Abstract
T helper (Th) cells and their cytokines play a pleiotropic role in the pathogenesis of systemic lupus erythematosus (SLE). Recently, a new effector T cell subset, Th9 cells, which preferentially secrete IL-9, has been identified. IL-9 is mainly produced by several T cell subsets including Th9 and Th17, and effective on the functions of Th cells and mast cell. However, there are no unambiguous conclusions that IL-9 contributes to the pathogenesis of SLE. Recently, IL-9 was reported to mediate profound anti-inflammatory effects in several cells or experimental autoimmune models. In particular, IL-9 production seemed to be important in mast cell recruitment. Defect in IL-9/IL-9R axis exhibited a more severe course of Experimental Autoimmune Encephalomyelitis (EAE) and enhanced activity of Tregs, phenotypes reminiscent of SLE. Consistently, IL-9 was implicated in the proliferation of several types of CD4+ T cells, indicating that IL-9 may be therapeutically relevant in SLE. In this article, we briefly discuss the biological features of IL-9 and summarize recent advances on the role of IL-9 in the pathogenesis and treatment of SLE.
Keywords: T helper (Th) cells, systemic lupus erythematosus (SLE), Th9, Th17, IL-9
Introduction
Systemic lupus erythematosus (SLE) is a disorder of generalized autoimmunity, characterized by multisystemic organ involvement, polyclonal B-cell activation and the production of autoantibodies. It has been reported that T helper (Th) cells and dysregulation of proinflammatory cytokine secretion play a pivotal role in its pathogenesis. However, up to date, the exact mechanisms that lead to the development of SLE remain ill defined. Initially, IFN-γ-producing Th1 cells and, more recently, IL-17- producing Th17 cells have been implicated in the pathogenesis of SLE, but whether Th1 or Th17 cells are the key culprits of SLE remains controversial [1]. Recently, a new effector T cell subset, Th9 cells, which preferentially secrete IL-9, has been identified [2-3]. It has been reported that the Th9 cells may regulate chronic allergic inflammation [4]. However, whether this T cell subset plays a role in autoimmune diseases like SLE has not been determined. The ability of the Th9 cell subset to induce autoimmune disease were mostly investigated in Experimental Autoimmune Encephalomyelitis (EAE), a model of human multiple sclerosis [5]. The data demonstrated that Th9 cells induced central nervous system (CNS) autoimmunity, which correlated for the first time Th9/IL-9 with autoimmune disease [5]. Recent review of IL-9 signaling pathways in CD4+ T cells concisely showed implications of this cytokine in autoimmunity [6]. However, the precise roles that IL-9 plays in the pathogenesis of SLE have not been clearly described. Given that the Th cell subsets express more IL-9 than other cytokines such as IL-17, IL-4, IL-12, it is logical to put the role of IL-9 in the context of Th cell-mediated autoimmune inflammatory diseases. In this review, we will summarize the biological features of IL-9, and then focus on two conflicting properties of IL-9 on lupus: both pro-inflammatory and anti-inflammatory. Finally we will discuss some recent advances on the potential roles of IL-9 in the pathogenesis of lupus.
IL-9/ IL-9R
IL-9 belongs to a member of the four-helix bundle cytokine family. Its gene has been shown to reside within the Th2 cytokine cluster in the region q31-35 on chromosome 5 [7]. Recent study showed that IL-9 was produced by several CD4+T cell subset, such as Th17 cells [5], regulatory T cells (Treg) cells [8], and Th9 cells [2,3]. IL-9 exerts its biological activity through interaction with a heterodimer receptor complex composed of IL-9Rα and a common γ chain (γC). IL-9Rα is a specific IL-9 receptor chain produced in both soluble and member-bound forms. The γC chain, however, is shared by IL-2, IL-4, IL-7, and IL-15 receptor complex [9]. Past studies have showed that IL-9R was expressed by several cell types such as mast cells [10], macrophages [11], dendritic cells [12] immature neurons [13], and human microglia [14]. More recently, evidences suggested that this cytokine receptor were also expressed on NKT cells [15], NK cells [16], Th9 [3], Th17 and Treg cells [17]. The wide expression of IL-9 receptor on the multiple cell types is consistent with the diverse activities of IL-9 [6]. Although the precise signal pathway of IL-9/IL-9R axis and its biology function remain elusive, signal transduction in IL-9/IL-9R has been shown to play critical roles in cell growth, survival, and differentiation of many types of cells [18,19]. Moreover, the association of JAK1 with IL-9R and the presence of IL-9-induced activation complexes containing STAT1, STAT3, and STAT5 have been noted in several studies [18-20].
