VEGFR inhibitor, demonstrated persistent normalization of the vasculature. This change in the vascularization pattern—from highly abnormal to normalized vessels—is suggestive of increased infiltration and local vessel co-option rather than rebound angiogenesis after cessation of antiangiogenic treatment.8 However, the mechanisms for treatment evasion and tumor invasion are largely uncharacterized.8 Preclinical evidence pointed toward a key role for treatment-induced recruitment of protumor bone marrow–derived cells (BMDCs).7
Myeloid BMDCs, including tumor-associated macrophages (TAMs), have been implicated in tumor progression after antiangiogenic therapy.9 Macrophages have been implicated in tumor inflammation, tumor angiogenesis, tumor cell invasion, migration and metastasis, and treatment evasion.9,10 Specific TAMs found in the microenvironment of solid tumors are believed to promote tumorigenesis because TAMs are associated with poor prognosis in both clinical and preclinical studies of breast, bladder, and cervical carcinomas.10–12 In GBM, the role of TAMs is poorly defined. Although some studies have reported an association of TAMs with histological grade and vessel density, other clinical and preclinical studies failed to detect any correlation.13–17
More recently, attention has focused on the role of TAMs and other myeloid BMDCs as a mechanism of resistance to antiangiogenic therapy. In preclinical models of non-CNS tumors, resistance to antiangiogenic therapy is driven by infiltration of CD11b+Gr-1+ myeloid cells.18 In patients with rGBM, a decrease in circulating VEGF receptor 1 (VEGFR1)+ monocytes was associated with responsiveness, and an increase in matrix metalloproteinase 9 (MMP9) levels (usually secreted by TAMs) was associated with resistance to aflibercept, an anti-VEGF and anti–placental growth factor (PlGF) drug.19 Our autopsy study of patients with rGBM treated with cediranib reported an increase in CD68+ TAMs in the tumor bulk. Of interest, in this small cohort, the 2 patients with the highest number of TAMs had the shortest OS.8
This study sought to specifically characterize the role of TAMs in rGBM in a larger cohort of autopsy samples. Autopsy analysis is a powerful technique that allows specific, spatially defined measurements of tumor progression. To this end, we examined whether antiangiogenic therapy increases the number of TAMs and whether this increase is associated with resistance to treatment and reduced OS.
Materials and Methods
Tissue Specimens
Formalin-fixed, paraffin-embedded (FFPE) samples from 28 autopsies and 12 surgical specimens from patients with rGBM were evaluated. Twenty autopsies were from patients treated with chemoradiation and at least one anti-angiogenic agent (AAT+). These autopsy cases were compared with post-mortem tissue samples from 8 patients with rGBM who received no treatment or received chemotherapy and/or radiation without anti-angiogenic therapy (AAT–). We compared autopsy tissue samples from 4 AAT+ patients with their original tumor specimen obtained at the time of initial diagnosis. We also compared surgical tumor specimens before and after antiangiogenic therapy in a separate cohort of 4 patients with rGBM.
All tissue specimens were obtained from the Department of Pathology and/or from the neuro-oncology repository at Massachusetts General Hospital after obtaining informed consent. Tissue samples were reviewed and confirmed by a neuropathologist (M.S.). Clinical information was gathered from the patients' medical records. This research project was approved by the Institutional Review Board at Massachusetts General Hospital.
Histological Analysis
Each autopsy was reviewed by a neuropathologist (M.S.) to ensure that specific blocks selected for the study were representative of the tumor. Specific regions of interest included the (i) tumor bulk, defined as areas of tumor cells with no recognizable intervening normal tissue; (ii) infiltrative tumor, regions of tumor cells and normal tissue; and (iii) normal tissue from the contralateral brain. For surgical specimens, only tumor bulk was available for analysis.
