Abstract
Tuberculosis (TB) is a major health problem requiring sustained immunity to inhibit Mycobacterium tuberculosis growth and appropriate antimicrobial therapy to prevent dissemination and drug resistance. Cell-mediated immune responses to M. tuberculosis involve the activation of cytokines such as Tumor Necrosis Factor (TNF) which is critical for granuloma formation and host resistance against TB. TNF inhibition, used as therapy for the treatment of inflammatory diseases, disrupts granuloma allowing replication of mycobacteria which may increase the efficacy of TB chemotherapy. To test this hypothesis mice infected with M. tuberculosis were treated with isoniazid (INH) and rifampicin (RMP) in the presence or absence of Enbrel, a soluble TNF receptor antagonist during three phases of M. tuberculosis infection. Inhibition of TNF with Enbrel augmented the efficacy of TB chemotherapy as shown by enhanced mycobacterial clearance from the lung of acute and established infection as well as in chronically infected mice. Furthermore, TNF inhibition significantly reduced lung pathology as compared to TB chemotherapy alone. Therefore, the experimental data suggest that TB chemotherapy may be more effective in the presence of a TNF inhibitor, which may be relevant to eradicate mycobacteria during chronic M. tuberculosis infection or reactivation.
Keywords: Mycobacterium tuberculosis, chemotherapy, isoniazid (INH), rifampicin (RMP), tumor necrosis factor (TNF), TNF inhibitors, granulomas
Introduction
Mycobacterium tuberculosis infection is a major health problem with an estimated 8.7 million new cases and 1.4 million deaths in 2011 (WHO). Co-infection with HIV caused 13 % new active infected people resulting in a high mortality rate (48.6 %) among these patients [1]. M. tuberculosis multidrug-resistant strains which are resistant to first-line tuberculosis (TB) drugs isoniazid and rifampicin (INH/RMP) affects more than 4% of new patients annually and largely contribute to the present TB epidemic [2,3]. One-third of the global population is considered to be infected with M. tuberculosis in a latent form that can reactivate years after a primary infection when host immunity declines [4].
The long-term chemotherapy for M. tuberculosis infection and often insufficient compliance of the patients to take medicines contribute to incomplete treatment, infection relapse and emergence of multi-drug resistance [5,6]. Therefore reduction of the duration of TB chemotherapy is an important issue, and the development of new therapies that shorten the duration of M. tuberculosis treatment is a major objective [7]. Several possibilities are currently explored including the co-administration of new drugs to first line drugs for chemotherapy of TB [8]. An alternative for intervention discussed recently consisted in modification of host immune responses that could potentially alter M. tuberculosis transition from a dormant state, as found within granulomatous lesions, to a resuscitation or replicating state that may allow better drug accessibility [9]. This hypothesis was tested using an inhibitor of macrophage responses combined to Isoniazid (INH) resulting in reduced lung pathology and better bacillary clearance compared to INH therapy alone [10,11]. These studies suggest that modification of host immune system may affect bacillus elimination by chemotherapy.
Tumor Necrosis Factor (TNF) is a critical cytokine for cell-mediated host immunity against mycobacteria and other infectious agents but, on the other hand, TNF is a main mediator of inflammatory processes in which its deregulated expression may cause immunopathology [12]. TNF neutralizing therapies have emerged as the most efficacious treatments against several autoimmune inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis [13]. However, TNF blockade, mainly using anti-TNF antibodies, in treatments for chronic diseases has been associated with immunosuppression, reactivation of latent TB [14-16] and a risk of new M. tuberculosis infection in particular in countries with a high incidence of TB [17]. Anti-TNF effects are related to cell death, granuloma disruption and extracellular bacteria that can multiply and disseminate [18-20]. Granuloma formation plays a key role in host protection against mycobacterial infections and their breakdown is thought to contribute to exacerbated TB [21]. However, granulomas can be also a niche where mycobacteria might persist in the latent form until decline in host immunity provides chance to reactivate [22]. M. tuberculosis, which possesses mechanisms to subvert the host immune system, induces exacerbated inflammatory responses associated to important tissue lesions and dissemination of the bacilli into the airways [23]. Dysregulated TNF expression regulated by pro and anti-eicosanoids has been associated with defective host immunity due to excessive or inefficient inflammation [24]. The overall paradoxical activity of TNF provides a target for studies on combined therapy in selected TB patients. In HIV patients with pulmonary TB, a clinical trial combining TNF inhibitors with anti-TB drugs showed that TNF inhibitors can be safely administrated during TB treatment and, in addition, higher responses to TB treatment were observed in the group of Enbrel (etanercept, soluble TNFR2-Fc) treated patients [25].