Anti-inflammatory roles of IL-9
IL-9 was believed to have an immunosuppressive function that indirectly inhibits the production of pro-inflammatory cytokines. Since the maintenance of immune homeostasis and the prevention of immunopathology are mediated by Treg subset, and the exaggerated responses of Th1, Th2, and Th17 cells can induce tissue inflammation, abnormal functions of the Tregs will lead to proliferation of Th cells and disregulated expression of cytokines. Along these lines, Elyamana et al demonstrated that IL-9 (here as a Th17 cell-associated cytokine) acted on Tregs and enhanced their suppressive function both in vitro and in vivo. Furthermore, IL-9 was shown to promote the survival of Tregs and induce the activation of STAT3 and STAT5 signaling in Tregs [17].
Serum IL-23 was known to be upregulated in lupus [21] and correlated with SLE disease activity index (SLEDAI) [22,23]. Recently, it was reported that Th17 cells exposed to IL-23 during a secondary stimulation resulted in a significantly reduced production of IL-9, and the Th17 cells from IL-23R-/- mice produced significantly higher levels of IL-9 [17]. These findings suggested that IL-23 is a negative regulator of IL-9 secretion by Th17 cells, and the reverse association of IL-23 with IL-9 also implied a potential protective role of IL-9 in lupus. Furthermore, IL-9 production was found to be important in recruitment of mast cells [24], which has the potential to exert both pro-inflammatory and anti-inflammatory effects, depending on different environmental factors it encounters [25].
Pro-inflammatory roles of IL-9
Extensive studies have been done in recent years on the T cell subsets that produce IL-9, a cytokine that was initially thought to be a T cell-derived factor preferentially expressed by Th2 cells [24]. The newly identified Th9 cells that predominantly produce IL-9 and IL-10 changed this conception. However, unlike Th2 cells, the Th9 cells do not exhibit any regulatory properties [2,3], indicating that IL-9/IL-10-producing T cells are not regulatory T cells but effector T cells that induce tissue inflammation [17]. Consistently, an early study reported that Th9 cells induced severe colitis and peripheral neuritis upon adoptive transfer into immune deficient hosts [2]. Recently, Ja¨ger et al have developed protocols to generate myelin oligodendrocyte glycoprotein-specific Th cells in vitro including Th9 cells, and shown that not only Th1 and Th17 cells, but also Th9 cells, are capable of inducing EAE upon adoptive transfer [5]. These findings provided important insight into the roles of IL-9 in the pathogenesis of autoimmunity.
Over the last few years, several elegant studies have advanced our understanding of the involvement of Th17 cells in tissue inflammation [25-27]. There has been an increasing focus on the role of Th17 in SLE, and many studies have indicated that Th17 cell might be a promising therapeutic target for SLE [1]. Ja¨ger et al observed that Th17 cells secreted large amount of IL-9 and showed that Th17 are capable of inducing EAE upon adoptive transfer [5]. Elyamana et al also found that IL-9 can be produced by Th17 cells, and synergized with TGF-beta1 to differentiate naïve CD4+T cells into Th17 cells in vitro, which was independent of IL-6 signaling. Meanwhile, they suggested that IL-9 might normally contribute to Th17 differentiation in spite of the fact that Th17 cells were differentiated by other cytokine cocktails (TGF-beta plus IL-6 or TGF-beta plus IL-21) [17]. These findings suggested that IL-9 might play an important role in the pathogenesis of SLE via regulating Th17 cells.
More recently, in the murine model of rheumatoid arthritis (RA), serum levels of IL-9 accompanied by sustained activation of STAT3 and pathologic cytokines, were elevated after the second immunization [28,29]. Multiplex kits were use to test serial plasma samples from six RA patients at baseline as well as several time points (3, 6, and 9 months) following the treatment with rituximab. While down-regulation of inflammatory cytokines such as TNFalpha, IL-1beta, IFN gamma, IL-10, IL-6, and IL-13 were evident by rituximab, the profile of significantly elevated immunomodulators included IL-9 [28], indicating a pro-inflammatory role for this Th9 cytokine.
