Abstract
An overview of the current literature is showing that autoantibodies (AutoAbs) against cytokines are produced in several pathological conditions, including autoimmune diseases, but can also be detected in healthy individuals. In autoimmune diseases, these AutoAbs may also be prognostic markers, either negative (such as AutoAbs to IL-8 and IL-1α in rheumatoid arthritis) or positive (such as AutoAbs to IL-6 in systemic sclerosis and those to osteopontin in rheumatoid arthritis). They may have neutralizing activity and influence the course of the physiological and pathological immune responses. High levels of AutoAbs against cytokines may even lead to immunodeficiency, such as those to IL-17 in autoimmune polyendocrine syndrome type I or those to IFN-γ in mycobacterial infections. Their role in human therapy may be exploited not only through passive immunization but also through vaccination, which may improve the costs for long lasting treatments of autoimmune diseases. Detection and quantification of these AutoAbs can be profoundly influenced by the technique used and standardization of these methods is needed to increase the value of their analysis.
Keywords: Cytokines, autoimmune diseases, autoantibodies, vaccines
Introduction
Autoimmunity raises from failure of the immune self-tolerance and may involve both T and B cells [1]. In most autoimmune diseases, T cells play a pivotal role in both dysregulation and autoimmune aggression, but autoantibodies (AutoAbs) are also widely produced. These autoAbs play a key pathogenic role in diseases such as Graves disease, autoimmune hemocytopenias, and systemic lupus erythemathosus (SLE), but are also present in other diseases, where they may play a minor pathogenic role, but can be used as valuable diagnostic markers [2-4]. In addition to T cells and AutoAbs, also cytokines play a key role in development of the (auto)immune response and proinflammatory cytokines are deeply involved in the autoimmune damage. Interestingly, it has been noted that an anti-cytokine antibody response is spontaneously produced by healthy subjects and seems to be a physiologic mechanism to control the immune response. This modulating effect seems to be time-limited since the levels of these AutoAbs increase in response to cytokine levels exceeding a threshold and, then, decrease after few weeks. This phenomenon is present also in several pathological conditions, including autoimmunity and some data suggest that endogenous anti-cytokine antibodies may be used as prognostic markers monitoring the disease [5-7]. In this context, cytokines are pivotal therapeutic targets of antagonistic drugs blocking their pathogenic effects in the disease; in this line, passive immunization with monoclonal antibodies (mAbs) against TNF-α [8], IL-1 [9], and IL-6 [10] are effective in rheumatoid arthritis (RA). However, most autoimmune diseases require long term treatment, which substantially raises the cost of therapies that are already expensive in terms of drug synthesis, development and implementation. An alternative approach is given by active immunization protocols aimed to support and enhance the endogenous production of anti-cytokine AutoAbs which would stably counteract the pathological effects of the cytokine. It has been shown that production of these AutoAbs can be effectively induced or boosted by active immunization with recombinant cytokines using either the protein or the naked DNA. The increasing interest on this topic is demonstrated by the growing literature published in the last 10 years (Figure 1) that this review will summarize and discuss.
Figure 1.
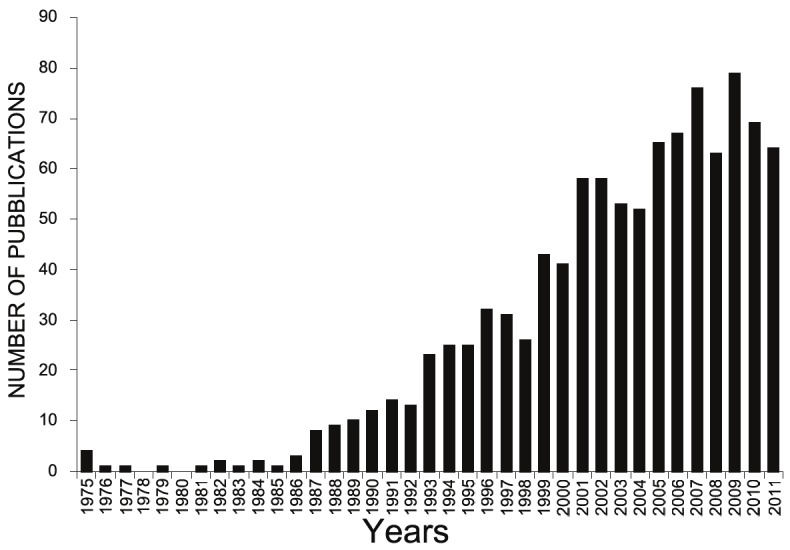
Number of publications focused on AutoAbs against cytokines between 1975 and 2011 (http://www.ncbi.nlm.nih.gov/pubmed/).
Natural AutoAbs against cytokines in human autoimmune diseases
AutoAbs against cytokines have been described in various autoimmune diseases, such as RA, systemic sclerosis (SSc), multiple sclerosis (MS), SLE, autoimmune polyendocrine syndrome type I (APS-1), but their frequency and role has been extensively debated. The Table 1 reports the frequency of AutoAbs against cytokines in various autoimmune diseases compared to healthy controls, as detected by different authors. Since their analysis used several different techniques, it cannot be ruled out whether the wide differences between the studies are ascribable to the different population analyzed or to the technique used to detect the AutoAbs (see the methods section).
Table 1.
