Abstract
Sphingosine 1-phosphate (S1P) is a lipid metabolite with intra- and extracellular signalling properties. It activates five G protein-coupled cell surface receptors designated S1P-receptors type 1-5 (S1P1-5) that transmit extracellular signals into cells, and it modulates intracellular signalling as a cofactor. The analysis of sphingosine kinases (SphK) type 1 and 2, the key enzymes for S1P production, in different infection models point to an important role for the activation of different immune cells like macrophages, mast cells, and dendritic cells. S1P additionally influences local and systemic lymphocyte circulation and positioning, the vascular tone, and blood pressure. Modulation of S1P-mediated signalling pathways therefore results either in local immune cell activation or systemic immune suppression, or both. Pharmacological approaches that modulate certain S1P-mediated signalling pathways while leaving others untouched appear to be promising new avenues for next generation pharmaceuticals. This review summarizes current strategies to modulate S1P signalling for immune intervention with the clear focus on the specificity of the different principles applied. Known local and systemic effects of S1P on immunity are discussed as potential pharmaceutical targets to combat immune and autoimmune diseases and sepsis.
Keywords: Lymphocyte egress, sphingosine kinase, S1P-lyase, sphingolipid metabolism, lymphopenia, FTY720, rheumatoid arthritis, multiple sclerosis, asthma, anaphylaxis
Introduction
S1P is a signalling molecule with an extraordinary broad functional repertoire [1,2]. Surprisingly the importance of this molecule in immunity was barely recognized until a small molecule called FTY720 was shown to induce profound immune suppression in rodents by a totally new mechanism at that time (Figure 1) [3]. Although early investigations reported that this substance induced apoptosis [3-6], subsequent studies revealed disruption of lymphocyte emigration from thymus and lymph nodes as its predominant mode of action [7-9]. FTY720 shared structural similarities with sphingosine, the unphosphorylated precursor of S1P (Figure 1). While FTY720 itself was a prodrug and not inhibiting lymphocyte emigration, it was phosphorylated in vivo by sphingosine kinases (SphK), the major S1P producing enzymes, to the respective phosphate FTY-P (Figure 1), which turned out to be the active compound that binds to four out of five S1P receptors except S1P2 [10,11]. Of the two known sphingosine kinases, SphK2 was the predominant one involved in FTY720 phosphorylation [12-16]. At first activation of S1P receptors was considered to establish an insuperable barrier for exiting lymphocytes [17,18]. The analysis of several different S1P receptor deficient mice however demonstrated that loss-of-function of S1P1 in T and B cells was critical to interrupt their circulation, emphasizing activation-induced S1P1 receptor internalization as the most important function of FTY-P in order to block lymphocyte emigration from thymus and lymph nodes [19-21]. Consequently S1P in blood and lymph was established as the predominant exit signal for emigrating lymphocytes [22]. Further studies have shown that locally produced S1P in the microenvironment of thymus and lymph nodes is also important for efficient lymphocyte emigration [23-25], and the expression of different S1P receptors in various leukocyte subsets led to the rapid expansion of immune functions mediated by S1P and FTY-P [1,2], why the latter is now called immunomodulator rather than immunosuppressant. Different functions of the five known S1P receptors together with the presence of local and systemic pools of S1P and a vivid regulation of S1P receptor surface expression and S1P metabolism attracted a lot of attention from pharmacologists to modulate S1P receptor function and S1P metabolism for targeted immune intervention [26,27]. This review discusses the principles of S1P and S1P receptor signalling in different immune conditions and disease states.
Figure 1.
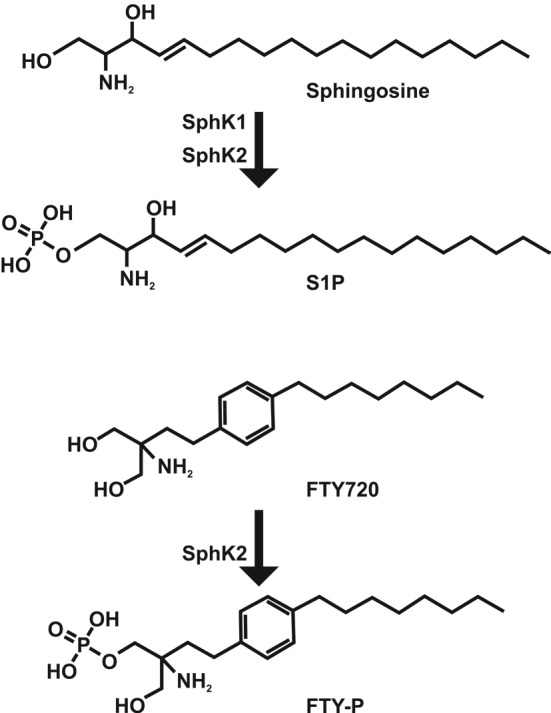
Phosphorylation and structure of sphingosine and FTY720. While sphingosine is phosphorylated by SphK1 and SphK2 to S1P, FTY720 is predominantly phosphorylated by SphK2 in vivo.