Immunohistochemistry
Five-micron thick FFPE sections were immunostained according to the manufacturer's recommendations and standard protocols with the following antibodies: mouse anti-CD68 (prediluted, Neomarkers), rabbit anti-CD11b (1 : 200, Abcam), mouse anti-CD163 (1 : 100, Leica Microsystems), mouse anti-CD14 (1 : 100, Leica Microsystems), mouse anti-CD45 (1 : 50, DAKO), and rabbit anti-CSF1R (1 : 50, LifeSciences). Antibody binding was detected by a horseradish peroxidase/3,3′ diaminobenzidine (HRP/DAB) system.
Each autopsy and surgical specimen was independently reviewed by a neuro-oncologist (C.L.E.) and a neuropathologist (M.S.) blinded to treatment group (AAT+ or AAT–). For autopsy material, each stain was performed on at least 2 representative sections, 2 × 1.5 cm of tissue each. For surgical biopsy specimens, each stain was performed on one best section of clinical material available. For surgical biopsies, amount of tissue varied from small biopsies (0.4 × 0.3 cm of tissue) to resections (1 × 1 cm). Both observers (C.L.E. and M.S.) independently performed semiquantitative analysis of the intensity of the immunohistochemical stains, as used routinely in pathology practice, with use of light microscopy, as described previously.8 We estimated the number of positive cells with use of a scale of 0 (0% of cells stained), 1 (1%–10% of cells stained), 2 (11%–50% cells stained), 3 (51%–90% cells stained), and 4 (>90% cells stained). Both observers scored tissues independently and then compared their results. In case of disagreement, the final score was reached as a mean of scores for the area analyzed. This analysis was performed for each area of interest (tumor bulk, infiltrative area, and normal brain) for each case. Areas of necrosis were excluded from the analysis.
Flow Cytometry
Freshly dissected tumor tissue was harvested from autopsy tissue within 8 h post-mortem. Tissue was digested for 30 min at 37°C in 1.5 mg/mL collagenase (Sigma, C2674-1G), 1.5 mg/mL hyaluronase (Sigma, H3506-5G), and 2 mg/mL DNase (Sigma, D5025-150KU) in DMEM (1 g/L glucose, L-glutamine and sodium pyruvate, Cellgro, 0-014-CV) supplemented with 10% fetal bovine serum (premium select, Atlanta Biologicals, 5115508) and strained to achieve a single cell suspension. After blocking with human FCR blocking reagent (Miltenyi Biotec, 120-000-442), cells were immunostained with fluorescently labeled antibodies against CD45 (CD45-APC-H7, 641399), CD11b (CD11b-APC, 340937) and CD14 (CD14-PE-CY7, 557742; all BD Biosciences), CD163 (CD163-PE, FAB1607P, R&D systems), and CD68 (CD68-FITC, Invitrogen, GM4152). Data were acquired on an LSRII flow cytometer (Becton Dickinson) and analyzed using FACSDiva software.
Statistical Analysis
Comparison of immunohistochemical scores was performed using the exact Mann-Whitney U test. Duration of OS was calculated for those patients with rGBM who first received anti-angiogenic agents at recurrence, counting from the start of anti-angiogenic therapy. Correlation of OS with TAM scores was quantified using Spearman's correlation coefficients and tested using Monte Carlo method.20
Results
Patient Characteristics
As presented in Table 1, 20 patients with GBM who received chemoradiation and at least 1 anti-angiogenic agent (AAT+) and 8 patients with GBM who received no treatment or chemotherapy and/or radiation without an anti-angiogenic agent (AAT–) underwent post-mortem examination. Median age of the AAT+ group was 54.5 years (range, 27–76 years), with a median Karnofsky performance status (KPS) of 90. In the AAT– group, the median age was 68.5 years (range, 16–82 years). Detailed clinical information was only available for 3 of the 8 patients, with median KPS being 70. Four patients (20%) in the AAT+ group and 2 (25%) in the AAT- group had gross total resections, with other patients undergoing subtotal resections or biopsy.
Table 1.