Summary of AutoAbs frequencies reported in human autoimmune diseases and healthy controls
| Anti-cytokine Antibody | Disease(s) | Frequencies in patients vs healthy controls | Prognostic value | References |
|---|---|---|---|---|
| IL-1α | RA | 16,6% vs 5,6% | YES | [24] |
| 36% vs 38% | [91] | |||
| 18,9% vs 9,7% | YES | [26] | ||
| 42,9% vs 15% | [26] | |||
| RA/ ILD | 56,2% vs 15% | [25] | ||
| SLE | 4,7% vs 5,6% | [24] | ||
| SSc | 6,3% vs 5,6% | [24] | ||
| IL-6 | SSc | 17,3% vs 1,9% | YES | [33] |
| OPN | T1DM | 7,8% vs 3,9 % | [47] | |
| RA | 15% vs 0% | YES | [48] | |
| GM-CSF | FS | 12,4% vs 0% | [36] | |
| FS | 3,2% vs 0% | [92] | ||
| MS | 2,3% vs 0% | [36] | ||
| IL-12 | RA/SLE | 17,6% vs 0% | [93] | |
| IL-3 | FS | 3,1% vs 1,3% | [94] | |
| IFN-β | MS | 4% vs 0% | [93] | |
| RA/SLE | 5,9% vs 0% | [93] | ||
| IFN-ω | MS | 4% vs 0% | [93] | |
| RA/SLE | 5,9% vs 0% | [93] | ||
| IFN-α | RA/SLE | 29,4% vs 1,4% | [93] | |
| IL-17A | APS-I | 66,7% vs 0% | [6] | |
| IL-17F | APS-I | 93,9% vs 0% | [6] | |
| IL-22 | APS-I | 90,9% vs 0% | [6] | |
| TNF-α | RA | 61% vs 80% (8%)† | [12] | |
| SLE | - | YES | [17] |
Abbreviations: RA, Rheumatoid Arthritis; SLE, Systemic lupus erythematosus; SSc, Systemic sclerosis; T1DM, type 1 diabetes mellitus; FS, Felty’s syndrome; MS, Multiple Sclerosis; APS-I, Autoimmune polyendocrine syndrome type I; ILD, interstitial lung disease; RD, rheumatic diseases.
The authors confirmed a strong reaction in 8% of controls only.
Anti-cytokine AutoAbs seem to be more frequently directed against proinflammatory cytokines, such as IL-1α [11], TNF-α [12], IL-6 [13], IL-8 [14] and GM-CSF [15], whereas those against anti-inflammatory cytokines, such as IL-10 [16] and TGF-β [17] have been rarely reported. Unfortunately, most reports do not provide data about the functional effects of these AutoAbs, which would be helpful to define their role in the disease and the possibility to exploit them in therapy. However, these AutoAbs have been suggested to have a protective role in some diseases and may be used as prognostic markers. For instance, high levels of anti-IL-1α AutoAbs correlated with a mild disease course in patients with RA [7] and high levels of those against IFN-γ were associated with recovery in Guillain-Barré syndrome [18].
Four pro-inflammatory cytokines have been shown to play a key role in joint aggression in RA, i.e. TNF-α [19], IL-1α, IL-1β [19-21], and IL-6 [22] and the imbalance in the network of these cytokines have been suggested to play an orchestral role in the RA’s initiation and progression [23]. Suzuki et al [24] and Maniwa et al [25] reported increased levels of AutoAbs against IL-1α (but not IL-1β) in the sera of RA patients and showed that they were higher in those with nondestructive arthritis than in those with destructive arthritis. These data have been partly debated by other authors who detected these antibodies with similar frequencies in RA patients and healthy controls [26,27]. Nevertheless, they suggested that these AutoAbs may be used as a positive prognostic marker if detected early in RA [26,27], since several indices of disease activity and severity were significantly lower in the patients with high levels of anti-IL-1α AutoAbs than in those with low levels of these autoAbs. In line with the clinical data, these AutoAbs displayed neutralizing activity on IL1-α function in vitro by inhibiting proliferation of thymocytes [28] and IL-6 secretion of synoviocytes induced by IL-1α, and blocked binding of IL-1α to its receptor [29]. Anti-IL-1α AutoAbs have been also detected in patients with SSc, an autoimmune disease characterized by dermal and visceral fibrosis and vasculopathy [30,31]. These AutoAbs have been ascribed to the high amount of IL-1α secreted in the skin [24]. Intriguingly, high titers of anti-IL-1α AutoAbs have been also detected in two other dermal diseases, i.e. psoriasis and pemphigus, which suggests their involvement in regulation of cutaneous inflammation [32]. Moreover, SSc patients also display AutoAbs against IL-6 and these have been mainly associated with the cutaneous form of the disease compared to the visceral one, which suggests that they may influence the clinical outcome [33]. AutoAbs against IL-1α [24] have been described also in patients with SLE, a systemic autoimmune disease due to deposition of immune complexes in several tissues, including skin, kidney, and joints [34], but no clinical correlations were found in this disease [5]. Moreover, SLE patients also displayed AutoAbs against TNF-α, and their levels were lower in the patients with active disease compared to those with inactive disease, which suggests that their presence marks exacerbations of the autoimmune aggression [17]. The same group also found high serum levels of anti-TGF-β AutoAbs and suggested that these may contribute to dysregulate the immune homeostasis [17]. Finally, anti-GM-CSF AutoAbs have been detected in SLE patients with neutropenia and their levels inversely correlated with the neutrophil counts, which suggested that they might inhibit the hemopoietic function of this cytokine [35].
AutoAbs against proinflammatory cytokines are also found in MS, an autoimmune disease affecting the central nervous system (CNS) in young adults. MS patients may display high plasma levels of AutoAbs against TNF-α and IFN-γ, and low levels of AutoAbs against IL-4, and IL-10 [1,16] but their biological role is not clarified yet. By contrast, anti-GM-CSF autoAbs have been rarely detected in MS patients [36], but a vaccine developed to neutralize this cytokine showed promising results in the treatment of experimental autoimmune encephalomyelitis (EAE) [37], the animal model for MS; this result might be related to inhibition of the GM-CSF effect on glial cells and the function of other cytokines in CNS [38].
Beside these “classical” cytokines, new frontiers have been opened by the demonstration of the pathogenic role of other anti-cytokine AutoAbs, such as those against Osteopontin (OPN), IL-17, and IL-8, in autoimmunity.