Immune suppression
FTY720 was first tested in clinical trials as an immunosuppressant after renal transplantation [28-31]. Although FTY720 was able to induce apoptosis in lymphocytes [3-6], it turned out that concentrations reached in patients were not high enough to substantially eradicate lymphocytes [8,32]. The predominant mode of action was subsequently attributed to disrupted lymphocyte circulation due to prevention of their egress from thymus and lymph nodes [7-9]. The finding that FTY720 had to be phosphorylated in order to be active in vivo and that it was structurally similar to the naturally occurring lipid metabolite sphingosine suddenly attracted notice to S1P and its receptors as potential target molecules of FTY720 [10,11]. Efficient activation of four out of five S1P receptors except S1P2 by FTY-P led to the hypothesis that S1P receptor activation prevented lymphocyte exit by establishing endothelial cell barriers [17,18]. Parallel investigations however demonstrated that FTY720 inhibited T and B cell chemotaxis to S1P even in the absence of endothelial cell barriers simply by internalization and degradation of the S1P1 receptor in lymphocytes [20,33,34]. The analysis of S1P1 deficient fetal liver chimeric and T cell-specific conditional knockout mice supported the notion that S1P1 expression in T and B cells was required in order to exit thymus and lymph nodes [19,21]. Additional support provided the analysis of SphK2 deficient mice with an inducible deletion of SphK1 preferentially in hematopoietic and vascular endothelial cells (VEC), resulting in almost complete depletion of circulating S1P and therefore referred to as “S1P-less mice” [22]. The absence of circulating S1P in these mice induced lymphopenia due to a block of T and B cell emigration from thymus and lymph nodes and resembled the phenotype of lymphocyte-specific S1P1 receptor deletion [22]. These results constituted a system where S1P in blood and lymph served as an exit signal for T and B cells in thymus and lymph nodes. The latter expressed the S1P1 receptor on their cell surface in order to sense S1P for exiting [19-21,35,36]. This general picture was further specified by studies indicating that locally produced S1P within thymus and lymph nodes supported lymphocyte egress most likely via establishment of local S1P gradients at the respective exit sites [23-25], although the existence of such gradients was never directly shown due to analytical constraints. Main sources for S1P were shown to be red blood cells (RBC) and VEC in blood [22,37,38], lymphatic endothelial cells (LEC) in lymph [39], and pericytes in thymus [25]. Regulation of S1P1 receptor surface expression on lymphocytes turned out to be critical for maintaining lymphocyte circulation [36]. Surprisingly not only surface expression of S1P1 in thymus and lymph nodes was important for T and B cell emigration, but also its internalization and desensitization to re-enter lymphoid organs [40]. Prevention of S1P1 receptor desensitization trapped lymphocytes in circulation [40]. While S1P1 was the predominant exit signal-sensing receptor on T and B cells, natural killer (NK) cells were shown to emigrate from lymph nodes and bone marrow mainly via activation of the S1P5 receptor [41,42]. An interesting finding was that S1P5 signalling on NK cells was not influenced by CD69 expression [41], while S1P1 was inhibited upon CD69 upregulation through direct interaction [43,44]. This could be an important functional characteristic of NK cells to escape CD69-mediated retardation of lymphocytes in lymph nodes in order to enable their participation in the early immune defense against infections [45].
Interfering with the outlined regulation of lymphocyte egress from lymphoid organs has evolved as a new concept for immune suppression [46]. A major advantage of this approach is the complimentary mode of action in combination with classical immunosuppressants like cyclosporine or tacrolimus, which would theoretically allow for better efficacy and lower toxicity in combinatorial treatment. But there are some obstacles that turned out to be counteractive. One major problem was the rather ubiquitous expression of S1P1 which resulted in various side effects like transient bradycardia, impaired renal function, and the development of macula edema [28]. More specific agonists and antagonists for S1P1 were developed to decrease the list of unwanted side effects. But while FTY720 was predominantly activated via phosphorylation by SphK2 in tissues, which led to more than 100-fold higher FTY-P concentrations in tissues than in blood [47], direct agonists and antagonists were present in blood at high concentrations and interfered with the maintenance of the vascular tone by blood-borne S1P and S1P1 receptor stimulation on VEC [48]. Thus application of FTY720 as a prodrug turned out to be beneficial to prevent increased vascular leak. Novel strategies need to be established to circumvent these pitfalls of currently available small molecules in order to be regarded as competitive immunosuppressive candidates in the current pharmaceutical market.