Patient characteristics
| Patient characteristics | No antiangiogenic therapy (AAT–); n = 8 | Antiangiogenic therapy (AAT+); n = 20 |
|---|---|---|
| AGE (years) | ||
| Median (range) | 68.5 (16–82) | 54 (27–76) |
| Sex | ||
| Male | 3 | 13 |
| Female | 5 | 7 |
| MEDIAN KPS | 70 (n = 3a) | 90 |
| INITIAL SURGERY | (n = 6a) | 4 |
| Gross total resection | 2 | 11 |
| Partial resection | 2 | 5 |
| Biopsy | 1 | 0 |
| Diagnosis at autopsy | 1 | |
| PRIOR DIAGNOSIS OF GLIOMA | (n = 5a) | |
| Glioblastoma | 5 | 17 |
| Other glioma | 0 | 3 |
| TREATMENT | None (n = 3) | XRT/Chemotherapy plusb: |
| XRT (n = 3) | Bevacizumab (n = 17) | |
| XRT/Chemotherapy (n = 2) | Cediranib (n = 7) | |
| Cabozantinib (n = 4) | ||
| Thalidomide (n = 1) | ||
| Vatalanib (n = 1) | ||
aLimited clinical information from some patients.
bSome AAT+ patients received multiple antiangiogenic agents.
Abbreviations: KPS, Karnofsky performance score; XRT, radiation therapy.
Chemoradiation consisted of the standard fractionated radiation therapy with concurrent temozolomide at 75 mg/m2.
All patients in the AAT+ group received standard fractionated radiation with concurrent temozolomide, followed by adjuvant temozolomide. In addition, 7 patients (35%) received an antiangiogenic agent in conjunction with chemoradiation and/or with postradiation temolozomide. Mean duration of antiangiogenic treatment in the AAT+ group was 8.2 months. Thirteen (65%) of 20 patients received an antiangiogenic agent only at recurrence, with a median length of treatment of 4.8 months. Eleven (55%) of 20 patients received >1 antiangiogenic agent during their course of treatment, with 4 of these patients receiving their treatment only at recurrence. Sixteen (80%) of 20 patients were still receiving an antiangiogenic agent within 6 weeks before their death. In the AAT– group, 3 patients (38%) did not receive any treatment. Three patients (38%) only received radiation, and 2 patients (24%) received chemotherapy and radiation.
In the AAT+ group, steroid data was available for 20 of 20 patients. Six patients (30%) were not receiving steroids at the time of death, whereas the other 14 patients (70%) were receiving steroids at the time of their last office visit, which was within 1 month of time of death. Median dose of dexamethasone was 6 mg daily. In the AAT- group, steroid data was available for 7 of 8 patients. All patients were receiving steroids at the time of their last note, which ranged from 2 years to a few days from the time of death. Median dose of dexamethasone was 12 mg daily.
Antiangiogenic Therapy Increases TAMs in Autopsy Specimens
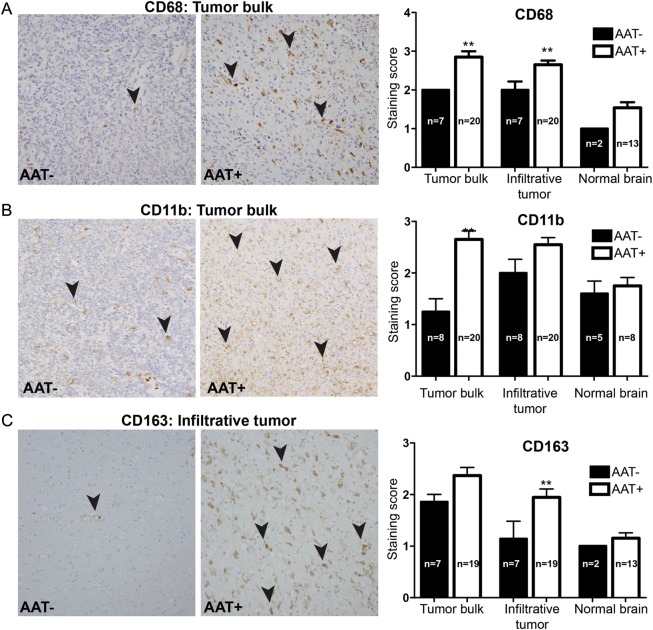