The proposed model of IL-9 production by Th9 and Th17 cells as well as its downstream effects on SLE is shown in Figure 1.
Figure 1.
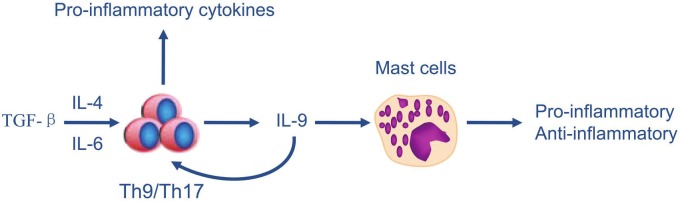
Proposed model of IL-9 production by Th9/Th17 cells and its downstream effects on SLE. TGF-β and IL-4 induces the differentiation of Th9, whereas TGF-β and IL-6 induces the differentiation of Th17. The Th9/Th17 cells then exhibit two effects: i. produce pro-inflammatory cytokines; ii. secrete IL-9 to act on Th cells and mast cells, which have the potential to exert both pro-inflammatory and anti-inflammatory effects, depending on different environmental factors it encounters.
Roles and therapeutic potential of IL-9 in SLE
Cytokine-mediated immunity plays an important role in the pathogenesis of SLE and other autoimmune diseases. Elyaman et al showed that IL-9R−/− EAE mice had a higher frequency of Th1 cells and Th17 cells when compared with WT mice. They also showed that Treg cells from IL-9R−/− mice exhibited a defect in their suppressive activity in comparison to their counterparts from WT mice [17]. These data suggested that IL-9 pathway might have a protective role in SLE. However, Nowak et al demonstrated that IL-9 receptor deficiency and IL-9 neutralization attenuated EAE, which correlated with decreases of Th17 cells and IL-6-producing macrophages in the central nervous system, as well as the reduction of mast cell numbers in the regional lymph nodes [30]. The heterogeneous effects of IL-9 could be explained by the differential expression of its receptor on multiple cell types. The net outcomes of IL-9 signaling, however, may be pro-inflammatory in view of attenuation of EAE with IL-9 neutralization. Along this line, it has been reported that serum IL-9 levels were increased in patients with SLE (50.7 ± 52.0 pg/ml) compared to healthy individuals (40.4 ± 41.7 pg/ml; p < 0.05) [31].
The symptoms of SLE ranged from rash and arthritis to neuropathies and severe renal disease. Mast cell infiltrates were present in the affected tissue from patients with SLE-mediated glomerulonephritis, which strongly indicated an influence of mast cells [32]. Indeed, there are accumulating evidences to suggest that IL-9 is associated with the recruitment and/or accumulation of mast cells. For example, Forbes et al demonstrated that IL-9-deficient mice failed to develop experimental oral antigen-induced intestinal anaphylaxis, and the intestinal IL-9 overexpression induced an intestinal anaphylaxis phenotype (intestinal mastocytosis, intestinal permeability, and intravascular leakage). In addition, intestinal IL-9 overexpression was predisposed to oral antigen sensitization, which required mast cells and increased intestinal permeability [33]. Collectively, these findings suggest that blocking IL-9-mast cell pathway could be an effective strategy to attenuate lupus phenotype.
Conclusion
Currently, the direct evidence from animal and clinical studies for lupus is still scarce. The available literature regarding IL-9 biology suggested that IL-9/IL-9R pathway have the potential to exert both pro-inflammatory and anti-inflammatory effect although the net outcomes of IL-9/IL-9R signaling may be biased towards pro-inflammatory roles. Therefore, it is not surprising to propose IL-9 as possible mediator in autoimmune disease. However, following points should be taken into consideration in this regard: First, IL-9 has pleiotropic functions, in particular when heterogeneity was observed in IL-9-induced Th cell responses. Blocking IL-9 for Th17 cell may also have a cross-effect on other T cells. Second, much of the data regarding IL-9 were obtained from murine models, which may not be applicable to human (or other experimental) SLE. Therefore, further studies are required, especially in human systems, to comprehensively explore the therapeutic potential of IL-9 in SLE.
Acknowledgements
This work was partly supported by grants from the National Natural Science Foundation of China (30830089, 81102192), and National Health and Medical Research Council of Australia (1006428).