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system. Although the cause of this disease remains unknown, it is evident that lymphocytes cross the blood-brain-barrier and cause inflammation around the axons of the brain and the spinal cord, leading to demyelination, neuroaxonal injury, astrogliosis, and finally neurodegeneration [49]. The initial finding that FTY720 had a therapeutic effect on experimental autoimmune encephalomyelitis (EAE) in mice [50-53], an established animal model for MS, led to the exploration of its therapeutic potential in clinical trials for MS [54,55]. FTY720 was approved in the United States in 2010 and in Europe in 2011 for treatment of the relapsing form of MS under the brandname Gilenya [56]. It was the first orally available treatment for MS. The initial finding that FTY720 induced lymphopenia shaped the hypothesis that lymphocytes did not reach the inflammatory sites in the brain anymore, which would result in decreased inflammation and potentially lower destruction of neuronal tissue [50]. More recent studies documented that predominantly naïve and central memory T cells including interleukin 17 producing (Th17) T cells were reduced in peripheral blood whereas effector memory T cell counts were normal [57,58]. It was suggested that effector memory T cells were not trapped in lymph nodes by the mechanism outlined in “immune suppression” because they did not circulate through lymph nodes due to the lack of the chemokine receptor CCR7, which was involved in redirecting T cells from peripheral blood into lymph nodes [57,58]. While the predominant deletion of Th17 cells in peripheral blood was considered as the most important event for its therapeutic efficacy in MS [58], FTY720 may also modulate the function of astrocytes in the brain [59,60]. Mice carrying a genetic deficiency of the S1P1 gene in glial fibrillary acidic protein (GFAP) expressing astrocytes not only showed attenuated EAE, but FTY720 had also no additional effect on EAE progression [59]. These data suggest that the efficacy of FTY720 in MS treatment may at least partially depend on its local activity on astrocytes in the brain rather than immune cell trafficking. Notably the conditional genetic deletion of S1P1 in GFAP expressing astrocytes also showed diminished lymphocyte infiltration without alterations of peripheral lymphocyte counts [59]. Besides general immune suppression by impairment of lymphocyte circulation, disruption of local S1P1 signalling in astrocytes could be an alternative treatment option for MS, which may also be effective for primary progressive forms of MS that are insensitive for current immune therapies. A correspondent phase III clinical trial for FTY720 (INFORMS) is ongoing [56]. While S1P1 inhibition in astrocytes could be an effective treatment option for MS, the endogenous role of S1P and S1P1 receptor signalling in astrocytes and other cells of the central nervous system (CNS) remains enigmatic. A better understanding of the basic functions of S1P in the CNS may not only explain the unforeseen efficacy of FTY720 for MS treatment, but may offer additional and more CNS-specific target molecules for better treatment options.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic disease, characterized by an inflammatory synovitis that can lead to functional impairment and destruction of joints. Although the cause of this disease is unknown, autoimmunity plays a pivotal role in RA pathology [61]. Targeting S1P-driven lymphocyte circulation seems to be a promising strategy to combat this disease. In fact application of FTY720 in SKG mice, which spontaneously develop T cell-mediated chronic autoimmune arthritis due to a mutation in ZAP-70, proved to have therapeutic potential [62]. While FTY720 has not yet been tested for RA treatment in the clinical setting, a different strategy evolved that targets the metabolism of S1P. Inhibition of the retro-aldolase S1P-lyase (SGPL1), which irreversibly cleaves S1P into hexadecenal and phosphoethanolamine, resulted in more than 100-fold accumulation of S1P in lymphoid organs [63-65]. Consequently the S1P-gradient between blood and lymph with high S1P levels and lymphoid organs with low S1P levels was annulled, and surface expression of S1P1 on lymphocytes was prevented in thymus and lymph nodes. Exit-mediating S1P1 signalling was ultimately abrogated in these