TAMs are usually characterized as CD11b+CD68+ or CD11b+CD14+ cells, and CD163 is often considered as an M2-like TAM marker.21 Before analysis, we confirmed that cells positive for CD68, CD11b, CD163, and CD14 immunostaining showed characteristic morphological features of TAMs. CD68+ TAMs were subsequently included in the immunohistochemical analysis, because they provided the best quality and specificity of the staining. Comparison of autopsy specimens from AAT+ patients and AAT– patients revealed an increase in TAMs in the tumor bulk and infiltrative areas of patients exposed to antiangiogenic therapy (Fig. 1). There was a significant increase in CD68+ cells in the tumor bulk (P < .01) and infiltrative regions (P = .02) in the AAT+ patients. There were also increased numbers of CD11b+ cells in the tumor bulk (P < .01) and a trend toward increase in infiltrative regions (P = .09) in AAT+ tumors. Finally, there was an increase in CD163+ TAMs in infiltrative tumor (P = .02) and a similar trend for CD163+ cells in the tumor bulk (P = .09) in the AAT+ patients.
Fig. 1.
TAM measurement by immunohistochemistry. TAMs were morphologically identified and then semiquantitatively scored on the basis of the following markers: CD68, CD11b, and CD163. The staining score was defined as follows: 0 (0% of cells stained), 1 (1%–10% of cells stained), 2 (11%–50% cells stained), 3 (51%–90% cells stained), and 4 (>90% cells stained). Microscopically, an increase in the number of TAMs was seen in AAT+ patients. (A) An increase in CD68+ TAMs (black arrowheads) was seen in tumor bulk (P < .01) and infiltrative tumor (P < .05) in AAT+ patients than in AAT– patients. (B) An increase in CD11b+ TAMs (black arrowheads) was seen in tumor bulk (P < .01) of AAT+ patients than in that of AAT– patients. (C) An increase in CD163+ TAMs (black arrowheads) was seen in infiltrating tumor (P < .05) in AAT+ patients.
We did not find any significant difference in CD68+ TAMs, CD163+ M2-like TAMs, and CD11b+ myeloid cells between patients who received only 1 antiangiogenic agent and those who received multiple antiangiogenic agents. Of intrigue, the TAM levels measured in AAT- patients were comparable among patients regardless of whether they received no treatment, chemoradiation, or radiotherapy alone, further supporting the notion that increases in TAMs is not a feature of natural history in GBM, nor a result of radiation or standard chemotherapy.
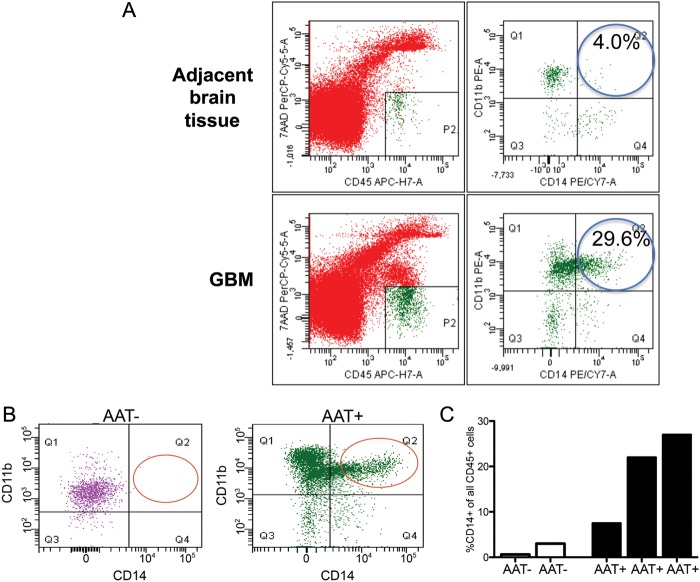
To further examine the specificity of the IHC staining for TAMs in GBM, autopsy samples were examined by flow cytometry. When tissue was available within 6 h post-mortem, we verified the presence of TAMs in dissociated tumor tissues with use of a series of markers, including CD45, CD11b, CD68, CD163, and CD14. We detected a distinct CD45+CD11b + CD68+ population of TAMs in the tumor (Fig. 2A). In the TAM population, the macrophage marker CD14 was the most specific across flow cytometry measurements (Fig. 2B). Thus, we used CD14 to compare TAMs between AAT– and AAT+ samples. We detected a distinct CD45+/CD11b+/CD14+ population of TAMs in the tumor (Fig. 2A). We found that tumor tissue from AAT+ patients contained a notable increase in TAMs, compared with tissue from AAT- patients (Fig. 2B and C), consistent with the results of our immunohistochemical analysis.
