Abstract
Since crystallins in the human lens do not turnover, they are susceptible to modification by reactive molecules over time. Methylation is a major post-translational lens modification, however the source of the methyl group is not known and the extent of modification across all crystallins has yet to be determined. Sites of methylation in human lens proteins were determined using HPLC/mass spectrometry following digestion with trypsin. The overall extent of protein methylation increased with age, and there was little difference in the extent of modification between soluble and insoluble crystallins. Several different cysteine and histidine residues in crystallins from adult lenses were found to be methylated with one cysteine (Cys 110 in γD crystallin) at a level approaching 70%, however, methylation of crystallins was not detected in fetal or newborn lenses. S-adenosylmethionine (SAM) was quantified at significant (10–50 μM) levels in lenses, and in model experiments SAM reacted readily with N-α-tBoc-cysteine and N-α-tBoc-histidine, as well as βA3-crystallin. The pattern of lens protein methylation seen in the human lens was consistent with non-enzymatic alkylation. The in vitro data shows that SAM can act directly to methylate lens proteins and SAM was present in significant concentrations in human lens. Thus, non-enzymatic methylation of crystallins by SAM offers a possible explanation for this major human lens modification.
Keywords: methylation, post-translational modification, age, lens protein
1. Introduction
Lens proteins do not turnover, and the center of the human lens, formed prenatally, can undergo post-translation modifications that reflect an individual’s lifetime exposure to reactive molecules. The central lens region, known as the nucleus, is devoid of organelles and for the enzymes that have been tested, contains little, or no, residual enzyme activity in adults (Dovrat et al., 1984; Truscott and Zhu, 2010; Zhu et al., 2010). Thus, even rare modifications can accumulate over decades if the protein adducts formed are stable under physiological conditions.
S-methylation, an unusual post-translational modification (PTM), was first reported in human lens nearly a decade ago, (Lapko et al., 2002, 2003, 2005; Searle et al., 2005) but no plausible mechanism was proposed. More recent work (Wilmarth et al., 2006) on modifications of aged human lens crystallins indicated that methylation was found at other sites and was an abundant lens PTM; again no mechanism was suggested. Given the high abundance of crystallins in lens nuclei, decades of exposure, and sensitivity of modern mass spectrometry PTM detection, testing reasonable mechanisms for lens modifications, such as methylation, can still be extremely challenging.
In this study, we wanted to characterize the sites and extent of methylation in human lenses and to search for plausible mechanisms that could explain this major lens PTM. We examined long-lived proteins in human lenses for evidence of methylation and most of the major crystallins in older lenses were found to contain methylated cysteine and/or histidine residues. We found that methylation was largely absent in young lenses, and that the extent of methylation increased with age. One major methyl donor, S-adenosylmethionine (SAM), has been shown to non-enzymatically methylate DNA (Barrows and Magee, 1982) and we detected significant levels of SAM in aged human lenses. In vitro studies found that SAM alone readily methylated Cys and His derivatives, and could, therefore, offer a potential mechanism for the methylation observed in human lens.
2. Methods and materials
2.1. Lenses
Australian lenses were donated to the Lions Eye Bank, Sydney Eye Hospital. Enucleation occurred within 12 h of death and lenses were stored at −80 °C until use. Tissue was handled in accordance with the tenets of the declaration of Helsinki and ethical approval was obtained from Sydney and Wollongong Universities. Lenses were dissected into cortex and nucleus using a trephine with diameter of 4.5 mm. A cold scalpel was used to remove ~1 mm from each end from the nuclear core. Whole fetal lenses were used without dissection into regions. Australian lenses were used for the determination of SAM levels, measurement of methyltransferase activity (Table 1) and analysis of methylation of gamma S crystallin (Figs. 1 and 2a). Cataract lenses were obtained from India. American lenses from the Lions Eye Bank of Oregon were used to determine the extent of methylation of proteins as a function of age (Figs. 1 and 2b).
Table 1.
Methyltransferase activity in human lens regions as a function of age. Enzyme activity was measured in the outer cortex (newly synthesized) region and the centre (nucleus) of human lenses. (ND = not detected).
| Age | Outer cortex (nmol/min/mg) | Nucleus (nmol/min/mg) |
|---|---|---|
| 16 | 11.3 | 3.4 |
| 19 | 7.3 | 5.7 |
| 30 | 3.9 | 1.4 |
| 32 | 4.4 | 3.1 |
| 54 | 1.8 | ND |
| 61 | 1.2 | ND |
| 74 | 10.5 | ND |
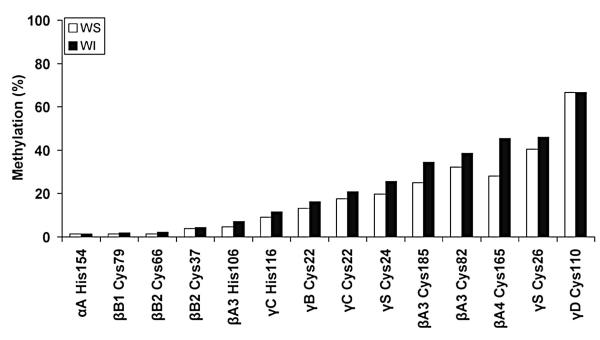
Fig. 1.