cells, and lymphopenia was developing similar to that seen after FTY720 treatment [63-65]. The SGPL1 inhibitor LX2931 was developed by Lexicon Pharmaceuticals and is currently tested in phase II clinical trials for RA treatment [66]. First results demonstrated low efficacy of this compound compared to placebo controls, why an additional dose escalation trial was initiated (Lexicon Pharmaceuticals). LX2931 is structurally similar to 2-acetyl-4-tetrahydroxybutylimidazole (THI), a compound found in the food additive caramel colour III (E150c, Figure 2) [67]. Inhibition of SGPL1 by THI was evident in mice and could be antagonized by vitamin B6 supplementation, indicating that THI blocked the functionally important interaction of SGPL1 with its prosthetic group pyridoxal phosphate (PLP) [63,67-70]. It is very likely that LX2931 also functions as an antagonist of PLP, which implicates that vitamin B6 consumption may partially neutralize the pharmacological effect of LX2931 in patients. Thus, although inhibition of SGPL1 proved to be a promising approach for RA treatment, PLP-independent direct inhibitors for this metabolic enzyme are still missing. Compared to FTY720 treatment, SGPL1 inhibition had at least one remarkable advantage: S1P accumulation was predominantly seen in lymphoid organs, and less or no accumulation of S1P was observed in most other peripheral organs like heart, eye, or liver [66]. This profile decisively increased the specificity to lymphoid compartments, which was probably a major reason for the favourable safety of LX2931 demonstrated in clinical trials.
Figure 2.
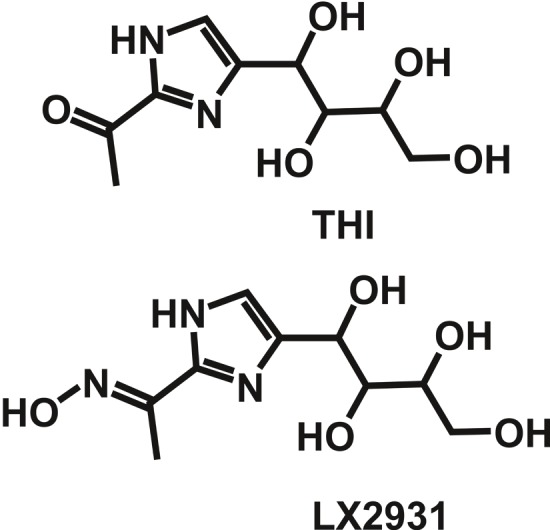
Structure of THI and LX2931. THI is a component of caramel colour III (E150c), while LX2931 was developed by Lexicon Pharmaceuticals for treatment of RA.
Asthma
The role of S1P in allergic responses is probably best demonstrated for asthma, which is a chronic inflammatory disease of the airways [71]. Airway smooth muscle cells are thought to play a key role in asthmatic attacks, and the finding that S1P was not only increased in airways of asthmatic patients, but also induced contraction of human airway smooth muscle cells prompted researchers to take a closer look into the regulation of S1P and its signalling in allergic asthma [72]. Notably administration of S1P increased bronchial hyperresponsiveness in mice [73], while inhalation of FTY720 and sphingosine kinase inhibitors abrogated experimental asthma [74,75]. The main S1P receptor involved in the induction of airway hyperreactivity was S1P3 [76]. Besides the contraction of smooth muscle cells, different immune cells were modulated by S1P as well, including dendritic cells (DC) [74], eosinophils [77,78], and mast cells [79]. Activated mast cells produced S1P [80], and human eosinophils upregulated expression of S1P receptors type 1-3, the chemokine receptor CCR3, and its ligand CCL5 upon S1P stimulation, and supported their recruitment to inflamed sites [77]. Inhalation of S1P and FTY720 did not result in systemic lymphopenia and immune suppression, but inhibited the migration of lung DC to mediastinal lymph nodes, which abrogated the development of allergen-specific Th2 cells in respective lymph nodes even during ongoing allergen challenge [74]. Notably FTY720 also reduced the capability of DC to form an immunological synapse with naïve and effector Th2 cells, which additionally impaired the allergen-specific immune response [74]. While the mechanistic details of this impairment remained unknown, activation of S1P3 downstream of the protease-activated receptor 1 (PAR1) in DC also promoted inflammation, although in a very different setting of systemic inflammatory response syndromes [81]. The involvement of S1P3 in the promotion of other DC functions like the above mentioned activation of Th2 cells may be possible. Importantly S1P signalling not only modulated DC migration, but also improved DC function [74,81]. All different cell types involved seemed to have one characteristic in common: They all signal via S1P. Abrogation of S1P signalling in the local environment of the lung could therefore be a promising approach to combat asthma. Antagonists for S1P1 and/or S1P3 seem to be attractive candidates, which could be delivered locally by inhalation. Although this concept has not yet been evaluated in the clinical setting, it might be a reasonable future approach.