Fig. 2.
Flow cytometry analysis of TAMs. (A) CD11b+ cells were measured in the tumor bulk but not found in the adjacent brain tissue. (B) The macrophage marker CD14+ showed a strong staining for macrophages from autopsy samples. (C and D) CD14+ TAMs were distinctly increased in autopsies from antiangiogenic treated (AAT+) patients, compared with patients who did not receive antiangiogenic therapy (AAT–). All data are shown as a fraction of hematopoietic cells (after gating on the CD45-positive cell population).
Antiangiogenic Therapy Leads to Increase in TAMs when Compared with Initial Diagnostic Tissue
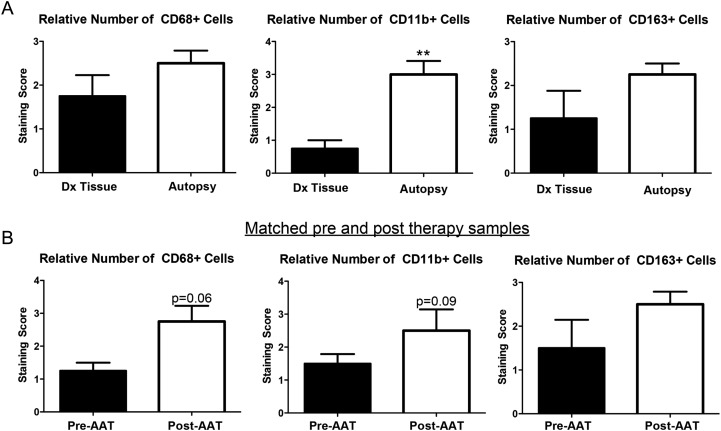
To confirm that the increase in TAMs at autopsy was specifically attributable to antiangiogenic treatment, we analyzed initial diagnostic tissue samples from patients who underwent autopsy. Of the 20 patients with analyzed autopsies, 4 patients had their initial diagnostic surgical specimens available. When comparing autopsy specimens with these prior surgical specimens, each of these patients demonstrated an increase in the number of CD11b+ myeloid cells, CD68+ total TAMs, and CD163+ M2-like TAMs (Fig. 3A). Of note, this increase was not seen in the one AAT– patient who had an initial diagnostic surgical specimen available for comparison with autopsy tissue.
Fig. 3.
TAM analysis in matched samples. (A) When available, the initial diagnostic tissue of AAT+ patients was examined and compared with their autopsy tissue. A relative increase in CD68+, CD11b+, and CD163+ TAMs were seen in autopsy, as compared with their initial diagnostic tissue, with significance reached for CD11b+ cells (P < .01). (B) A separate cohort of patients treated with antiangiogenic therapy was examined. Pretreatment tissue was compared with posttreatment tissue. Although statistical significance was not reached, likely because of the limited number of samples (n = 4), there was a trend for an increase in CD68+ TAMs (P = .06) and CD11b+ cells (P = .09).