OPN is a pleiotropic cytokine secreted by activated macrophages, leukocytes, and activated T lymphocytes and is present in the extracellular fluids, at the sites of inflammation, and in the extracellular matrix of mineralized tissues [39,40]. High OPN serum levels have been reported in patients with various autoimmune diseases, such as autoimmune lymphoproliferative syndrome (ALPS), MS, SLE [41-44]; in RA [45] and juvenile idiopathic arthritis [46], they may play a role in supporting inflammation and bone erosion [47]. Moreover, AutoAbs against OPN have been reported in RA patients and their level inversely correlated with markers of disease activity, such as serum levels of rheumatoid factor and C-reactive protein, and the erythrocyte sedimentation rate [48]. Intriguingly, passive immunization with antibodies against a cryptic epitope of the N-terminal portion of OPN exerted beneficial effects in mouse and primate models of RA [49]. Presence of anti-OPN AutoAbs is not exclusive of RA since it has been detected also in patients with type 1 diabetes [47] and mice with EAE. Steinman et al [50] showed that EAE induction triggered production of anti-OPN AutoAbs and remission occurred when their titers peaked. Moreover, they showed that mouse immunization with a plasmid encoding for OPN boosted production of these AutoAbs and ameliorated the chronic course of EAE when administered before EAE induction. In line with these data, we recently detected high levels of anti-OPN AutoAbs in patients with MS, but clinical correlations are not available yet (unpublished data).
Recently, the Th1/Th2 paradigm of the T helper response has been challenged by identification of another T helper cell type (Th17), which may play a role in several autoimmune diseases [51] by secreting cytokines such as IL-17A, IL-17F, IL-21, and IL-22 [51]. Autoimmune polyendocrine syndrome type I (APS-I), also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), is a rare inherited autoimmune disease due to mutations of the gene of AIRE, involved in thymocyte selection [52,53]. High levels of antoantibodies against IL-17A, IL-17F, and IL-22 have been detected in APS-I patients displaying chronic mucocutaneous candidiasis [6] which suggests that they may inhibit the Th17 cell capacity to counteract this infection. Presence of anti-IL-17 AutoAbs in APS-I has been confirmed using a robust high-throughput radioligand binding assay (RLBA) that has been compared to the ELISA assay [54]. These findings support the hypothesis that anti-cytokine AutoAbs might favor candida infection by causing a partial immune deficiency. This is also supported by other reports describing development of mycobacterial or lysterial infections in patients with various autoimmune diseases (RA, Chron’s diseases, psoriasis) treated with anti-TNF-α antibodies [55].
IL-8 is involved in chemotactic recruitment of inflammatory cells and is secreted by several cell types including synoviocytes participating in the destructive process of the joint synovial [56]. In RA, AutoAbs against IL-8 correlate with several inflammatory parameters, such as serum levels of C-reactive protein and immune complexes, and have been suggested to be a useful marker of disease severity in the patients with extra-articular manifestations [14].
Methods to detect AutoAbs against cytokines
Different reports display substantial discrepancies about the presence and the levels of AutoAbs against cytokines in both patients and healthy controls. It is difficult to rule out whether these differences are due to heterogeneity of the analyzed subjects or to the method used to detect the autoAbs. Two main methods have been used by most authors, i.e. ELISA and Western Blot (WB). Both of them use, as bait, recombinant cytokines that are either immobilized to plastic wells (ELISA) or separated by size on a nitrocellulose filter (WB). The bait cytokines are bound by the AutoAbs present in the proband’s serum and are then detected by horseradish peroxidase- or alkaline phosphatase-conjugated secondary antibodies. Anti-human IgG polyclonal antibodies are generally used to detect the memory response and avoid the aspecific binding of low affinity IgM AutoAbs [57]. The set up of a reliable immunological test for the AutoAbs should take into consideration, and possibly avoid, a number of technical bias that could be introduced. A first problem arises from the formulation of the bait recombinant cytokines used for the test. These are often provided in solution with bovine serum albumin (BSA), used as a stabilizer. BSA, indeed, may cause aspecific signals because of the frequent reactivity of serum Ig with this “dietary” antigen. Sjöwall et al [58] revised their previous results concerning levels of anti-TNF-α AutoAbs in RA patients by demonstrating that anti-BSA antibodies were responsible for several false positives [58].
A second problem concerns the features of the epitope(s) recognized by the AutoAbs, since conformational epitopes, such as those reported for OPN in RA patients [48], may be lost in the assay. For instance, WB is inadequate to detect conformational epitopes since the bait cytokine is denatured in the SDS/PAGE run. Although ELISA may detect conformational epitopes, some of them may be lost as well due to distortions of the three-dimensional structure following the absorption of the bait cytokine to the plastic well. Moreover, the recombinant cytokine may lack epitopes displayed by the wild-type proteins, such as the glycosilated epitopes which are absent in the recombinant cytokines produced in procariotic cells. Finally, detection of the AutoAbs can be difficult in the serum because of the formation of immuno-complexes between the AutoAbs and the endogenous cytokines [59].
The specificity and sensitivity of the test could also be limited by the presence of heterophilic antibodies. These are multispecific antibodies of the early immune response or interfering antibodies of unknown origin [60]. These antibodies can bind Fc fragments of the human antibodies used in the immunoassay, giving false positive signals. To reduce this interference, their removal by PEG precipitation or heat treatment has been suggested by DeFroge et al [61].
To overcome some of the technical issues described above, innovative approaches have been recently developed such as those involving the conjugation of the bait cytokines with fluorescently labeled microsphere [62] or radio iodine [63]. The particle-based multiplex assays have the advantages to detect the AutoAbs using small volumes of serum and to target multiple AutoAbs simultaneously in the same sample, by using microspheres conjugated with different fluorochromes and, then, detected by dedicated instruments or flow cytometry. Moreover, binding to the microspheres seems to have less distortion effects on the bait cytokine, than binding to a microtiter well. This method was successful for detection of AutoAbs in celiac disease, hypothyroidism or anti-phospholipid syndrome [64]. In Radio Immuno Assay (RIA) the soluble cytokine is radiolabeled [63] and allows to discriminate the saturable from the non-saturable binding because of the addition of a molar excess of the unlabeled cytokine which inhibits binding of the tracer [65-68]. Indeed, this method has been used to confirm the presence of anti-IL-17, -IL-22, and -IFN-α-β-γ AutoAbs in APS-I patients [54,69].