Here, we investigated the effect of combined TNF inhibition and chemotherapy for TB on bacterial clearance and immunopathology compared to M. tuberculosis chemotherapy alone in murine models of pulmonary experimental TB infection. Our data demonstrate an enhanced bacillary clearance and reduced lung pathology in mice receiving a combination of TNF neutralization and INH and RMP chemotherapy during acute, established and chronic M. tuberculosis infection.
Material and methods
Ethics statement
All animal experimental protocols complied with French regulations. Approval was obtained from the “Ethics Committee for Animal Experimentation of CNRS Campus Orleans” (CCO), registered (N°3) by the French National Committee of Ethical Reflexion for Animal Experimentation, under the N° CLE CCO 2011-029 in compliance with the French ethical and animal experiments regulations (see Charte Nationale, Code Rural R 214-122, 124).
Mice
C57BL/6 mice and TNF deficient mice [26] were bred under specific pathogen free conditions at the Transgenic Institute (CNRS UPS44, Orleans, France) and used between 8-12 weeks of age. Infected animals were maintained in sterile isolators in a biohazard animal unit.
Mycobacterial infection of mice and treatments
A frozen aliquot of M. tuberculosis H37Rv (Pasteur Institute, Paris) was rapidly thawed at room temperature and passed 30x through a 26G needle and diluted in sterile saline. Pulmonary infection with M. tuberculosis was performed by delivering 300-500 CFU/lung of H37Rv intra nasally (40 μl) under xylazine-ketamine anaesthesia as described [27]. M. tuberculosis infected mice were killed at different time points as indicated. Treatment with anti-TB drugs, isoniazid and rifampicin (INH/RMP) (Sigma-Aldrich) at concentration 0.1 mg/mL each was delivered in drinking water as previously reported [28]. Enbrel at 20 mg/kg, diluted in saline, was administered intraperitoneally twice a week and control mice were injected with similar volumes of saline [29]. Figure 1 represents the three experimental protocols used in this work corresponding to treatments at different infection phases, acute, established and chronic infection, during which TB chemotherapy may differently affect mycobacterial elimination: (1) treatment with TB chemotherapy and anti-TNF (Enbrel) from day 29 to day 40 post-infection and mouse killing on day 43; (2) treatment with antibiotics and Enbrel from day 60 to day 84 after infection and sacrifice on day 95; (3) treatment from day 162 to day 186 and sacrifice on day 198.
Figure 1.
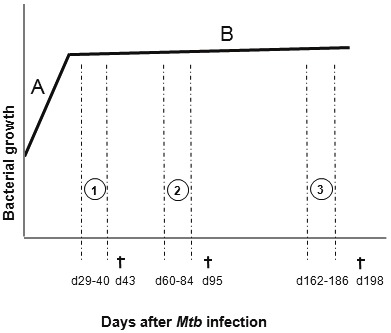
Graphic representation of 3 different protocols developed in this study during acute (1), established (2), and chronic (3) M. tuberculosis infection. TNF neutralizing and INH/RPM treatments are indicated by vertical discontinuous lines. Day of sacrifice is indicated by. A. Theoretical early bacterial replication between days 1 to 21 post-infection. B. Steady state phase of the infection in the absence of treatment.
Determination of CFU from infected organs
The number of viable bacteria recovered from lungs of mice sacrificed at different time points was scored by plating 10-fold serial dilutions of fresh-frozen lung homogenates onto Middlebrook 7H11 agar plates as previously reported [30].
Histological analyses of pulmonary lesions
Lungs were fixed in 4% buffered formaldehyde and embedded in paraffin for subsequent hematoxylin/eosin (H&E) staining. Histopathological parameters peribronchiolitis, perivasculitis, inflammation and granuloma formation were semiquantitatively scored as followed: absence: 0, minimal: 1, slight: 2, moderate: 3, marked: 4, and strong: 5 [31]. Each parameter was evaluated in three different pulmonary lobe sections from five mice for protocol number 1 (at 43 days post infection) and from three mice for the other protocols. A mean pathological score from two independent evaluators is given.