To analyze the temporal correlation between the increase in number of TAMs and exposure to antiangiogenic agents in patients with rGBM, we evaluated tissue samples from a separate cohort of patients who underwent a second resection after treatment with antiangiogenic agents. These patients underwent a second surgical procedure because of concern for radiographic progression during antiangiogenic therapy. Using immunohistochemistry, we detected a trend for increase in CD68+ (P = .06) and CD11b+ (P = .09) cells after antiangiogenic treatment in the tumor bulk (Fig. 3B). These data are supportive of the hypothesis that the influx of TAMs may be selectively associated with antiangiogenic therapy.
Increase in Number of TAMs at Autopsy Correlates with Decreased OS among Patients with rGBM
Because TAMs have been associated with poor prognosis in other solid tumors, we explored the relationship between the number of TAMs at autopsy and OS among 13 patients with GBM who first received an antiangiogenic agent at recurrence (Table 2). We detected a negative correlation between OS and the number of CD11b+ cells in tumor bulk and infiltrative areas and OS (both P = .05). The number of CD68+ TAMs in tumor bulk and infiltrative tumors showed a similar trend with the combined number of CD68+ TAMs in tumor bulk and infiltrative tumors correlating with decreased OS (P = .03).
Table 2.
The amount of tumor-associated macrophages (TAMs) inversely correlates with overall survival in patients with recurrent glioblastoma (GBM).
| TAM Type/Location | Rho | P value |
|---|---|---|
| CD68+ bulk | −0.6382 | .040 |
| CD68+ infiltrative | −0.5379 | .094 |
| CD68+ normal | 0.1309 | NS |
| CD11b+ bulk | −0.5765 | .066 |
| CD11b+ infiltrative | −0.5606 | .076 |
| CD11b+ normal | N/A | N/A |
| CD163+ bulk | 0.1418 | NS |
| CD163+ infiltrative | 0.4119 | NS |
| CD163+ normal | 0.2474 | NS |
TAM scores were correlated with overall survival in patients with recurrent GBM who first received antiangiogenic treatment at recurrence. Patients with decreased survival had an increased number of CD68+ TAMs (P = .05) and CD11b+ cells (P = .04) in infiltrating tumor. Decreased survival was also seen in patients with increased number of CD11b+ TAMs in tumor bulk (P = .04).
Discussion
The benefits derived from antiangiogenic therapy are transient in many solid tumors, including in GBM.7,22–25 One of the mechanisms of resistance suggested by preclinical studies is the recruitment of TAMs that promote tumor progression and spread despite vascular targeting. We identified populations of TAMs with use of a variety of macrophage markers in the tumor microenvironment. By comparing autopsies of patients with rGBM treated with antiangiogenic agents with a control group, we showed that antiangiogenic therapy is associated with significant increases in the number of CD68+ TAMs and CD11b+ myeloid cells in tumor bulk and CD68+ TAMs and CD163+ M2-like TAMs in the infiltrative edges of the tumor. This increase in TAMs is induced by antiangiogenic therapy, because the AAT– autopsy cohort exhibited relatively few TAMs, despite being treated with radiotherapy and/or chemotherapy. Moreover, comparison of the AAT+ autopsy tissue with their initial diagnostic tissue and evaluation of surgical tissue before and after antiangiogenic treatment revealed an increase in TAMs. This increase in TAMs is associated with inferior survival. Collectively, these results suggest that posttreatment recruitment of tumor-promoting TAMs may be an evasion mechanism in patients with rGBM after antiangiogenic therapy.