References
- 1.Pan HF, Ye DQ, Li XP. Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4:352–335. doi: 10.1038/ncprheum0815. [DOI] [PubMed] [Google Scholar]
- 2.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+T cells and, together with TGF-beta, generates IL-9+IL-10+Foxp3-effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 4.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Rostami A. IL-9: Basic Biology, Signaling Pathways in CD4+ T Cells and Implications for Autoimmunity. J Neuroimmune Pharmacol. 2010;5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci USA. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, Sugamura K. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int Immunol. 1995;7:115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Hultner L, Kolsch S, Stassen M, Kaspers U, Kremer JP, Mailhammer R, Moeller J, Broszeit H, Schmitt E. In activated mast cells, IL-1 upregulates the production of several Th2-related cytokines including IL-9. J Immunol. 2000;164:5556–5563. doi: 10.4049/jimmunol.164.11.5556. [DOI] [PubMed] [Google Scholar]
- 11.Druez C, Coulie P, Uyttenhove C, Van Snick J. Functional and biochemical characterization of mouse P40/IL-9 receptors. J Immunol. 1990;145:2494–2499. [PubMed] [Google Scholar]
- 12.Mohamadzadeh M, Ariizumi K, Sugamura K, Bergstresser PR, Takashima A. Expression of the common cytokine receptor gamma chain by murine dendritic cells including epidermal Langerhans cells. Eur J Immunol. 1996;26:156–160. doi: 10.1002/eji.1830260124. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine RH, Cases O, Lelievre V, Mesplès B, Renauld JC, Loron G, Degos V, Dournaud P, Baud O, Gressens P. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ. 2008;15:1542–1552. doi: 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]
- 14.Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto T, Min B, Sugimoto T, Hayashi N, Ishikawa Y, Sasaki Y, Hata H, Takeda K, Okumura K, Van Kaer L, Paul WE, Nakanishi K. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197:997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meissner U, Blum H, Schnare M, Röllinghoff M, Gessner A. A soluble form of the murine common gamma chain is present at high concentrations in vivo and suppresses cytokine signaling. Blood. 2001;97:183–191. doi: 10.1182/blood.v97.1.183. [DOI] [PubMed] [Google Scholar]
- 17.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demoulin JB, Uyttenhove C, Van Roost E, De-Lestré B, Donckers D, Van Snick J, Renauld JC. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol. 1996;16:4710–4716. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamson AS, Collins K, Laurence A, O'Shea JJ. The current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21:161–166. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornakova T, Staerk J, Royer Y, Flex E, Tartaglia M, Constantinescu SN, Koops L, Renauld JC. Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/ STAT pathway via interleukin-9 receptor alpha homodimers. J Biol Chem. 2009;284:6773–6781. doi: 10.1074/jbc.M807531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604–606. doi: 10.1136/ard.2008.097089. [DOI] [PubMed] [Google Scholar]
- 22.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17- mediated inflammation in autoimmunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Huang XF, Hua J, Shen N, Chen S. Dysregulated expression of interleukin-23 and interleukin-12 subunits in systemic lupus erythematosus patients. Mod Rheumatol. 2007;17:220–223. doi: 10.1007/s10165-007-0568-9. [DOI] [PubMed] [Google Scholar]
- 24.Knoops L, Renauld JC. IL-9 and its receptor: From signal transduction to tumorigenesis. Growth factors. 2004;22:207–215. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 27.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan IH, Krishnan VV, Ziman M, Janatpour K, Wun T, Luciw PA, Tuscano J. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76:159–168. doi: 10.1002/cyto.b.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji F, Yoshimi M, Katsuta O, Takai M, Ishihara K, Aono H. Point mutation of tyrosine 759 of the IL-6 family cytokine receptor, gp130, augments collagen-induced arthritis in DBA/1J mice. BMC Musculoskelet Disord. 2009;10:23. doi: 10.1186/1471-2474-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. J Rheumatol. 2011;38:2193–2197. doi: 10.3899/jrheum.110268. [DOI] [PubMed] [Google Scholar]
- 32.Hiromura K, Kurosawa M, Yano S, Naruse T. Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kidney Dis. 1998;32:593–599. doi: 10.1016/s0272-6386(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 33.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]