Percent methylation at different sites within human crystallins. Whole human lenses were extracted and digested with trypsin prior to separation of the peptides by LC/MS. WS, water-soluble proteins (◻); WI, water-insoluble proteins (∎).
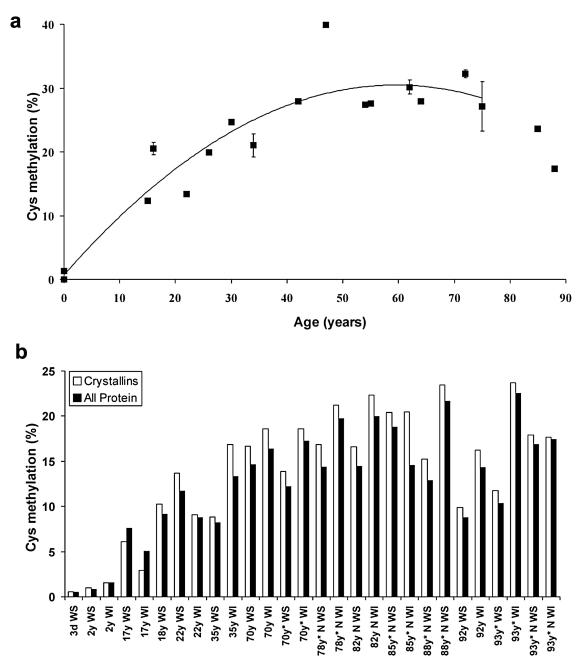
Fig. 2.
Percent methylation of lens proteins as a function of age. a) Methylation of γS crystallin from the lens nucleus. The extent of methylation of one tryptic peptide (21–37) as a function of age. This peptide contains 3 Cys residues and the proportion of the dimethylated form, as a proportion of the total amount of all forms of that peptide, based on ion counts, is shown. The increase with age is statistically significant (y = 0.242384 × + 11.324641, r2 = 0.428779, Two-sided P = 0.0032). b) Soluble and insoluble proteins from whole lens and lens nuclei of different ages were compared for the degree of Cys methylation. Whole cataract lenses, and cataract lens nuclei, (indicated by the * symbol), are shown for comparison. WS, water-soluble protein; WI, water-insoluble protein; N, nucleus. The increase in methylation of normal lenses with age was significant. (WS y = 0.104044 × + 4.511831, r2 = 0.507326, two-sided P = 0.0313, WI y = 0.179908 × + 2.725751, r2 = 0.821256 two-sided P = 0.0019).
2.2. Reagents
All organic solvents and acids were HPLC grade (Ajax, NSW, Australia) and Milli-Q® water was used in preparation of all solutions. N-α-t-Boc-l-cystine (Boc-Cys), N-α-t-Bocl-histidine (Boc-His), formic acid, trifluoroacetic acid, sodium hydride (60%), methyliodide (≥99%), S- (5′-adenosyl)-l-methionine chloride (SAM·HCl) were from Sigma–Aldrich. 3-Methyl-N-α-t-Boc-l-histidine (3-Me-Boc-His) was purchased from Chem-Impex International, USA.
2.3. Synthesis of N-α-(tert-butoxycarbonyl)-1-methyl-l-histidine (1-me-Boc-His)
1-Me-Boc-His was synthesized using the method of Kaur et al. (Kaur et al., 2004).
2.4. Incubation of Boc-Cys and Boc-His with SAM
Boc-Cys (0.075 mmol) was dissolved in oxygen-free 0.1 M MOPS buffer (pH 7, 0.5 ml) and SAM·HCl (0.04 mmol) was added and the pH adjusted to 7.0 using 0.1 M NaOH. The resulting suspension was placed at 37 °C in the dark. Boc-Cys (0.075 mmol) and SAM (0.03 mmol) were incubated separately as controls. Chloroform (2–3 drops) was added to all solutions as an anti-bacterial agent. Aliquots (20 μl) were taken at time intervals, diluted in 2% CH3CN/0.1% formic acid (v/v) and examined by LC–MS/MS.
Similarly, Boc-His (0.07 mmol) was incubated with SAM·HCl (0.04 mmol) under the above conditions with Boc-His (0.07 mmol) and SAM (0.03 mmol) incubated separately as controls.
2.5. Analysis of Cys and His methylation by HPLC
Incubations of SAM with Boc-His and Boc-Cys were analysed with a Shimadzu HPLC equipped with LC-10 pumps, a SIL-10 autoinjector and SPD-M10 diode array detector using an Alltech (C18, 5 μm, 4.6 × 250 mm) column fitted with a Phenomenex (4 μm, 2 × 4 mm) guard column. Buffer A (H2O/0.05% TFA, v/v) and buffer B (80% CH3CN/0.05% TFA, v/v) were used at a flow rate of 1 ml min−1 and the eluent monitored at 210 nm. The mobile phase gradient was as follows: 0–5 min (5% buffer B), 5–30 min (5–80% buffer B), 30–35 min (80% buffer B). S-Me-Boc-Cys eluted at 25.8 min, 3-Me-Boc-His at 15.0 min and 1-Me-Boc-His at 16.1 min.