Vaccination against cytokines: the therapeutic use of auto-antibodies
Current treatments of autoimmune diseases are based on administration of aspecific immunosuppressive reagents; they display a wide suppressive activity on multiple effector mechanisms of the immune response including those that may be minimally involved in the disease and should be preserved to counteract infections [70]. As an alternative to this approach, many efforts showed that passive immunotherapy using anti-cytokine mAbs may be a valuable therapeutic tool focusing the immunosuppression toward the effector mechanisms that are directly involved in the disease pathogenesis (reviewed in [71]).
In humans, the first effective anti-cytokine drug has been an anti-TNF-α (human/murine) chimeric mAb (Infliximab) initially used in RA, Crohn’s disease, ulcerative colitis, psoriasis, and ankylosing spondylitis [72]. Then, this approach has been extended to other anti-TNF-α reagents and a fully human antibody (Golimumab) has been recently approved by FDA for treatment of acute arthritis [73]. Moreover, use of anti-TNF-α [8] antibodies has been flanked by use of anti-IL-1 [9] and anti-IL-6 [10] mAb in RA. Recently, a phase III trial reported the efficacy of a fully human antibody against IL-17A (AIN457) on psoriasis, RA, and uveitis [74]. Furthermore, a study evaluating the safety, tolerability, and efficacy of AIN457 in patients with relapsing-remitting MS is in progress to date (www.clinicaltrials.gov, Novartis).
The main side effects exerted by anti-cytokine passive immunization have been increased susceptibility to infections, risk of anaphylactic reactions, and decrease of the therapeutic efficacy due to production of anti-drug antagonistic antibodies [71]. A further severe problem is the high cost of this therapy that dramatically increased the cost per patient of these diseases.
Part of these problems might be overcome by moving to active immunization against cytokines, that is a novel promising approach for autoimmune disease treatment (reviewed in [75]). This strategy is aimed to transiently induce or increase the levels of neutralizing AutoAbs against a pathogenic cytokine in order to antagonize the cytokine harmful effects. To break the tolerance, the self-protein is often coupled to an antigenic carrier producing a hetero-complex (cytokine plus carrier protein) able to induce a carrier-specific T helper response that efficiently helps the autoreactive anti-cytokine B cell response. Carriers used to this aim have been synthetic virus like particles (VLPs) [76], keyhole limpet hemocyanin (KLH) [77], and ovalbumin (OVA) [78].
Extensive overviews of anti-cytokines vaccination have been published by Ratsimandresy et al [79], Delavallee et al [80] and Uyttenhove et al [81]. Dalum et al [82] has been the first to report successful vaccination against TNF-α in mice affected with collagen-induced arthritis (CIA) using a TNF-α protein modified by replacement with OVA sequences. Later on, Uyttenhove used IL-9 and IL-12 cross-linked to OVA to induce the respective AutoAbs and evaluate the role of these cytokines in Leishmania major infection [83]. The same strategy was effective in inducing anti-IL-12 AutoAbs which protected mice against EAE [84]. Moreover, protein vaccination against anti-IL-17A conferred protection against EAE [85]. All data reported to date are based on results obtained in animal models and showed that cytokine vaccination can be effective in controlling several autoimmune diseases and seems to be safe since no severe side effects, including increased susceptibility to infections, were reported [81].
Because of these promising results, new clinical trials have been developed for human therapy using kinoids, which are non toxic immunogenic cytokine derivatives inducing high titer of neutralizing Abs. They are produced as heterocomplexes of the cytokine coupled, by aldheide treatment, to a foreign T helper carrier protein such as KLH or tetanus toxoid, both approved by FDA for clinical human use [86]. The human TNF-α coupled to KLH, previously shown to induce the production of AutoAbs against TNF-α in TTG mice (transgenic for human TNF-α) with positive effects on their spontaneous arthritis [87], is being used in a clinical trial in RA patients resistant to conventional TNF-α antagonist. Moreover, a clinical trial is evaluating the safety and immunogenicity of a TNF-α-kinoid in patients with Crohn’s Disease and an IFN-α-kinoid in SLE patients. (www.clinical.trials.gov).
DNA vaccination is another approach to induce AutoAbs against cytokines. DNA-based vaccines can activate the immune system using CpG sequences binding Toll-like receptor 9 (TLR9) as an adjuvant [88]. DNA vaccination against human TNF-α prevented CIA in mice [89]. A DNA vaccine encoding for IL-18 conferred protection against the lupus-like autoimmune syndrome displayed by MRLlpr/lpr mice with decreased lymphoproliferation (a hallmark of the lpr disease), IFN-γ production, glomerulonephritis, renal damage, and mortality [90]. Data obtained in the animal models suggest that DNA vaccination is a powerful strategy and encourages its use in humans. However, DNA vaccination has potential risks limiting its use in humans, including development of malignances due to integration of the plasmid DNA, or autoimmune responses against the tissue expressing the DNA vaccine, and difficulties in controlling the expression of the encoded molecules.
Conclusions
AutoAbs against cytokines are emerging as a novel tool in the follow up and prognostic evaluation of autoimmune diseases. However, a standard detection method(s) of these AutoAbs is required with the aim to avoid discordant results obtained by different research groups. Moreover, the anti-cytokine AutoAbs may be exploited to control the disease activity using inverse vaccination techniques that may substitute expensive passive immunization with anti-cytokine antibodies. This possibility is supported by encouraging results obtained in experimental animal models of autoimmune diseases. However, the data suggesting that anti-cytokine AutoAbs may also be involved in increased susceptibility to infections raise the problem of safety of these treatments. Therefore, more work is needed to assess the kinetic of production of these AutoAbs during infections and vaccination in order to select protocols capable to induce a safe transient and reversible production of these immune modulators.
Acknowledgements
This work was supported by Fondazione Italiana Sclerosi Multipla 2012/R/12 (Genoa), Fondazione Cariplo (Milan), Associazione Italiana Ricerca sul Cancro (Milan), Regione Piemonte (Ricerca Sanitaria Finalizzata and Piattaforme Innovative), Fondazione Amici di Jean (Torino).