Recently, there has been increased focus on the role of TAMs in solid tumor progression, invasion, metastasis, and growth because of their ability to fulfill diverse roles. Traditionally, TAMs have been subdivided into 2 groups: M1, which exhibits a proinflammatory response, and M2, which exhibits an immunosuppressive phenotype.26,27 Recently, it has been shown that, although TAMs harbor immunoregulatory properties, they are more complex than what was proposed by the binary classification. Instead, there appears to be several populations of TAMs with overlapping features of M1, M2, and developmental macrophages9. These subpopulations mediate a spectrum of diverse functions, such as inflammation, immune regulation, angiogenesis, metastasis, intravasation, and invasion.9
In our examination of AAT+ autopsy specimens and post–antiangiogenic-treated surgical specimens, we did not observe an association of TAM infiltration with a rebound vascularization, consistent with di Tomaso et al.8 This suggests that TAMs may be involved in additional mechanisms other than upregulation of pro-angiogenic pathways when mediating escape from antiangiogenic therapy. Such alternative mechanisms may include tumor cell migration, invasion, and immunosuppression. Intravital imaging of preclinical models has documented tumor cells exiting their primary site when juxtaposed to TAMs and intravasation of tumor cells into blood vessels at sites of macrophage attachment to the abluminal sides of vessels.28 In murine mammary cancer, systemic depletion of macrophages resulted in reduced lung metastasis.12 Depletion of macrophages in the peritoneal cavity or liver resulted in reduced metastatic lesions when CC531 colon cancer cells were reintroduced at those sites, suggesting that macrophages facilitate tumor growth.29 In GBM, preclinical and clinical studies have documented a change in growth phenotype after exposure to antiangiogenic therapy,8,19,24,30–32 Although our study did not specifically examine invasion, we postulate that TAMs may be affecting the growth phenotype, because we observed significant increases in CD68+CD11b+ total TAMs in the bulk and CD68+CD163+ M2-like TAMs in the infiltrative portions of the tumor. These may represent 2 distinct populations of TAMs, with the CD68+CD163+ cells primarily mediating an increase in invasiveness of the GBM cells. Future molecular phenotyping of TAMs via fluorescence-activated cell sorting (FACS) will be necessary to dissect the function of TAMs in glioblastoma. For instance, expression of transforming growth factor beta, epidermal growth factor, macrophage colony–stimulating factor 1, C-C chemokine receptor type 2, and C-X-C chemokine receptor type 4 are associated with prometastatic effects of TAMs.9,33,34 As we develop new analytical methods for autopsy specimens from an immunohistochemistry approach, it will be equally as critical to develop methodologies in FACS to better characterize the immune components of GBM.
The prognostic implication of TAM recruitment to GBMs has been unclear. Yao et al. studied 50 glioma specimens, including low-grade gliomas and GBMs, and reported that those tumors positive for thymidine phosphorylase, attributed to macrophage origin, had a significantly decreased median survival.35 More recently, gene expression profiling found that patients with GBM with the shortest OS had significant enrichment of microglia and macrophage-selective genes.36 However, other studies of TAM density in gliomas reported no significant association with patient prognosis.15,17 Moreover, these previous studies did not examine TAMs in the context of antiangiogenic treatment. We report that the increased number of CD11b+ myeloid cells in tumor bulk and infiltrative areas and number of CD68+ TAMs in the infiltrative portion significantly correlated with decreased OS among patients who received an antiangiogenic agent at recurrence. Thus, TAM density after antiangiogenic treatment may be predictive of tumor progression and reduced survival.
Our study also shows that autopsy is a powerful tool to investigate the biological effects of targeted therapies on tumor biology. One of the strengths is the direct evidence at patient death from which we can glean potential causes of death by the tumor. Of more importance, it offers the opportunity to examine the biological effects of various treatments on the tumor and its microenvironment. Furthermore, autopsy analysis reveals the spatial heterogeneity of the tumor and its microenvironment. To gauge the impact of treatment, we usually rely on clinical parameters and radiographic appearance. Unfortunately, these represent gross approximations from which we infer the underlying tumor biology. Examination of post-mortem specimens offers histological confirmation of the effects of treatments on the tumor and serves as a better guide to understanding patterns of tumor resistance and progression.