IFNγ assay
IFNγ lung concentration was evaluated by ELISA (Duoset, R&D Systems, Abingdon, UK) from supernatants of tissue homogenates (one full right lobe per mL PBS) after filtration through 0.22 um [32].
Statistical analyses
The unpaired Student’s t-test was used for analyses. P values < 0.05 were considered as statistically significant.
Results
Enbrel treatment enhances bacterial clearance by INH/RMP chemotherapy and reduces lung pathology during acute M. tuberculosis infection
To test whether TNF neutralization improves the efficacy of TB chemotherapy, mice were infected with M. tuberculosis H37Rv (500 CFU). At day 29 post-infection C57BL/6 mice were divided in four therapeutic groups: saline treated controls, treated with Enbrel (etanercept, a soluble human TNFR2-Fc blocking TNF), treated with isoniazid and rifampicin (INH/RMP), TB drugs exerting different activities against M. tuberculosis [33], and the fourth group was treated with INH/RMP plus Enbrel. Body weight measurement of C57BL/6 mice did not show significant differences between groups during treatments in contrast to a group of infected but untreated TNF knock-out (KO) mice which showed weight loss due to unrestricted bacterial growth confirming early susceptibility to the infection (data not shown). On day 43 of infection mice were sacrificed and lung analyzed for pathology and mycobacterial load. Lung weight, a surrogate measure of lung inflammation, decreased in animals treated with INH/RMP with or without Enbrel compared to control and Enbrel-treated mice suggesting a reduction in lung inflammation (Figure 2A). Pulmonary bacillary loads were significantly decreased in mice treated with INH/RMP compared to control mice (Figure 2B). The group of mice receiving combined INH/RMP and Enbrel treatment showed lower bacterial counts (p<0.05) compared to the antibiotic treatment alone (Figure 2B). Histological examination of the lungs on day 43 documented a reduced inflammatory pathology in mice treated with antibiotics which was further decreased in mice treated with INH/RMP plus Enbrel (Figure 2C). Semi-quantification of pulmonary lesions confirmed that the combination of Enbrel plus INH/RMP treatment resulted in significantly reduced pathology in terms of perivasculitis, inflammation and extent of granulomatous lesions, as compared to treatment with INH/RMP alone (Figure 2D). Therefore, the data demonstrate that TNF neutralization with Enbrel enhances the efficacy of chemotherapy induced bacterial clearance and reduces inflammatory lung pathology in acute M. tuberculosis infection.
Figure 2.
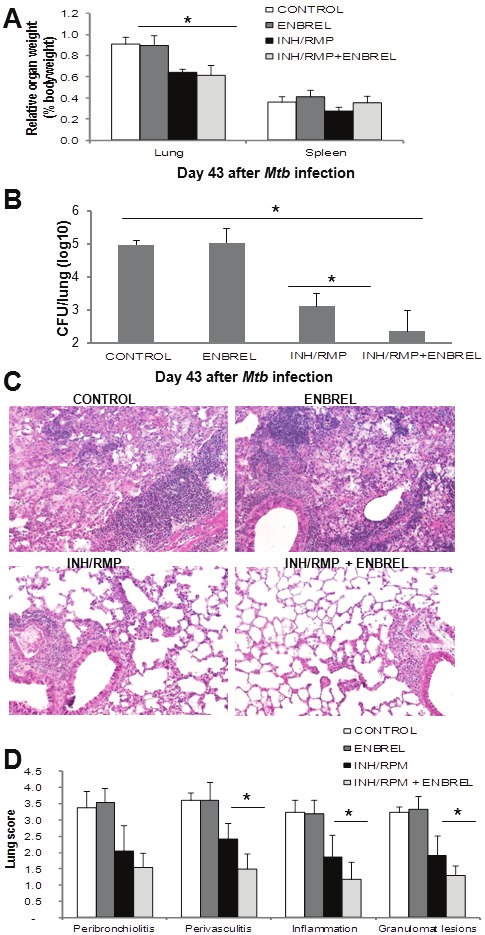
TNF inhibition and chemotherapy during acute M. tuberculosis infection reduce bacterial load and lung pathology. A. C57BL/6 mice were infected with M. tuberculosis H37Rv (500 CFU) treated with saline (CONTROL), with Enbrel (ENBREL), with antibiotics (INH/RMP) or the combination of INH/RMP plus Enbrel (INH/RMP+ENBREL) from day 29 to day 40 post-infection, killed at day 43 post-infection and their relative lung and spleen weight changes determined (n=5). B. Lung CFU evaluation at day 43 after infection. Results are represented as mean CFU±SD from one experiment with 4-5 mice per group. C. Lung sections from mice infected with M. tuberculosis for 43 days and treatment. Hematoxilin & eosin staining of lung sections with magnification 200X. Pictures are representative of five mice per group. D. Scores of lung pathology at day 43 postinfection monitored on total sections from 2-3 lung lobes per mice. Data are represented as means scores ± SD from five mice per group.