2.6. Liquid chromatography–mass spectrometry (LC–MS)
LC–MS was used to quantify SAM in lens extracts and LC–MS/MS to confirm the identification of compounds in the amino acid + SAM incubations. Samples were run on a Shimadzu LC–MS-2010EV electrospray ionisation mass spectrometer (ES-MS). A Phenomenex column (C18, 4 μm, 2 × 150 mm) was used at 30 °C. For Boc-Cys and Boc-His incubations, the mobile phase system consisted of buffer A, H2O/0.1% formic acid (v/v) and buffer B, CH3CN/0.1% formic acid (v/v). The mobile phase gradient was as follows: 0–10 min (0% buffer B), 10–25 min (0–80% buffer B), 25–30 min (80% buffer B). Analysis of SAM was performed using buffer A, 10 mM heptafluorobutyric (HFBA) acid-NH4OH (pH 3.2) and buffer B, aqueous 90% MeOH/10 mM HFBA acid-NH4OH (pH 3.2) and the following mobile phase gradient: 0–5 min (0% buffer B), 5–35 min (0–80% buffer B), 35–40 min (80% buffer B). The flow rate was 0.2 ml min−1 and the source temperature was maintained at 200 °C. All spectra were acquired in positive ion mode and monitored at 210 nm (Boc-Cys and Boc-His) and 254 nm (SAM).
LC–MS/MS was conducted using electrospray ionisation MS (ESI-MS) in positive ion mode using a Finnigan Surveyor Thermo LCQ DECA instrument. For tandem MS (ES-MS/MS) analyses, ions were subjected to collision energies between 10 and 25 eV.
2.7. Methyltransferase assays
Fresh human lenses (within 12 h of death) were dissected into cortex and nucleus and ~20–30 mg tissue homogenised in 10 mM phosphate buffer pH 7.0 (500 μl). Samples were centrifuged (10 000 g, 4 °C) for 10 min and the supernatants filtered through a 10 kDa filter. The filter was washed with phosphate buffer (100 μl) to remove residual S-adenosylhomocysteine. Proteins were suspended in phosphate buffer (100 μl) and 10 μl was assayed for methyltransferease activity using a commercial assay kit (Cayman 700140).
2.8. Extraction of SAM from human lenses
Normal human lenses were separated into cortex and nucleus as described and the ends of each nuclear core were added to the cortex. SAM was extracted as described (Kim and Kim, 2005). Nuclear and cortical regions were homogenised in 150 μl and 300 μl water, respectively, and proteins precipitated with ethanol (final 70%). Solutions were incubated at −20 °C for 15 min and centrifuged (13 000 g, 20 min, 4 °C). The supernatant was removed and stored at −20 °C while the pellets were re-extracted twice more with 70% (v/v) ethanol. The supernatants were combined and lyophilised. The residue was redissolved in ammonium acetate (150 μl, 25 mM) and applied to a C18 Sep-Pak cartridge (Waters). The cartridge was rinsed with water (1 ml) and then SAM eluted with methanol-water (15%: 85%, 1 ml) containing formic acid (0.1%). Quantitation was by reference to a HPLC standard curve with detection at 254 nm. Identification of HPLC peaks was confirmed by LC–MS/MS.
To determine the extraction efficiency, SAM (0.5 mg) was homogenised with bovine lens proteins (68 mg) in water (300 μl) and treated as described above. Values were corrected for the recovery of SAM (57.5%).
2.9. Methylation of crystallins in aged human lenses
The relative percent of methylation at specific Cys and His residues in crystallins of whole aged human lenses and the overall extent of Cys methylation as a function of donor age were determined by digestion of lens samples with trypsin and two-dimensional liquid chromatography tandem mass spectrometric (2D-LC–MS/MS) analysis. The relative percent of Cys and His methylation in the water-soluble and water-insoluble crystallins of 3 (70-year normal lens, 70-year mild cataract, and 93-year old moderate cataract whole human lenses (Fig. 1) was determined by the ratios of assigned MS/MS spectra to the methylated and unmethylated forms of each Cys containing peptide; details are given in Wilmarth et al. (Wilmarth et al., 2006)). The analysis of overall Cys methylation in whole lens and lens nuclear fractions as a function of age (Fig. 2b) used lens samples previously described (Dasari et al., 2009), except lens nuclei were dissected using a trephine and scalpel as described above. The relative extent of protein methylation was estimated using spectral counting of SEQUEST (version 28, revision 12, Thermo Scientific, San Jose, CA USA) results. To detect sites of Cys methylation, the program was configured to search for Cys with a static modification of +57 and a differential modification of −43. The −43 value being the difference between the +14 mass increase caused by Cys methylation and the +57 mass increase caused by cysteine carboxyamidomethylation, the latter only occurring at unmethylated Cys residues during alkylation with iodoacetamide during sample preparation. A differential search of +16 was also performed on methionine residues to detect oxidation. The searches were performed against the human Sprot database (version 57.15) with concatenated reversed sequences. Monoisotopic parent ion mass tolerance was 2.5 Da and monoisotopic fragment ion tolerance was 1.0 Da. Search results processing was performed using in-house software (Wilmarth et al., 2009), and the relative percent of Cys methylation was estimated from the ratios of assigned modified MS/MS spectra to the total of modified and unmodified MS/MS spectra of Cys containing peptides within each lens sample as previously described (Wilmarth et al., 2006). Comparing apparent modification rates, a within sample normalization, avoided the need for normalizations between samples.