References
- 1.Kearney JF. Formation of autoantibodies, including anti-cytokine antibodies, is a hallmark of the immune response of early B cells. J Interferon Res. 1994;14:151–152. doi: 10.1089/jir.1994.14.151. [DOI] [PubMed] [Google Scholar]
- 2.Özen S, Berk Ö, Şimşek DG, Darcan S. Clinical course of Hashimoto’s thyroiditis and effects of levothyroxine therapy on the clinical course of the disease in children and adolescents. J Clin Res Pediatr Endocrinol. 2011;3:192–197. doi: 10.4274/jcrpe.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee A, Peyerl F. TSI assay utilization: impact on costs of Graves’hyperthyroidism diagnosis. Am J Manag Care. 2012;18:e1–14. [PubMed] [Google Scholar]
- 4.Sblattero D, Berti I, Trevisiol C, Marzari R, Tommasini A, Bradbury A, Fasano A, Ventura A, Not T. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000;95:1253–1257. doi: 10.1111/j.1572-0241.2000.02018.x. [DOI] [PubMed] [Google Scholar]
- 5.Slavikova M, Schmeisser H, Kontsekova E, Mateicka F, Borecky L, Kontsek P. Incidence of autoantibodies against type I and type II interferons in a cohort of systemic lupus erythematosus patients in Slovakia. J Interferon Cytokine Res. 2003;23:143–147. doi: 10.1089/107999003321532475. [DOI] [PubMed] [Google Scholar]
- 6.Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougnères PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graudal NA, Svenson M, Tarp U, Garred P, Jurik AG, Bendtzen K. Autoantibodies against interleukin 1 alpha in rheumatoid arthritis: association with long term radiographic outcome. Ann Rheum Dis. 2002;61:598–602. doi: 10.1136/ard.61.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Juanatey C, Llorca J, Sanchez Andrade A, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. Short-term adalimumab therapy improves endothelial function in patients with rheumatoid arthritis refractory to infliximab. Clinical and Experimental Rheumatology. 2006;24:309–312. [PubMed] [Google Scholar]
- 9.Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, Roy S. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;5:e1–18. doi: 10.2165/11599820-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, Kishimoto T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 11.Svenson M, Poulsen LK, Fomsgaard A, Bendtzen K. IgG autoantibodies against interleukin 1 alpha in sera of normal individuals. Scand J Immunol. 1989;29:489–492. doi: 10.1111/j.1365-3083.1989.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 12.Fomsgaard A, Svenson M, Bendtzen K. Autoantibodies to tumour necrosis factor alpha in healthy humans and patients with inflammatory diseases and gram-negative bacterial infections. Scand J Immunol. 1989;30:219–223. doi: 10.1111/j.1365-3083.1989.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MB, Svenson M, Diamant M, Bendtzen K. Anti-interleukin-6 antibodies in normal human serum. Scand J Immunol. 1991;33:777–781. doi: 10.1111/j.1365-3083.1991.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 14.Peichl P, Pursch E, Bröll H, Lindley IJ. Anti-IL-8 autoantibodies and complexes in rheumatoid arthritis: polyclonal activation in chronic synovial tissue inflammation. Rheumatol Int. 1999;18:141–145. doi: 10.1007/s002960050073. [DOI] [PubMed] [Google Scholar]
- 15.Svenson M, Hansen MB, Ross C, Diamant M, Rieneck K, Nielsen H, Bendtzen K. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054–2061. [PubMed] [Google Scholar]
- 16.Elkarim RA, Mustafa M, Kivisäkk P, Link H, Bakhiet M. Cytokine autoantibodies in multiple sclerosis, aseptic meningitis and stroke. Eur J Clin Invest. 1998;28:295–299. doi: 10.1046/j.1365-2362.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 17.Sjöwall C, Ernerudh J, Bengtsson AA, Sturfelt G, Skogh T. Reduced anti-TNFalpha autoantibody levels coincide with flare in systemic lupus erythematosus. J Autoimmun. 2004;22:315–323. doi: 10.1016/j.jaut.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Elkarim RA, Dahle C, Mustafa M, Press R, Zou LP, Ekerfelt C, Ernerudh J, Link H, Bakhiet M. Recovery from Guillain-Barré syndrome is associated with increased levels of neutralizing autoantibodies to interferon-gamma. Clin Immunol Immunopathol. 1998;88:241–8. doi: 10.1006/clin.1998.4573. [DOI] [PubMed] [Google Scholar]
- 19.Arend WF, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor a in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 21.Joosten LA, Helsen MM, Saxne T, van de Loo FA, Heinegard D, van den Berg WB. IL-1alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 22.Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. IL-6 Blockade Attenuates the Development of Murine Sclerodermatous Chronic Graft-Versus-Host Disease. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.226. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Ayabe T, Kamimura J, Kashiwagi H. Anti-IL-1 alpha autoantibodies in patients with rheumatic diseases and in healthy subjects. Clin Exp Immunol. 1991;85:407–412. doi: 10.1111/j.1365-2249.1991.tb05740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniwa K, Ogushi F, Tani K, Ohmoto Y, Muraguchi M, Sone S. Increased incidence of autoantibodies to interleukin-1a in rheumatoid arthritis with interstitial lung disease. Respirology. 2000;5:315–320. [PubMed] [Google Scholar]
- 26.Jouvenne P, Fossiez F, Banchereau J, Miossec P. High levels of neutralizing autoantibodies against IL-1 alpha are associated with a better prognosis in chronic polyarthritis: a follow-up study. Scand J Immunol. 1997;46:413–418. doi: 10.1046/j.1365-3083.1997.d01-139.x. [DOI] [PubMed] [Google Scholar]