Enbrel treatment potentiates INH/RMP chemotherapy effects during established M. tuberculosis infection
To explore whether TNF neutralizing therapy potentiates the efficacy of antibiotic chemotherapy during established M. tuberculosis infection, mice infected with M. tuberculosis H37Rv (300 CFU) were treated with antibiotics in the presence or absence of Enbrel from day 60 to day 84 and sacrificed at day 95 postinfection (Figure 1). No difference in body weight between groups was observed (data not shown). Mice treated with INH/RMP and combined INH/RMP and Enbrel showed a reduced pathology on day 95 of infection as observed macroscopically (Figure 3A), with a decreased lung weight as compared to infected, non-treated mice (Figure 3B). Pulmonary bacterial burden was significantly lower in the group receiving combined INH/RPM - Enbrel treatment as compared to the INH/RMP only group (Figure 3C). Histopathological analyses of infected lungs at day 95 post-infection revealed large lesions in untreated control mice as shown in Figure 3D. In contrast, the extent of the pulmonary lesions was attenuated in INH/RPM and in INH/RPM plus Enbrel treated mice. Enbrel combined with antibiotics further reduced the general pattern of lung pathology compared to antibiotics alone. Granulomatous lesions were larger in mice treated with antibiotics and contained accumulation of lymphoid cells which were reduced in the combined INH/RPM and Enbrel group. Semi-quantitative analysis of pulmonary lesions confirmed a significant reduction in peribronchiolitis, perivasculitis, inflammation foci and the extent of granulomatous lesions in the combined INH/RPM and Enbrel group as compared to the INH/RPM group (Figure 3E).
Figure 3.
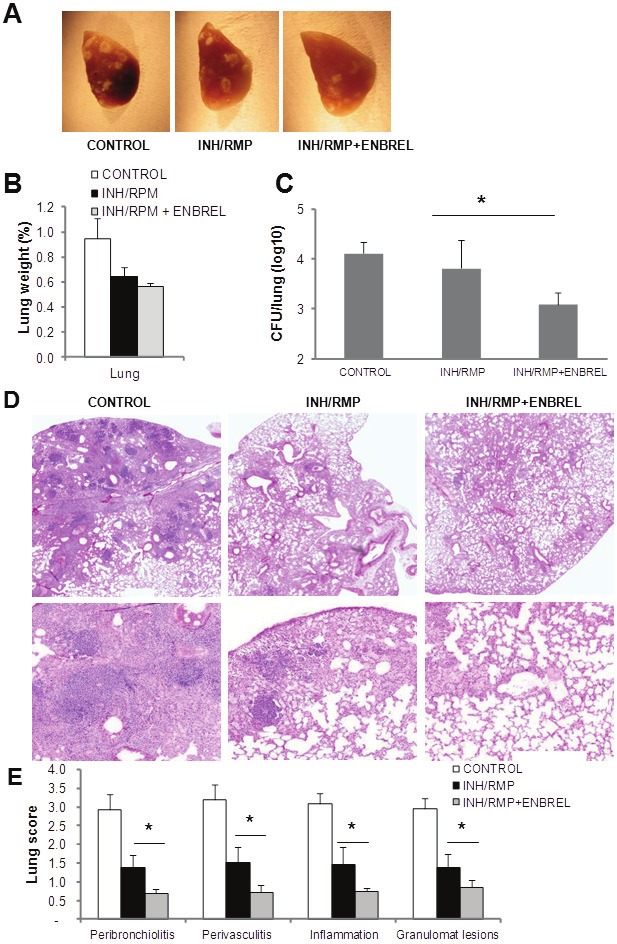
Effect of TNF neutralization and chemotherapy during established M. tuberculosis infection. Mice were infected with M. tuberculosis H37Rv (300 CFU) and killed 95 days after infection. A. Pulmonary lesions observed in mice infected with M. tuberculosis and treated with INH/RMP alone or with INH/RMP+ENBREL. B. Relative lung weight changes of M. tuberculosis-infected mice at day 95 postinfection. C. Lung CFU evaluation from mice treated with INH/RMP alone or with INH/RMP+ENBREL. Data are represented as mean CFU±SD from 3 mice per group. *p=0.05, differences between INH/RMP and INH/RMP+ENBREL groups. D. Lung sections from mice infected with M. tuberculosis for 95 days, hematoxilin & eosin staining of lung sections with magnification 20X (up) and 200X (bottom). E. Scores of lung pathology at day 95 postinfection monitored on total sections from 2-3 lung lobes per mice. Data are represented as means scores ± SD from three mice per group, *p<0.01 compared to control group.