The extent of methylation of peptide 21–37 of γS-crystallin in the lens nucleus as a function of donor age (Fig. 2a) was determined by analysis of ion currents for the respective modified and unmodified forms of the peptide in MS scans as previously described (Hains and Truscott, 2008), except that analysis was by LC–MS/MS using an Ultima API hybrid Q-TOF tandem mass spectrometer (Micromass, Manchester, UK).
Statistical analyses were performed using statsDirect software Version 2.7.7 (StatsDirect Software Inc., San Diego, CA, USA). Simple linear regression analysis was performed on normal lens samples to analyse Cys methylation as a function of age for soluble and insoluble protein. A two-sided P value of less than 0.05 was considered statistically significant.
2.10. Modification of human βA3-crystallin by SAM
Recombinant human βA3-crystallin was expressed and purified without a tag as previously described (Takata et al., 2007). Incubation with SAM (Sigma A7007) was performed at 37 °C for 5 days under argon in a 13 μl mixture containing 200 pmol human βA3-crystallin, 75 mM MOPS (pH 7), 3.8 mM SAM, 1.5 mM Tris[2-carboxyethyl] phosphine (TCEP), and 0.075% sodium azide. Control samples were simultaneously incubated, but without SAM. After incubation, samples were trypsinized following reduction and alkylation with dithiothreitol and iodoacetamide, respectively. Digests (75 pmol) were then analyzed by LC–MS/MS using an LTQ linear ion trap (ThermoScientific) as previously described (Bassnett et al., 2009). The MS/MS spectra generated from peptides containing methylated and unmethylated Cys residues were identified as described above, except lists of peptides and proteins were assembled using the program Scaffold (version 02_04_00, Proteome Software, Portland, OR), and peptide and protein probabilities were set at 95 and 99.9%, respectively.
3. Results
3.1. Human lenses
Human lenses were examined for the degree of methylation by proteomic analyses involving tryptic digestion of extracted proteins followed by mass spectrometry (Fig. 1). Only Cys and His residues were found to be methylated, and the degree of methylation of His was lower than that for Cys. Residue C110 in γD crystallin was the most highly methylated site with approximately 70% of this residue present in the S-methyl form. Methylation was largely confined to the β and γ crystallins with only one site detected in the two α crystallin polypeptides (His 154 in αA crystallin). This result may reflect the fact that human αB crystallin has no Cys residues and that αA crystallin contains just two Cys, whereas the β and γ crystallins are more Cys rich. The extent of methylation appeared to be greater in the acidic β crystallins with three sites of methylation detected in βA3-crystallin.
As the lens ages, a greater proportion of proteins become insoluble (Coghlan and Augusteyn, 1977) presumably due to denaturation. In a study of lenses from American donors, soluble proteins were compared with the insoluble proteins from the same lens. Overall the degree of methylation in the soluble proteins was less at each site than in the insoluble protein fraction, however the differences were small (Fig. 1). This may indicate that methylation does not markedly alter the structure of the crystallins. If methylation of Cys or His is present in fetal or young lenses, the levels were negligible.
3.2. Methylation of crystallins increases with age
Since little or no methylation was detected in fetal lenses (which correspond to the nuclei of adult lenses) and yet high levels were found in adults, we examined the time course of methylation using human lenses of varying age. The results for a highly methylated site on one crystallin are depicted in Fig. 2a. Methylation of γS crystallin was found to increase progressively in the nucleus reaching a plateau at approximately age 50–60. In a separate study, whole lenses and lens nuclei were examined for the degree of Cys methylation (Fig. 2b). For both crystallins and other proteins, methylation increased with age and the extent of methylation for proteins from cataract and normal lenses was indistinguishable.
3.3. Methylation of Cys and His
The major methyl donor in biology is SAM, which is the cofactor for several methyltransferases (Paik et al., 2007). Since SAM had been shown to non-enzymatically methylate DNA, it was of interest to determine if SAM were also able to transfer its methyl group to Cys and His in the absence of methyltransferase enzymes. tBoc-Cys and tBoc-His were incubated separately with SAM in phosphate buffer pH 7. As shown in Fig. 3, SAM decomposed quite rapidly under these conditions. Major degradation products detected by LC/MS were S-adenosylhomocysteine, methylthioadenosine and adenine (data not shown).