Some limitations to this retrospective autopsy series include the difficulty to recruit a well-matched control group. With the introduction of bevacizumab for rGBM into standard oncology practice, it is becoming increasingly difficult to obtain autopsy specimens from antiangiogenic therapy–naive patients. Our study included historical controls from the pretemozolomide era; thus, clinical data, including steroid use at the time of death, was limited. In more recent times, patients who forego antiangiogenic agents are inherently older or more frail than the typical patient who is offered these treatments, thus raising the possibility of selection bias. In this study, we attempted to address this by also looking at pre- and posttreatment surgical specimens unaffected by antiangiogenic therapy to determine whether TAM recruitment is an effect of other treatments, including steroids.
In conclusion, on the basis of our analyses of autopsy and biopsy GBM tissues, we propose that TAMs play a direct role in tumor progression after antiangiogenic therapy. Moreover, our data indicate that the specific distribution of various TAM populations appears to affect OS among patients with rGBM who first receive antiangiogenic treatment after recurrence. This highlights TAMs as a potential biomarker and/or therapeutic target to improve outcomes in patients treated with antiangiogenic agents.
Funding
This work was funded by a the National Foundation for Cancer Research grant (to R.K.J.), the National Institutes of Health (grants R01CA129371, U01CA062490, K24CA125440A to T.T.B.; P01CA080124, R01CA163815 to R.K.J.; R01CA159258 to D.G.D.); Proton Beam/Federal Share Program (to R.K.J. and D.G.D.), and the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers).
Conflict of interest statement. R.K.J. has received research grants from Dyax, MedImmune, and Roche; has received consultant fees from Dyax, Enlight, Noxxon, and SynDevRx; owns equity in Enlight, SynDevRx, and XTuit; serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. No reagents or funds from these organizations were used in this study. All other authors declare no conflicts.
Acknowledgments
We thank Carolyn Smith for outstanding technical support for histology studies. This work was presented at the Annual 2012 ASCO meeting and 2012 SNO meeting.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 4.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinot O. Phase III trial of bevacizumab added to standard radiotherapy and temozolomide for newly diagnosed glioblastoma: mature progression free survival and preliminary overall survival results.. AVAglio, Society for Neuro-Oncology 17th Annual Scientific Meeting and Education Day; Washington DC.. Neuro-Oncology; 2012. [Google Scholar]
- 7.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 12.Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 14.Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 15.Rossi ML, Jones NR, Candy E, et al. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989;78:189–193. doi: 10.1007/BF00688208. [DOI] [PubMed] [Google Scholar]
- 16.Galarneau H, Villeneuve J, Gowing G, et al. Increased Glioma Growth in Mice Depleted of Macrophages. Cancer Res. 2007;67:8874–8881. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Saio M, Kito Y, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 18.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 19.de Groot JF, Piao Y, Tran H, et al. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clin Cancer Res. 2011;17:4872–4881. doi: 10.1158/1078-0432.CCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hothorn T, Hornik K, van de Wiel MA, et al. A Lego System for Conditional Inference. The American Statistician. 2006;60:257–263. [Google Scholar]
- 21.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 25.Shirai K, Siedow MR, Chakravarti A. Antiangiogenic therapy for patients with recurrent and newly diagnosed malignant gliomas. J Oncol. 2012;2012:193436. doi: 10.1155/2012/193436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 29.Oosterling SJ, van der Bij GJ, Meijer GA, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147–155. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 30.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18:2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 33.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiratsuka S, Duda DG, Huang Y, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proceedings of the National Academy of Sciences. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y, Kubota T, Sato K, et al. Macrophage Infiltration-associated Thymidine Phosphorylase Expression Correlates with Increased Microvessel Density and Poor Prognosis in Astrocytic Tumors. Clinical Cancer Research. 2001;7:4021–4026. [PubMed] [Google Scholar]
- 36.Engler JR, Robinson AE, Smirnov I, et al. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS One. 2012;7:e43339. doi: 10.1371/journal.pone.0043339. [DOI] [PMC free article] [PubMed] [Google Scholar]