- 27.Hansen MB, Andersen V, Rohde K, Florescu A, Ross C, Svenson M, Bendtzen K. Cytokine autoantibodies in rheumatoid arthritis. Scand J Rheumatol. 1995;24:197–203. doi: 10.3109/03009749509100873. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Kamimura J, Ayabe T, Kashiwagi H. Demonstration of neutralizing autoantibodies against IL-1 alpha in sera from patients with rheumatoid arthritis. J Immunol. 1990;145:2140–2146. [PubMed] [Google Scholar]
- 29.Jouvenne P, Fossiez F, Garrone P, Djossou O, Banchereau J, Miossec P. Increased incidence of neutralizing autoantibodies against interleukin-1 alpha (IL-1 alpha) in nondestructive chronic polyarthritis. J Clin Immunol. 1996;16:283–290. doi: 10.1007/BF01541394. [DOI] [PubMed] [Google Scholar]
- 30.Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol. 2012;24:165–170. doi: 10.1097/BOR.0b013e32834ff2e8. [DOI] [PubMed] [Google Scholar]
- 31.Sakkas LI. New developments in the pathogenesis of systemic sclerosis. Autoimmunity. 2005;38:113–116. doi: 10.1080/16066350500095415. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani H, Ohmoto Y, Kupper TS, Shimizu M. Endogenous neutralizing anti-IL-1 alpha autoantibodies in inflammatory skin diseases: possible natural inhibitor for over expressed epidermal IL-1. J Dermatol Sci. 1998;20:63–71. doi: 10.1016/s0923-1811(98)00074-7. [DOI] [PubMed] [Google Scholar]
- 33.Takemura H, Suzuki H, Yoshizaki K, Ogata A, Yuhara T, Akama T, Yamane K, Kashiwagi H. Anti-interleukin-6 autoantibodies in rheumatic diseases. Increased frequency in the sera of patients with systemic sclerosis. Arthritis Rheum. 1992;35:940–943. doi: 10.1002/art.1780350814. [DOI] [PubMed] [Google Scholar]
- 34.Olsen NJ, Li QZ, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther. 2012;14:R174. doi: 10.1186/ar3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellmich B, Csernok E, Schatz H, Gross WL, Schnabel A. Autoantibodies against granulocyte colony-stimulating factor in Felty’s syndrome and neutropenic systemic lupus erythematosus. Arthritis Rheum. 2002;46:2384–2391. doi: 10.1002/art.10497. [DOI] [PubMed] [Google Scholar]
- 36.Meager A, Wadhwa M, Bird C, Dilger P, Thorpe R, Newsom-Davis J, Willcox N. Spontaneously occurring neutralizing antibodies against granulocyte-macrophage colony-stimulating factor in patients with autoimmune disease. Immunology. 1999;97:526–532. doi: 10.1046/j.1365-2567.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott DJ, Blanchfield JL, Martinson DA, Russell SC, Taslim N, Curtis AD, Mannie MD. Neuroantigen-specific, tolerogenic vaccines: GM-CSF is a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE) BMC Immunol. 2011;12:72. doi: 10.1186/1471-2172-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte-macrophage colony-stimulating factor crosses the blood--brain and blood--spinal cord barriers. Brain. 1997;120:2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- 39.Boggio E, Indelicato M, Orilieri E, Mesturini R, Mazzarino MC, Campagnoli MF, Ramenghi U, Dianzani U, Chiocchetti A. Role of tissue inhibitor of metalloproteinases-1 in the development of autoimmune lymphoproliferation. Haematologica. 2010;95:1897–1904. doi: 10.3324/haematol.2010.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stępień E, Wypasek E, Stopyra K, Konieczyńska M, Przybyło M, Pasowicz M. Increased levels of bone remodeling biomarkers (osteoprotegerin and osteopontin) in hypertensive individuals. Clin Biochem. 2011;44:826–831. doi: 10.1016/j.clinbiochem.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Chiocchetti A, Indelicato M, Bensi T, Mesturini R, Giordano M, Sametti S, Castelli L, Bottarel F, Mazzarino MC, Garbarini L, Giacopelli F, Valesini G, Santoro C, Dianzani I, Ramenghi U, Dianzani U. High levels of osteopontin associated with polymorphisms in its gene are a risk factor for development ofautoimmunity/lymphoproliferation. Blood. 2004;103:1376–1382. doi: 10.1182/blood-2003-05-1748. [DOI] [PubMed] [Google Scholar]
- 42.Chiocchetti A, Comi C, Indelicato M, Castelli L, Mesturini R, Bensi T, Mazzarino MC, Giordano M, D’Alfonso S, Momigliano-Richiardi P, Liguori M, Zorzon M, Amoroso A, Trojano M, Monaco F, Leone M, Magnani C, Dianzani U. Osteopontin-gene haplotypes correlate with multiple sclerosisdevelopment and progression. J Neuroimmunol. 2005;163:172–178. doi: 10.1016/j.jneuroim.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Barizzone N, Marchini M, Cappiello F, Chiocchetti A, Orilieri E, Ferrante D, Corrado L, Mellone S, Scorza R, Dianzani U, D’Alfonso S. Association of osteopontin regulatory polymorphisms with systemic sclerosis. Hum Immunol. 2011;72:930–934. doi: 10.1016/j.humimm.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 44.D’Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L, Indelicato M, Giacopelli F, Marchini M, Scorza R, Danieli MG, Cappelli M, Migliaresi S, Bigliardo B, Sabbadini MG, Baldissera E, Galeazzi M, Sebastiani GD, Minisola G, Ravazzolo R, Dianzani U, Momigliano-Richiardi P. Two single-nucleotide polymorphisms in the 5’ and 3’ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2005;52:539–547. doi: 10.1002/art.20808. [DOI] [PubMed] [Google Scholar]
- 45.Sennels H, Sørensen S, Ostergaard M, Knudsen L, Hansen M, Skjødt H, Peters N, Colic A, Grau K, Jacobsen S. Circulating levels of osteopontin, osteoprotegerin, total soluble receptor activator of nuclear factor-kappa B ligand, and high-sensitivity C-reactive protein in patients with active rheumatoid arthritis randomized to etanercept alone or in combination with methotrexate. Scand J Rheumatol. 2008;37:241–247. doi: 10.1080/03009740801910320. [DOI] [PubMed] [Google Scholar]