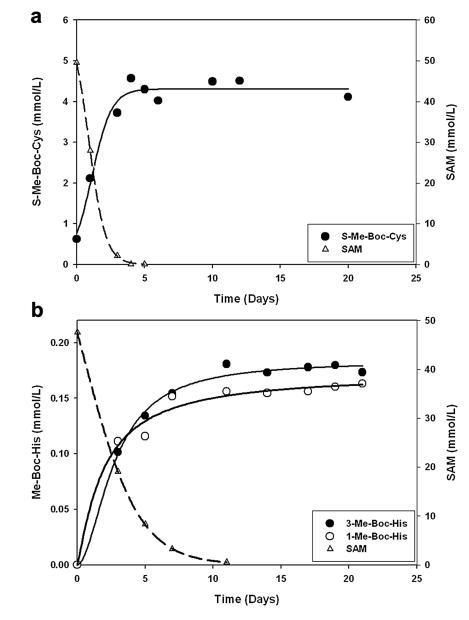
Fig. 3.
Reaction of SAM with Cys and His. a) N-α-tBoc Cys was incubated with SAM in phosphate buffer at pH 7. As SAM decreased (dashed line), the amount of S-methyl tBoc Cys (S-Me Boc-Cys) increased. b) N-α-tBoc His was incubated with SAM at pH 7. SAM decreased (dashed line) as the amount of both 1-methyl-tBoc-histidine (1-Me-Boc-His) and 3-methyl-tBoc-histidine (3-Me-Boc-His) increased.
As the levels of SAM in solution decreased, there was a corresponding increase in the concentration of tBoc-S-methyl Cys (Fig. 3a) and tBoc-N-methyl His (Fig. 3b). Their structures were confirmed using LC/MS/MS by comparison with the authentic standards. The rate of formation of the S-methyl Cys was faster than that for methyl His derivative, in accord with the relative nucleophilicity of the sulfhydryl and imidazole nitrogen groups. In the case of His, both 1-methyl and 3-methyl adducts were formed in approximately equal amounts (Fig. 3b). These experiments showed clearly that SAM is capable of directly methylating both His and Cys under physiological conditions.
3.4. SAM in human lenses
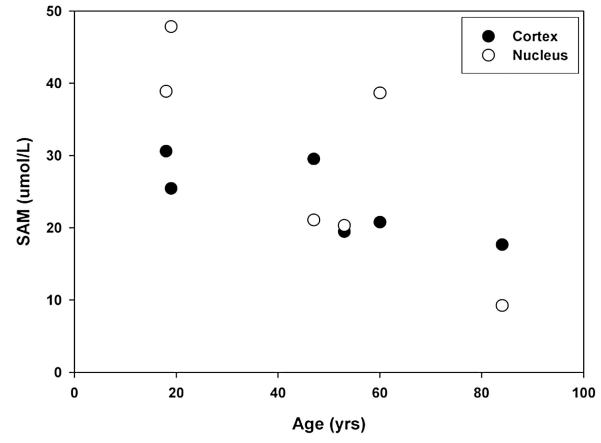
Significant methylation of crystallins was found in the center of adult lenses (Fig. 2), which is a region that contains few, if any, active enzymes (Dovrat et al., 1984; Zhu et al., 2010); however, it was not known if SAM were also present in this part of the lens. In order to examine this, an HPLC assay for SAM was developed based on previous studies (Friso et al., 2002). The results are shown in Fig. 4. Significant concentrations of SAM were found in both the cortex and the nucleus of adult human lenses, with a trend of decreasing levels with age. The levels found are in agreement with those found in animal lenses (Geller et al., 1988, 1986). In addition to SAM, S-adenosylhomocysteine was also found. This may indicate that methylation by SAM occurs within the lens, as was observed in the model studies with the tBoc amino acids.
Fig. 4.
SAM is present in human lenses. The concentration of SAM in the isolated nucleus and cortex of lenses of different ages was determined by HPLC.