Anaphylaxis
Anaphylaxis is a serious allergic response with a rapid onset. It typically starts upon allergen exposure with Immunoglobulin E (IgE) binding to an antigen of the allergen, which then activates FcεRI receptors on mast cells and basophils, although IgE-independent mechanisms also exist [82]. The release of inflammatory mediators like histamine subsequently impairs the function of multiple organ systems and induces e.g. vasodilation, vascular leak, bronchial smooth muscle contraction, and heart muscle depression [83]. As mentioned above, mast cells released S1P upon activation [80], and S1P in turn enhanced degranulation and histamine release via activation of S1P2 on mast cells in an autocrine manner, resulting in increased anaphylaxis [84]. SphK2 deficient mast cells revealed impaired effector functions like degranulation, suggesting that S1P production in mast cells predominantly occurred via SphK2 [80]. But in contrast to the impaired effector function of SphK2 deficient mast cells, SphK2 deficient mice had higher histamine concentrations in blood after induction of passive anaphylaxis than wild type control mice and SphK1 deficient mice [80]. It turned out that histamine concentrations in blood were not only dependent on the ability of mast cells to produce S1P via SphK2, but also on the amount of S1P in blood circulation, which may additionally activate mast cells in the event of an allergic response [80]. SphK1 deficient mice had lower S1P concentrations in blood compared to wild-type mice due to lower S1P production [12]. SphK2 deficient mice however exhibited higher S1P-levels in blood due to defective distribution and SGPL1-dependent degradation in peripheral tissues [85]. Further investigations demonstrated that increased S1P concentrations in blood of SphK2 deficient mice resulted in a faster recovery from an anaphylactic shock due to enhanced histamine clearance in blood [86]. The latter was mediated by an S1P2-dependent increase in blood pressure and pulse distension [86]. Furthermore blood-borne S1P regulated vascular integrity via stimulation of S1P1 signalling, most likely in endothelial cells [48,87]. Depletion of blood-borne S1P in the above mentioned S1P-less mice or abrogation of S1P1 signalling by activation-induced receptor internalization entailed increased vascular leak, a common phenotype of anaphylaxis [48]. Because of the counteracting roles of local (enhancing) and systemic (attenuating) S1P in early (enhancing) and late-stage (attenuating) anaphylaxis, S1P signalling pathways turned out to be rather difficult therapeutic targets for the treatment of anaphylaxis.
Sepsis
Sepsis is a systemic inflammatory response with an underlying infection that can cause organ dysfunction in its severe state [88]. Uncontrolled inflammation was typically regarded as the main cause of this disease. Current research however indicates that sepsis is much more complex than just being a deregulated immune response. Frequently a systemic inflammatory response is accompanied by an anergic phase, and both immune states are typically not severe enough to fully account for the observed high lethality rate [88]. Many factors contribute to the severity of this disease, and S1P might be a good candidate for therapeutic target molecules since it is involved in many processes that are relevant for sepsis onset and progression like regulation of vascular integrity [48,87], lymphocyte circulation [20,21], blood-borne antigen presentation [89,90], and cytokine secretion [91,92]. Probably the most challenging difficulty of targeting S1P signalling for sepsis treatment is to gain the required specificity for certain pathways at the right time. S1P1 signalling in endothelial cells for example was shown to be critically involved in cytokine amplification during influenza virus infection [91,92]. It may therefore also play a role in the release of cytokines during sepsis, which needs to be proven. Increased vascular permeability is a known complication during sepsis, and S1P in blood was shown to be an important regulator for the vascular tone [48,87]. Apolipoprotein M (ApoM), which is a binding molecule for S1P in plasma [93], was decreased in sepsis patients and may not only serve as a new diagnostic biomarker, but could also have therapeutically relevant functional consequences, e.g. by providing less S1P in plasma, which may increase vascular permeability and contribute to disease severity [94]. Lymphopenia is also frequently observed in sepsis patients [95]. Although lymphocyte apoptosis was attributed as the cause for lymphopenia [96], the involvement of other mechanisms like deregulation of S1P-mediated lymphocyte egress from lymphoid organs as outlined above cannot be excluded. Disruption of S1P gradients may also interfere with the presentation of blood-borne antigens by marginal zone B cells to follicular DC in the B cell zone of the spleen [89,90], which again needs to be investigated. Importantly S1P3 signalling in DC was a crucial event in the signalling cascade of PAR1, which sustained an inflammatory response [81]. Application of S1P3 antagonists could therefore be a first approach to interfere with sepsis progression.