- 46.Masi L, Ricci L, Zulian F, Del Monte F, Simonini G, Capannini S, De Martino M, Brandi ML, Falcini F. Serum osteopontin as a predictive marker of responsiveness to methotrexate in juvenileidiopathicarthritis. J Rheumatol. 2009;36:2308–2313. doi: 10.3899/jrheum.081156. [DOI] [PubMed] [Google Scholar]
- 47.Dodds RA, Connor JR, James IE, Rykaczewski EL, Appelbaum E, Dul E, Gowen M. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res. 1995;10:1666–1680. doi: 10.1002/jbmr.5650101109. [DOI] [PubMed] [Google Scholar]
- 48.Sakata M, Tsuruha JI, Masuko-Hongo K, Nakamura H, Matsui T, Sudo A, Nishioka K, Kato T. Autoantibodies to osteopontin in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2001;28:1492–1495. [PubMed] [Google Scholar]
- 49.Fan K, Dai J, Wang H, Wei H, Cao Z, Hou S, Qian W, Wang H, Li B, Zhao J, Xu H, Yang C, Guo Y. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum. 2008;58:2041–2052. doi: 10.1002/art.23490. [DOI] [PubMed] [Google Scholar]
- 50.Steinman L, Youssef S, Van Venrooij N, Chabas D, Baranzini SE, Rittling S, Denhardt D, Sobel RA, Lock C, Pedotti R, Oksenberg JR. Response to Comment on “The Influence of the Proinflammatory Cytokine, Osteopontin, on Autoimmune Demyelinating Disease”. Science. 2003;299:1845. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 51.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 52.Notarangelo LD, Mazza C, Forino C, Mazzolari E, Buzi F. AIRE and immunological tolerance: insights from the study of autoimmune polyendocrinopathy candidiasis and ectodermal dystrophy. Curr Opin Allergy Clin Immunol. 2004;4:491–496. doi: 10.1097/00130832-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Bøe AS, Knappskog PM, Myhre AG, Sørheim JI, Husebye ES. Mutational analysis of the autoimmune regulator (AIRE) gene in sporadic autoimmune Addison’s disease can reveal patients with unidentified autoimmune polyendocrine syndrome type I. Eur J Endocrinol. 2002;146:519–522. doi: 10.1530/eje.0.1460519. [DOI] [PubMed] [Google Scholar]
- 54.Oftedal BE, Kämpe O, Meager A, Ahlgren KM, Lobell A, Husebye ES, Wolff AS. Measuring autoantibodies against IL-17F and IL-22 in autoimmune polyendocrine syndrome type I by radioligand binding assay using fusion proteins. Scand J Immunol. 2011;74:327–333. doi: 10.1111/j.1365-3083.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 55.Harboe E, Damås JK, Omdal R, Frøland SS, Sjursen H. Risk of infection through use of selective immunomodulating drugs for rheumatoid arthritis. Tidsskr Nor Laegeforen. 2012;132:1867–1871. doi: 10.4045/tidsskr.12.0180. [DOI] [PubMed] [Google Scholar]
- 56.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis: passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 57.Joosten LA, Helsen MM, Saxne T, van de Loo FA, Heinegard D, van den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 58.Sjöwall C, Kastbom A, Almroth G, Wetterö J, Skogh T. Beware of antibodies todietary proteins in “antigen-specific” immunoassays! falsely positive anticytokine antibody tests due to reactivity with bovine serum albumin in rheumatoid arthritis (the Swedish TIRA project) J Rheumatol. 2011;38:215–220. doi: 10.3899/jrheum.100690. [DOI] [PubMed] [Google Scholar]
- 59.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Primus FJ, Kelley EA, Hansen HJ, Goldenberg DM. “Sandwich”-type immunoassay of carcinoembryonic antigen in patients receiving murine monoclonal antibodies for diagnosis and therapy. Clin Chem. 1988;34:261–264. [PubMed] [Google Scholar]
- 61.DeForge LE, Loyet KM, Delarosa D, Chinn J, Zamanian F, Chuntharapai A, Lee J, Hass P, Wei N, Townsend MJ, Wang J, Wong WL. Evaluation of heterophilic antibody blocking agents in reducing false positive interference in immunoassays for IL-17AA, IL-17FF, and IL-17AF. J Immunol Methods. 2010;362:70–81. doi: 10.1016/j.jim.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol. 2012;32:238–45. doi: 10.1007/s10875-011-9621-8. [DOI] [PubMed] [Google Scholar]
- 63.de LemosRieper C, Galle P, Svenson M, Pedersen BK, Hansen MB. Preparation and validation of radio iodinated recombinant human IL-10 for the measurement of natural human antibodies against IL-10. J Immunol Methods. 2009;350:46–53. doi: 10.1016/j.jim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Binder SR. Autoantibody detection using multiplex technologies. Lupus. 2006;15:412–21. doi: 10.1191/0961203306lu2326oa. [DOI] [PubMed] [Google Scholar]
- 65.Hansen MB. Human cytokine autoantibodies. Characteristics, test procedures and possible physiological and clinical significance. APMIS Suppl. 1996;59:1–33. [PubMed] [Google Scholar]
- 66.Svenson M, Hansen MB, Bendtzen K. Binding of cytokines to pharmaceutically prepared human immunoglobulin. J Clin Invest. 1993;92:2533–2539. doi: 10.1172/JCI116862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen MB, Svenson M, Bendtzen K. Serum-induced suppression of interferon (IFN) activity. Lack of evidence for the presence of specific autoantibodies to IFN-alpha in normal human sera. Clin Exp Immunol. 1992;88:559–562. doi: 10.1111/j.1365-2249.1992.tb06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansen MB, Svenson M, Abell K, Yasukawa K, Diamant M, Bendtzen K. Influence of interleukin-6 (IL-6) autoantibodies on IL-6 binding to cellular receptors. Eur J Immunol. 1995;25:348–354. doi: 10.1002/eji.1830250207. [DOI] [PubMed] [Google Scholar]
- 69.Hapnes L, Willcox N, Oftedal BE, Owe JF, Gilhus NE, Meager A, Husebye ES, Wolff AS. Radioligand-binding assay reveals distinct autoantibody preferences for type I interferons in APS I and myasthenia gravis subgroups. J Clin Immunol. 2012;32:230–7. doi: 10.1007/s10875-011-9617-4. [DOI] [PubMed] [Google Scholar]