3.5. Methylation of a lens crystallin by SAM
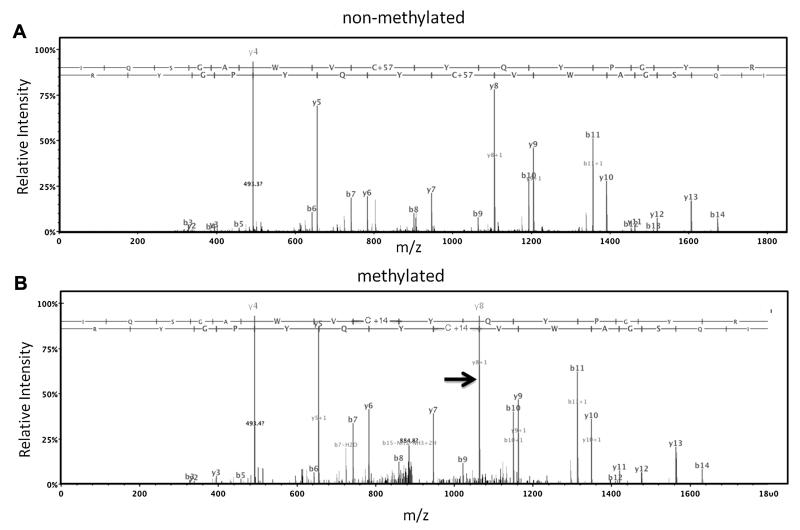
To investigate whether SAM was capable of methylating crystallins in vitro, 15 μM recombinant human βA3-crystallin was incubated with 3.8 mM SAM for 5 days at 37 °C under an argon atmosphere. Initial attempts to detect methylation in the intact protein by measuring the 14 unit mass shifts occurring with methylation were unsuccessful due to oxidation during the 5-day incubation. This resulted in 16 unit mass shifts that obscured the mass shifts due to methylation. Methylation was therefore detected by digesting the incubated βA3 with trypsin and analysis by LC–MS/MS. Peptides covering residues 1–197 comprising 98% of the protein sequence were identified, including methylated forms for all 5 Cys in the protein. Representative MS/MS spectra for βA3 peptide 163–177 containing Cys 170 are shown in Fig. 5. Fig. 5A is the spectrum derived from the doubly charged unmodified form of βA3 peptide 163–177 containing an alkylated Cys with a mass increase of 57 Da introduced following incubation by reaction with iodoacetamide prior to digestion. Fig. 5B is a MS/MS spectrum for the same peptide containing a methylated Cys with a mass increase of 14 Da. Similar MS/MS spectra confirmed methylation at the other 4 sites in incubated βA3 (data not shown). In contrast, no MS/MS spectra that indicated Cys methylation were identified in control samples incubated without addition of SAM. Attempts to quantify the extent of methylation in the SAM incubated samples were not successful due to the low ion currents for the methylated forms of the peptides. However, estimates derived by tallying the numbers of MS/MS spectra assigned to unmodified peptides and methylated peptides suggested that approximately 3% of each of the Cys residues underwent methylation after the 5 days of incubation. The reason for this low level of incorporation is most likely due to the instability of SAM (see Fig. 3).
Fig. 5.
Methylation of a purified lens crystallin by SAM. Recombinant human βA3-crystallin was incubated A) alone, or B) with SAM at pH 7. Proteomic analysis confirmed methylation at Cys 168 as shown by a 14 Da change in a major y8 ion in the MS/MS spectrum, indicated by the arrow (Fig. 5B).
4. Discussion
The ubiquitous distribution of SAM and its direct reaction with His and Cys under in vitro conditions, suggests that non-enzymatic methylation by SAM may contribute to posttranslational modifications of long-lived proteins, such as those in the lens. Most crystallins in adult human lenses were methylated at several sites. Methylation of Cys was observed at higher levels than His, with undetectable methylation of either Lys or Arg. No methylation was found in fetal lens proteins. Five crystallins (Fig. 1) contained Cys residues that were >25% methylated in 60–70 year old lenses: βA3, Cys 82 and Cys 185; βA4, Cys 165; γS, Cys 26 and γD, Cys 110. The only crystallins where methylation was not detected were βB3 and the chaperone protein, αB crystallin, which lacks Cys residues. This study extends earlier reports of Cys methylation in γS, γD and βA3-crystallins from human lenses (Lapko et al., 2002, 2003, 2005; Searle et al., 2005). In the case of β-crystallins, these authors detected substantial levels of S-methylcysteine only in βA1/A3-crystallin. In our study, the less abundant crystallin, βA4, was also found to contain significant levels of methylated Cys, with βB1 and βB2 crystallin methylated at much lower levels.
There was little difference in the percentage of methylation in soluble and insoluble proteins from the same lenses (Fig. 1). This is in contrast to modifications such as deamidation which was the only lens modification strongly correlated with loss of solubility in a previous study (Wilmarth et al., 2006) where most major lens modifications were characterized by tandem mass spectrometry of trypsin-digested lens proteins. This suggests that methylation of Cys or His may not compromise the structures of crystallins in the lens to the same extent that deamidation appears to. Age-related nuclear cataract is characterised by extensive oxidation of both Cys and Met residues (Truscott and Augusteyn, 1977). Crystallins from cataract lenses showed few differences (Fig. 2b) from those characterised in normal lenses implying that methylation is unlikely to protect lenses from age-related nuclear cataract. These observations provide circumstantial evidence that the disruption to lens function by methylation could be minor.