Conclusion and outlook
S1P signalling evolved as a clinically relevant therapeutic target for immune suppression, MS, and RA. Further applications may include asthma, anaphylaxis, and sepsis in the future. S1P was shown to modulate lymphocyte egress from lymphoid organs [20,21], vascular integrity [48,87], blood pressure [86], pulse distension [86], DC function [74,81], mast cell activation [80,84], eosinophil recruitment [77,78], antigen presentation [89,90], and cytokine secretion [91,92]. While some of its activities were relevant for normal operation (lymphocyte egress, vascular integrity, antigen presentation), others were only observed in certain disease states (blood pressure, pulse distension, DC function, mast cell activation, eosinophil recruitment, cytokine secretion). Current pharmacological target molecules include S1P receptors (agonists/antagonists like FTY720) [56], the degrading enzyme SGPL1 (inhibitors like LX2931) [66], the S1P producing enzymes SphK1 and SphK2 (inhibitors), the S1P transporter Spns2 (inhibitors) [97-100], and S1P itself (anti-S1P antibodies like iSONEP) [101,102]. FTY720 was already approved for MS treatment (Gilenya, Novartis), and LX2931 (Lexicon Pharmaceuticals) and iSONEP (LPath, Inc.) are currently tested in phase II clinical trials for treatment of RA and age-related macular degeneration, respectively. Further research is needed to decipher the network of S1P signalling in order to support the development of more selective and efficient pharmaceutical compounds for clinical use in immunity and sepsis.
Acknowledgements
The author thanks Constantin Bode, Max Berlin, and Franziska Röstel for scientific and technical support and the Deutsche Forschungs-gemeinschaft (DFG) for funding.
Abbreviations
- S1P
sphingosine 1-phosphate
- SGPL1
S1P-lyase
- SphK
sphingosine kinase
- RBC
red blood cells
- DC
dendritic cells
- MS
multiple sclerosis
- RA
rheumatoid arthritis
References
- 1.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, Hoshino Y, Chiba K. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200–205. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 4.Enosawa S, Suzuki S, Kakefuda T, Li XK, Amemiya H. Induction of selective cell death targeting on mature T-lymphocytes in rats by a novel immunosuppressant, FTY720. Immunopharmacology. 1996;34:171–179. doi: 10.1016/0162-3109(96)00132-4. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S, Li XK, Shinomiya T, Enosawa S, Amemiya H, Amari M, Naoe S. The in vivo induction of lymphocyte apoptosis in MRL-lpr/lpr mice treated with FTY720. Clin Exp Immunol. 1997;107:103–111. doi: 10.1046/j.1365-2249.1997.d01-885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki S, Li XK, Shinomiya T, Enosawa S, Kakefuda T, Mitsusada M, Amemiya H, Takahara S, Amari M, Naoe S, Hoshino Y, Chiba K. Induction of lymphocyte apoptosis and prolongation of allograft survival by FTY720. Transplant Proc. 1996;28:2049–2050. [PubMed] [Google Scholar]
- 7.Brinkmann V, Chen S, Feng L, Pinschewer D, Nikolova Z, Hof R. FTY720 alters lymphocyte homing and protects allografts without inducing general immunosuppression. Transplant Proc. 2001;33:530–531. doi: 10.1016/s0041-1345(00)02126-6. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Wilt C, Kristofic C, Nikolova Z, Hof RP, Chen S, Albert R, Cottens S. FTY720: dissection of membrane receptor-operated, stereospecific effects on cell migration from receptor-independent antiproliferative and apoptotic effects. Transplant Proc. 2001;33:3078–3080. doi: 10.1016/s0041-1345(01)02312-0. [DOI] [PubMed] [Google Scholar]
- 9.Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 11.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 12.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 13.Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 14.Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 15.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 16.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 17.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 18.Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 19.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 20.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 21.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 22.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 23.Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, Adams RH, Lafaille JJ, Escalante-Alcalde D, Morris AJ, Schwab SR. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267–1278. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensken SC, Nagarajan M, Bode C, Gräler MH. Local inactivation of sphingosine 1-phosphate in lymph nodes induces lymphopenia. J Immunol. 2011;186:3432–3440. doi: 10.4049/jimmunol.1002169. [DOI] [PubMed] [Google Scholar]