- 70.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 71.Prete M, Perosa F, Favoino E, Dammacco F. Biological therapy with monoclonal antibodies: a novel treatment approach to autoimmune disease. Clin Exp Med. 2005;5:141–160. doi: 10.1007/s10238-005-0080-5. [DOI] [PubMed] [Google Scholar]
- 72.Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, Ghrayeb J. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 73.Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, Pendley C, Jiao Q, Shankar G, Marciniak SJ, Cohen SB, Rahman MU, Baker D, Mascelli MA, Davis HM, Everitt DE. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47:383–396. doi: 10.1177/0091270006298188. [DOI] [PubMed] [Google Scholar]
- 74.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di Padova F Psoriasis Study Group; Rheumatoid Arthritis Study Group; Uveitis Study Group. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 75.Le Buanec H, Bensussan A, Bagot M, Gallo RC, Zagury D. Active and passive anticytokine immune therapies: current status and development. Adv Immunol. 2012;115:187–227. doi: 10.1016/B978-0-12-394299-9.00007-2. [DOI] [PubMed] [Google Scholar]
- 76.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 77.Delavallée L, Semerano L, Assier E, Vogel G, Vuagniaux G, Laborie M, Zagury D, Bessis N, Boissier MC. Active immunization to tumor necrosis factor-alpha is effective in treating chronic established inflammatory disease: a long-term study in a transgenic model of arthritis. Arthritis Res Ther. 2009;11:R195. doi: 10.1186/ar2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uyttenhove C, Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur J Immunol. 2006;36:2868–2874. doi: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- 79.Ratsimandresy RA, Rappaport J, Zagury JF. Anti-cytokine therapeutics: history and update. Curr Pharm Des. 2009;15:1998–2025. doi: 10.2174/138161209788453130. [DOI] [PubMed] [Google Scholar]
- 80.Delavallée L, Duvallet E, Semerano L, Assier E, Boissier MC. Anti-cytokine vaccination in autoimmune diseases. Swiss Med Wkly. 2010;140:w13108. doi: 10.4414/smw.2010.13108. [DOI] [PubMed] [Google Scholar]
- 81.Uyttenhove C, Van Snick J. Anti-cytokine auto-vaccinations as tools for the analysis of cytokine function in vivo. Cytokine Growth Factor Rev. 2012;23:1–6. doi: 10.1016/j.cytogfr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Dalum I, Butler DM, Jensen MR, Hindersson P, Steinaa L, Waterston AM, Grell SN, Feldmann M, Elsner HI, Mouritsen S. Therapeutic antibodies elicited by immunization against TNF-alpha. Nat Biotechnol. 1999;17:666–669. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 83.Arendse B, Van Snick J, Brombacher F. IL-9 is a susceptibility factor in Leishmania major infection by promoting detrimental Th2/type 2 responses. J Immunol. 2005;174:2205–2211. doi: 10.4049/jimmunol.174.4.2205. [DOI] [PubMed] [Google Scholar]
- 84.Uyttenhove C, Arendse B, Stroobant V, Brombacher F, Van Snick J. Development of an anti-IL-12 p40 auto-vaccine: protection in experimental autoimmune encephalomyelitis at the expense of increased sensitivity to infection. Eur J Immunol. 2004;34:3572–3581. doi: 10.1002/eji.200425443. [DOI] [PubMed] [Google Scholar]
- 85.Uyttenhove C, Sommereyns C, Théate I, Michiels T, Van Snick J. Anti-IL-17A autovaccination prevents clinical and histological manifestations of experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 2007;1110:330–336. doi: 10.1196/annals.1423.035. [DOI] [PubMed] [Google Scholar]
- 86.Bizzini B, Drouet B, Zagury D, Abitbol M, Burny A, Boissier MC. Kinoids: a family of immunogens for active anticytokine immunotherapy applied to autoimmune diseases and cancer. Immunotherapy. 2010;2:347–365. doi: 10.2217/imt.10.16. [DOI] [PubMed] [Google Scholar]
- 87.Semerano L, Assier E, Delavallée L, Boissier MC. Kinoid of human tumor necrosis factor-alpha for rheumatoid arthritis. Expert Opin Biol Ther. 2011;11:545–550. doi: 10.1517/14712598.2011.566856. [DOI] [PubMed] [Google Scholar]
- 88.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Shen Y, Chen J, Zhang X, Wu X, Xu Q. Human TNF-alpha gene vaccination prevents collagen-induced arthritis in mice. Int Immunopharmacol. 2007;7:1140–1149. doi: 10.1016/j.intimp.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Bossù P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen I, Fantuzzi GD. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci USA. 2003;100:14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hansen MB, Svenson M, Abell K, Varming K, Nielsen HP, Bertelsen A, Bendtzen K. Sex- and age-dependency of IgG auto-antibodies against IL-1 alpha in healthy humans. Eur J Clin Invest. 1994;24:212–218. doi: 10.1111/j.1365-2362.1994.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 92.Hellmich B, Ciaglo A, Schatz H, Coakley G. AutoAbs against granulocyte-macrophage colony stimulating factor and interleukin-3 are rare in patients with Felty’s syndrome. Ann Rheum Dis. 2004;63:862–866. doi: 10.1136/ard.2003.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, Willcox N. Anti-cytokine AutoAbs in autoimmunity: preponderance of neutralizing AutoAbs against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132:128–136. doi: 10.1046/j.1365-2249.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hellmich B, Ciaglo A, Schatz H, Coakley G. AutoAbs against granulocyte-macrophage colony stimulating factor and interleukin-3 are rare in patients with Felty’s syndrome. Ann Rheum Dis. 2004;63:862–866. doi: 10.1136/ard.2003.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]