The pattern of methylation observed in the crystallins is quite different from that found for proteins such as histones, where methyl groups are added enzymatically, and are typically present on Lys or Arg residues (Paik et al., 2007). Crystallins in the adult human lenses were characterised by higher levels of methyl Cys, with lower levels of methyl His and virtually undetectable methyl Lys and methyl Arg. Using shotgun proteomics, other investigators have detected methylated Lys and Arg in lens digests (MacCoss et al., 2002); however, this procedure did not allow for quantification. Our results suggest that methyl Lys or methyl Arg are present in human lens proteins at much lower levels than methyl His and methyl Cys. In the in vitro incubations, where SAM was acting as a methyl donor in the absence of enzymatic catalysis, the sulfhydryl group reacted faster than that of the imidazole nitrogen of His (Fig. 3). Non-enzymatic methylation of proteins using 14C-labelled SAM has been observed previously, although the products were not characterised (Paik et al., 1975).
Although the pattern of residues modified in lens proteins was not consistent with enzymatic modification, the inner and outer regions of human lenses were tested for the presence of methyltransferase activity. In all lenses, activity was detected in the outer part of the lens; however, methyltransferase was undetectable in the inner regions of older lenses (Table 1). This result is characteristic of human lenses where enzymatic activity is present in the nucleus of young lenses, but typically absent in the center of older lenses, presumably as a result of denaturation resulting from extended periods of time at physiological temperature and conditions (Dovrat et al., 1984; Truscott and Zhu, 2010; Zhu et al., 2010). Although methyltransferase activity was not present in the center of middle-aged and older lenses, the extent of crystallin methylation in this region appeared to increase up till age 50–60, where it reached a plateau (Fig. 2a).
Significant concentrations of SAM were measured in the center of human lenses (Fig. 4). In addition, comparable levels of S-adenosylhomocysteine were also observed by HPLC of these samples (data not shown) suggesting that SAM in the lens nucleus is involved in methylation. The lens contains high levels of GSH (Giblin, 2000) and, although the thiol group of GSH is likely to play a role in intercepting SAM as it does for other reactive molecules (Parker et al., 2007), our data showed that the human crystallins were methylated in an age-dependent manner. It is likely that this degree of protein methylation represents the outcome of ongoing competition between the sulfhydryl groups of crystallins and those of GSH. If methylation of GSH occurs, S-methylglutathione can presumably diffuse out of the lens; however, if methylation of proteins takes place, the modification is irreversible.
Our data suggest that the most likely source of methylation of Cys and His residues in lens proteins may be free SAM. In model studies at pH 7, SAM readily donated a methyl group to both of these nucleophiles (Fig. 3) as well as to an intact crystallin (Fig. 5). In addition, methyltransferase activity could not be detected in the centre of older adult lenses even though the extent of protein methylation continued to increase. Lastly, the pattern of amino acid methylation in proteins (i.e. high Cys and His methylation in the absence of Lys methylation) is not consistent with enzymatic methylation. Although free SAM is present at μM levels in the center of human lenses, we cannot rule out the possibility that other methyl donors may also contribute to the methylation of nucleophilic residues (Jung et al., 2008; Tornqvist et al., 1988). In monkey lenses, betaine homocysteine S-methyltransferase is abundant (Rao et al., 1998), and it is also present in human lenses; however, it is unlikely to catalyse methylation of thiol groups within proteins since the enzyme active site has specific recognition sites for the free carboxyl and amino groups of homocysteine that are lacking in protein-bound cysteine (Gonzalez et al., 2004).
Previous studies have shown that SAM can non-enzymatically methylate DNA and proteins (Barrows and Magee, 1982; Paik et al., 1975), and the data assembled in this study also suggests that SAM can methylate lens proteins in the absence of enzyme catalysis. While the presence of SAM and methylated crystallins does not prove cause and effect, it is a simple explanation that fits the data. Lens proteins do not turn over (Lynnerup et al., 2008) and are therefore continuously exposed to physiological concentrations of SAM. Thus, non-enzymatic methylation of lens proteins by SAM offers a plausible mechanism for one of the major age-related modifications observed in the human lens.
Acknowledgements
Peter Hains is thanked for developing some of the proteomic procedures, Ashok Reddy for portions of the LC–MS/MS analysis, Lucinda Robertson for preparation of lens samples, Kirsten Lampi for providing recombinant human βA3-crystallin, and Diala Abu-Hassan for assistance in SAM incubation of βA3-crystallin.
Funding Funding for this research was provided by NIH (EY013570, EY007755 and EY010572) and the National Health and Medical Research Council (1008667). RT is a NHMRC Senior Research Fellow.