- 25.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bode C, Gräler MH. Immune regulation by sphingosine 1-phosphate and its receptors. Arch Immunol Ther Exp (Warsz) 2012;60:3–12. doi: 10.1007/s00005-011-0159-5. [DOI] [PubMed] [Google Scholar]
- 27.Gräler MH. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell Physiol Biochem. 2010;26:79–86. doi: 10.1159/000315108. [DOI] [PubMed] [Google Scholar]
- 28.Budde K, Schutz M, Glander P, Peters H, Waiser J, Liefeldt L, Neumayer HH, Bohler T. FTY720 (fingolimod) in renal transplantation. Clin Transplant. 2006;20(Suppl 17):17–24. doi: 10.1111/j.1399-0012.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 29.Kahan BD, Karlix JL, Ferguson RM, Leichtman AB, Mulgaonkar S, Gonwa TA, Skerjanec A, Schmouder RL, Chodoff L. Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: a multicenter, randomized, placebo-controlled, phase I study. Transplantation. 2003;76:1079–1084. doi: 10.1097/01.TP.0000084822.01372.AC. [DOI] [PubMed] [Google Scholar]
- 30.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, Vanrenterghem Y. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2004;77:1826–1833. [PubMed] [Google Scholar]
- 31.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, Vanrenterghem Y. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2005;79:1553–1560. [PubMed] [Google Scholar]
- 32.Böhler T, Waiser J, Schütz M, Friedrich M, Schotschel R, Reinhold S, Schmouder R, Budde K, Neumayer HH. FTY 720A mediates reduction of lymphocyte counts in human renal allograft recipients by an apoptosis-independent mechanism. Transpl Int. 2000;13(Suppl 1):S311–313. doi: 10.1007/s001470050350. [DOI] [PubMed] [Google Scholar]
- 33.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 34.Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. Faseb J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 36.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hänel P, Andréani P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 38.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–1903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, Reiner SL, Miller SA, Weinmann AS, Goodnow CC, Lanier LL, Cyster JG, Chun J. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 43.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 45.Aktas E, Erten G, Kucuksezer UC, Deniz G. Natural killer cells: versatile roles in autoimmune and infectious diseases. Expert Rev Clin Immunol. 2009;5:405–420. doi: 10.1586/eci.09.27. [DOI] [PubMed] [Google Scholar]
- 46.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 47.Sensken SC, Bode C, Graler MH. Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2009;328:963–969. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]
- 48.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev. 2012;248:170–187. doi: 10.1111/j.1600-065X.2012.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 51.Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, Chiba K. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–448. [PubMed] [Google Scholar]
- 52.Rausch M, Hiestand P, Foster CA, Baumann DR, Cannet C, Rudin M. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- 53.Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Comi G, O’Connor P, Montalban X, Antel J, Radue EW, Karlsson G, Pohlmann H, Aradhye S, Kappos L. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler. 2010;16:197–207. doi: 10.1177/1352458509357065. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 56.Warnke C, Stuve O, Hartung HP, Fogdell-Hahn A, Kieseier BC. Critical appraisal of the role of fingolimod in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat. 2011;7:519–527. doi: 10.2147/NDT.S10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RL, Kappos L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 58.Mehling M, Lindberg R, Raulf F, Kuhle J, Hess C, Kappos L, Brinkmann V. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology. 2010;75:403–410. doi: 10.1212/WNL.0b013e3181ebdd64. [DOI] [PubMed] [Google Scholar]
- 59.Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 61.Colmegna I, Ohata BR, Menard HA. Current understanding of rheumatoid arthritis therapy. Clin Pharmacol Ther. 2012;91:607–620. doi: 10.1038/clpt.2011.325. [DOI] [PubMed] [Google Scholar]
- 62.Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol. 2010;136:197–204. doi: 10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- 63.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 64.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber C, Krueger A, Münk A, Bode C, Van Veldhoven PP, Gräler MH. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J Immunol. 2009;183:4292–4301. doi: 10.4049/jimmunol.0901724. [DOI] [PubMed] [Google Scholar]