Enbrel treatment enhances bacterial clearance by INH/RMP chemotherapy and reduces lung pathology during chronic M. tuberculosis infection
To further investigate the effects of Enbrel and INH/RMP co-administration during chronic infection, mice were infected with M. tuberculosis H37Rv and treated with antibiotics +/- Enbrel from day 162 to day 186. Lung macroscopic examination revealed a limited number of lesions in the group of mice treated with INH/RMP plus Enbrel on day 198 of infection (Figure 4A). In addition, the pulmonary bacterial load of mice treated with INH/RPM plus Enbrel was reduced as compared to mice treated with INH/RPM alone (Figure 4B). Pulmonary levels of IFNγ, measured as a surrogate marker of active immune response at this time point, revealed reduced IFNγ concentrations in the lungs of mice treated with antibiotics but no differences was found when Enbrel was administrated together with antibiotics (Figure 4C). The histopathology examination confirmed a reduction of pulmonary lesions in mice treated with INH/RPM plus Enbrel as compared with mice receiving only antibiotics (Figure 4D). Therefore, during M. tuberculosis chronic infection combination of TNF neutralization plus chemotherapy is more efficacious than chemotherapy alone to eliminate mycobacteria and to limit lung pathology.
Figure 4.
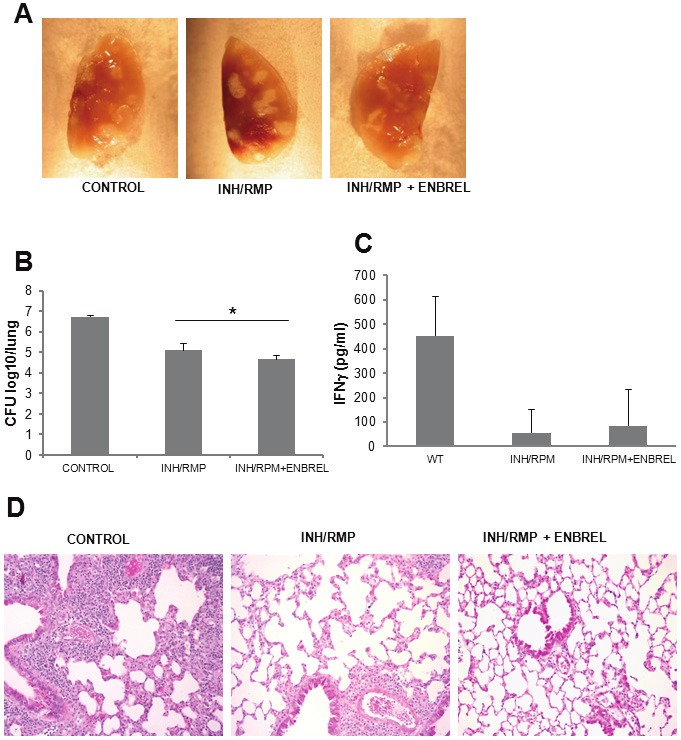
TNF inhibition and chemotherapy during chronic M. tuberculosis infection improve bacterial clearance and reduce pulmonary lesions. Mice were infected with M. tuberculosis H37Rv (300 CFU) and killed 198 days after infection. A. Pulmonary lesions observed in mice infected with M. tuberculosis and treated with INH/RMP alone or with INH/RMP+ENBREL. B. Lung CFU from mice treated with INH/RMP alone or with INH/RMP+ENBREL. Data are represented as mean CFU±SD from three mice per group. *p=0.05, differences between INH/RMP and INH/RMP+ENBREL groups. C. Levels of IFNγ in the lung of mice M. tuberculosis-infected and killed at day 198 after infection. Data are represented as mean pg of IFNγ/mL of lung homogenate ±SD from three mice per group. D. Lung sections from M. tuberculosis-infected mice for 198 days, hematoxilin & eosin staining of lung sections with magnification 200X. Pictures are representative of three mice per group.