References
- Barrows LR, Magee PN. Nonenzymatic methylation of DNA by S-adenosylmethionine in vitro. Carcinogenesis. 1982;3:349–351. doi: 10.1093/carcin/3.3.349. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol. Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- Coghlan SD, Augusteyn RC. Changes in the distribution of proteins in the aging human lens. Exp. Eye Res. 1977;25:603–611. doi: 10.1016/0014-4835(77)90139-7. [DOI] [PubMed] [Google Scholar]
- Dasari S, Wilmarth PA, Reddy AP, Robertson LJ, Nagalla SR, David LL. Quantification of isotopically overlapping deamidated and 18o-labeled peptides using isotopic envelope mixture modeling. J. Proteome Res. 2009;8:1263–1270. doi: 10.1021/pr801054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovrat A, Scharf J, Gershon D. Glyceraldehyde 3-phosphate dehydrogenase activity in rat and human lenses and the fate of enzyme molecules in the aging lens. Mech. Ageing Dev. 1984;28:187–191. doi: 10.1016/0047-6374(84)90019-8. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi SW, Dolnikowski GG, Selhub J. A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal. Chem. 2002;74:4526–4531. doi: 10.1021/ac020050h. [DOI] [PubMed] [Google Scholar]
- Geller AM, Kotb MY, Jernigan HM, Jr., Kredich NM. Methionine adenosyltransferase and S-adenosylmethionine in the developing rat lens. Exp. Eye Res. 1988;47:197–204. doi: 10.1016/0014-4835(88)90003-6. [DOI] [PubMed] [Google Scholar]
- Geller AM, Kotb MYS, Jernigan HM, Kredich NM. S-Adenosylmethionine in the developing rat lens. Fed. Proc. 1986;45:1731–1731. [Google Scholar]
- Giblin FJ. Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Pajares MA, Martinez-Ripoll M, Blundell TL, Sanz-Aparicio J. Crystal structure of rat liver betaine homocysteine s-methyltransferase reveals new oligomerization features and conformational changes upon substrate binding. J. Mol. Biol. 2004;338:771–782. doi: 10.1016/j.jmb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJ. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim. Biophys. Acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Jung SY, Li Y, Wang Y, Chen Y, Zhao Y, Qin J. Complications in the assignment of 14 and 28 Da mass shift detected by mass spectrometry as in vivo methylation from endogenous proteins. Anal. Chem. 2008;80:1721–1729. doi: 10.1021/ac7021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Monga V, Jain R. Facile one-step synthesis of N-a-Boc-1-alkyl-l-histidines. Tetrahedron Lett. 2004;45:6883–6885. [Google Scholar]
- Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J. Hepatol. 2005;42:907–913. doi: 10.1016/j.jhep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Lapko VN, Cerny RL, Smith DL, Smith JB. Modifications of human betaA1/betaA3-crystallins include S-methylation, glutathiolation, and truncation. Protein Sci. 2005;14:45–54. doi: 10.1110/ps.04738505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, Smith JB. S-methylated cysteines in human lens gamma S-crystallins. Biochemistry. 2002;41:14645–14651. doi: 10.1021/bi0267700. [DOI] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, Smith JB. Methylation and carbamylation of human gamma-crystallins. Protein Sci. 2003;12:1762–1774. doi: 10.1110/ps.0305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 2008;3:e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JI, Yates JR., 3rd Shotgun identification of protein modifications from protein complexes and lens tissue. Proc. Natl. Acad. Sci. U S A. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik WK, Lee HW, Kim S. Non-enzymatic methylation of proteins with S-adenosyl-L-methionine. FEBS Lett. 1975;58:39–42. doi: 10.1016/0014-5793(75)80220-1. [DOI] [PubMed] [Google Scholar]
- Paik WK, Paik DC, Kim S. Historical review: the field of protein methylation. Trends Biochem. Sci. 2007;32:146e152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Parker NR, Korlimbinis A, Jamie JF, Davies MJ, Truscott RJ. Reversible binding of kynurenine to lens proteins: potential protection by glutathione in young lenses. Invest. Ophthalmol. Vis. Sci. 2007;48:3705–3713. doi: 10.1167/iovs.06-1061. [DOI] [PubMed] [Google Scholar]
- Rao PV, Garrow TA, John F, Garland D, Millian NS, Zigler JS., Jr. Betainehomocysteine methyltransferase is a developmentally regulated enzyme crystallin in rhesus monkey lens. J. Biol. Chem. 1998;273:30669–30674. doi: 10.1074/jbc.273.46.30669. [DOI] [PubMed] [Google Scholar]
- Searle BC, Dasari S, Wilmarth PA, Turner M, Reddy AP, David LL, Nagalla SR. Identification of protein modifications using MS/MS de novo sequencing and the OpenSea alignment algorithm. J. Proteome Res. 2005;4:546–554. doi: 10.1021/pr049781j. [DOI] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Brandon TR, Lampi KJ. Deamidation alters the structure and decreases the stability of human lens betaA3-crystallin. Biochemistry. 2007;46:8861–8871. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist M, Osterman-Golkar S, Kautiainen A, Naslund M, Calleman CJ, Ehrenberg L. Methylations in human hemoglobin. Mutat. Res. 1988;204:521–529. doi: 10.1016/0165-1218(88)90046-8. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim. Biophys. Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Zhu X. Presbyopia and cataract: a question of heat and time. Prog. Retin. Eye Res. 2010;29:487–499. doi: 10.1016/j.preteyeres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J. Ocul. Biol. Dis. Infor. 2009;2:223–234. doi: 10.1007/s12177-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J. Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Korlimbinis A, Truscott RJ. Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation Res. 2010;13:553–560. doi: 10.1089/rej.2009.1009. [DOI] [PubMed] [Google Scholar]