- 66.Bagdanoff JT, Donoviel MS, Nouraldeen A, Carlsen M, Jessop TC, Tarver J, Aleem S, Dong L, Zhang H, Boteju L, Hazelwood J, Yan J, Bednarz M, Layek S, Owusu IB, Gopinathan S, Moran L, Lai Z, Kramer J, Kimball SD, Yalamanchili P, Heydorn WE, Frazier KS, Brooks B, Brown P, Wilson A, Sonnenburg WK, Main A, Carson KG, Oravecz T, Augeri DJ. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 67.Sinkeldam EJ, de Groot AP, van den Berg H, Chappel CI. The effect of pyridoxine on the number of lymphocytes in the blood of rats fed caramel colour (III) Food Chem Toxicol. 1988;26:195–203. doi: 10.1016/0278-6915(88)90119-6. [DOI] [PubMed] [Google Scholar]
- 68.Gobin SJ, Legg RF, Paine AJ, Phillips JA. The effect of 2-acetyl-4-tetrahydroxybutylimidazole on lymphocyte subsets in peripheral blood of the rat. Int J Immunopharmacol. 1989;11:937–946. doi: 10.1016/0192-0561(89)90116-1. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5’-phosphate binding domain exposed to the cytosol. Biochem Biophys Res Commun. 2004;325:338–343. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 70.Iscaro A, Mackay IR, O’Brien C. Lymphopenic effects on mice of a component of ammonia caramel, 2-acetyl-4(5)-tetrahydroxybutylimidazole (THI) Immunol Cell Biol. 1988;66:395–402. doi: 10.1038/icb.1988.51. [DOI] [PubMed] [Google Scholar]
- 71.Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol Sci. 2010;31:335–343. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. Faseb J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 73.Roviezzo F, D’Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, Orlotti D, D’Aiuto E, De Palma R, Rossi F, Sorrentino R, Cirino G. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol. 2010;42:572–577. doi: 10.1165/rcmb.2009-0108OC. [DOI] [PubMed] [Google Scholar]
- 74.Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 76.Trifilieff A, Fozard JR. Sphingosine-1-phosphate induced airway hyperreactivity in rodents is mediated by the S1P3 receptor. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.191585. [DOI] [PubMed] [Google Scholar]
- 77.Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, De Palma R. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sashio T, Kume H, Takeda N, Asano T, Tsuji S, Kondo M, Hasegawa Y, Shimokata K. Possible Involvement of Sphingosine-1-Phosphate/G(i)/RhoA Pathways in Adherence of Eosinophils to Pulmonary Endothelium. Allergol Int. 2012;61:283–293. doi: 10.2332/allergolint.10-OA-0299. [DOI] [PubMed] [Google Scholar]
- 79.Olivera A, Rivera J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv Exp Med Biol. 2011;716:123–142. doi: 10.1007/978-1-4419-9533-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 82.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 83.De Bisschop MB, Bellou A. Anaphylaxis. Curr Opin Crit Care. 2012 doi: 10.1097/MCC.0b013e3283557a63. [DOI] [PubMed] [Google Scholar]
- 84.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, Ryan JJ, Milstien S, Spiegel S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sensken SC, Bode C, Nagarajan M, Peest U, Pabst O, Gräler MH. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol. 2010;184:4133–4142. doi: 10.4049/jimmunol.0903358. [DOI] [PubMed] [Google Scholar]
- 86.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, Watford W, Meylan F, Diesner SC, Li L, Schnermann J, Proia RL, Rivera J. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 89.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 90.Cinamon G, Zachariah MA, Lam OM, Foss FW Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, Watanabe T, Hatta M, Shinya K, Suresh M, Kawaoka Y, Rosen H, Oldstone MB. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci USA. 2011;108:12018–12023. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumaraswamy SB, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care. 2012;16:R60. doi: 10.1186/cc11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wyllie DH, Bowler IC, Peto TE. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004;57:950–955. doi: 10.1136/jcp.2004.017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 97.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 Functions as a Sphingosine-1-Phosphate Transporter in Vascular Endothelial Cells. PLoS One. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L, Lucas M, Estabel J, Ryder E, Adissu H, Adams NC, Ramirez-Solis R, White JK, Steel KP, Dougan G, Hancock RE. The role of sphingosine-1-phosphate transporter spns2 in immune system function. J Immunol. 2012;189:102–111. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, Gentile A, Vekich J, Shestowsky W, Hiraiwa M, Matteo R, Cavalli A, Grotjahn D, Grant M, Hansen G, Campbell MA, Sabbadini R. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009;50:2245–2257. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wojciak JM, Zhu N, Schuerenberg KT, Moreno K, Shestowsky WS, Hiraiwa M, Sabbadini R, Huxford T. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc Natl Acad Sci USA. 2009;106:17717–17722. doi: 10.1073/pnas.0906153106. [DOI] [PMC free article] [PubMed] [Google Scholar]