Discussion
In this work we evaluated the effect of coadministration of a TNF inhibitor and INH/RPM in murine models of pulmonary tuberculosis to explore whether this combined treatment may improve bacterial clearance and potentially reduce the duration of chemotherapy. Using three different experimental protocols corresponding to different phases of M. tuberculosis infection in which the effect of TB chemotherapy can be different due to differential bacterial replication state, our data demonstrated that TNF neutralization combined with M. tuberculosis chemotherapy improved elimination of pulmonary bacilli better than using anti-TB drugs alone. The study showed that chemotherapy is more effective in eliminating mycobacteria during acute infection as compared to chronic infection. However, adjuvant therapy with Enbrel during both M. tuberculosis acute and chronic infections decreased the bacterial load and reduced lung pathology when compared to the effect of antibiotics alone. The amelioration of lung pathology in chronically infected mice by combined therapy was observed after two weeks without combined treatment and was associated with a reduction in bacterial load. Studies on patients treated with Enbrel for rheumatoid arthritis have shown modification of B and T cell populations and functions that may favour the reactivation of latent TB [18,34]. Prevention by chemoprophylaxis before anti-TNF treatment strongly reduced the probability of developing TB [35], however in countries with higher tuberculosis incidence, a proportion of patients still reactivate or are newly infected during anti-TNF treatment despite initial chemotherapy for M. tuberculosis infection [17]. In such cases the anti-TNF treatment is stopped and chemotherapy for TB started, but anti-TNF treatment withdrawal during active TB has shown paradoxical and unexpected clinical situations with worsening of tuberculosis severity [36]. Our study shows that TB chemotherapy can be improved by co-administration of anti-TNF treatment during different phases of M. tuberculosis infection, suggesting that in latent TB reactivation appearing upon TNF neutralization, anti M. tuberculosis chemotherapy could be administered without stopping TNF inhibition. A clinical trial in HIV patients with pulmonary TB combining TNF inhibitors with anti-TB drugs reported that TNF inhibitors can be safely administrated during M. tuberculosis treatment and Enbrel treated patients showed higher responses to tuberculosis treatment with reduced time of clinical responses [25]. Our data also indicates that a combination of TNF inhibition with anti-TB drugs may shorten the duration of treatment which is an important clinical issue. Similar results have recently been published confirming that anti-TNF as adjunctive chemotherapy can improve bacterial elimination in a murine model of necrotic TB granuloma [37].
We show here that co-treatment with anti-TNF and M. tuberculosis chemotherapy resulted in reduced lung pathology and enhanced clearance of bacilli from the lung during acute and chronic infection. The improved response is likely explained by a better access of anti-TB drugs to the infected cells. Considering that TNF, which is an essential cytokine produced by infected macrophages and T cells, plays an important role in granuloma formation, it can be expected that inhibition of TNF results in disorganization of granulomas [20]. At present, whether alteration of granuloma structure is advantageous for the efficacy of anti-TB drugs and intracellular mycobacteria destruction needs further investigation. Furthermore, TNF neutralization diminishes the inflammatory response at early phase of mycobacterial infection which may be followed by an enhanced pathology at later infection contributing to bacillus overgrowth [38,39]. In addition, M. tuberculosis chemotherapy in acutely infected TNF deficient mice had an enhanced effect in eliminating mycobacteria compared to wild-type mice as evidenced by a more rapid and increased M. tuberculosis clearance suggesting that TNF interferes with the effect of anti-TB drugs and impairs bacterial killing during this early phase of the infection [40].
Co-treatment with an anti-mouse TNF antibody and INH during acute M. tuberculosis infection did not change the bacillary load as compared to INH alone, while it improved lung pathology [10]. The type of TNF inhibitor as well as the dose may account for the different results as the two categories of TNF blocking agents are known to act differently [41-43]. Enbrel is known to be less effective than anti-TNF antibodies for the treatment of certain human pathologies [41,44]. However, M. tuberculosis reactivation are less frequently reported in patients treated with Enbrel than with anti-TNF antibodies, although reactivation or enhanced sensitivity to TB has been observed with both therapeutic agents, and at different infectious status of patients [44,45]. Enbrel has been used in different mouse studies but it cannot be excluded that long term treatment can result in mouse antibody production against the human molecule. However, we have previously shown that blockade of soluble TNF and membrane TNF by Enbrel alone during murine acute M. tuberculosis infection compromised host defense, worsened lung pathology and increased bacillary burden proving their efficient TNF neutralizing activity during infection [29]. We also reported that selective inhibition of soluble TNF sparing membrane TNF by Dominant-Negative (DN)-TNF biologics preserves host immunity to M. tuberculosis infection yet maintaining a strong anti-inflammatory activity as mice are well protected from hepatitis [29,46]. Selective inhibition of soluble TNF by DN-TNF biologics (sparing membrane TNF) combined to M. tuberculosis chemotherapy would represent an interesting approach to better maintain host immunity during TB chemotherapy and needs further investigation to evaluate the efficacy of combined treatments for improving chemotherapy.
In conclusion, this work emphasizes the complex activity of TNF in mycobacterial infections which is clearly required for host defense mechanisms against mycobacteria, but may also provide evasion of mycobacteria in granulomas with reduced accessibility to chemotherapy. Therefore, TNF neutralisation with granuloma dissolution will increase efficacy of chemotherapy. This work strongly suggests that M. tuberculosis chemotherapy may be more efficacious in the presence of a TNF inhibitor to clear bacilli and reduce lung pathology, which may be considered in acute and chronic M. tuberculosis infection.
Acknowledgements
Financial support: Swiss National Science Foundation (grant 3200A0-118196), Ligue Pulmonaire Genevoise, CNRS and University of Orleans through International Associated Laboratory «TB IMMUNITY» (LIA N°236), and Le Studium, Orléans, France (Research fellowship to I.G.). The authors thank Pr. M. Jacobs for helpful discussion.
References
- 1.Organization WHO. Global tuberculosis report 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2.Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931–936. doi: 10.1056/NEJMra1205429. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med. 2005;11:S33–44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 5.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168:443–447. doi: 10.1164/rccm.200303-390OC. [DOI] [PubMed] [Google Scholar]
- 6.Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: a meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clin Infect Dis. 2009;49:1883–1889. doi: 10.1086/647944. [DOI] [PubMed] [Google Scholar]
- 7.Khasnobis S, Escuyer VE, Chatterjee D. Emerging therapeutic targets in tuberculosis: post-genomic era. Expert Opin Ther Targets. 2002;6:21–40. doi: 10.1517/14728222.6.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian V, Solapure S, Gaonkar S, Mahesh Kumar KN, Shandil RK, Deshpande A, Kumar N, Vishwas KG, Panduga V, Reddy J, Ganguly S, Louie A, Drusano GL. Effect of Coadministration of Moxifloxacin and Rifampin on Mycobacterium tuberculosis in a Murine Aerosol Infection Model. Antimicrob Agents Chemother. 2012;56:3054–3057. doi: 10.1128/AAC.06383-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 10.Koo MS, Manca C, Yang G, O’Brien P, Sung N, Tsenova L, Subbian S, Fallows D, Muller G, Ehrt S, Kaplan G. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One. 2011;6:e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbian S, Tsenova L, O’Brien P, Yang G, Koo MS, Peixoto B, Fallows D, Zeldis JB, Muller G, Kaplan G. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol. 2011;179:289–301. doi: 10.1016/j.ajpath.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–6961. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 14.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 15.Ehlers S. Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J Rheumatol Suppl. 2005;74:35–39. [PubMed] [Google Scholar]
- 16.Garcia I, Olleros ML, Quesniaux VF, Jacobs M, Allie N, Nedospasov SA, Szymkowski DE, Ryffel B. Roles of soluble and membrane TNF and related ligands in mycobacterial infections: effects of selective and non-selective TNF inhibitors during infection. Adv Exp Med Biol. 2011;691:187–201. doi: 10.1007/978-1-4419-6612-4_20. [DOI] [PubMed] [Google Scholar]
- 17.Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127–1132. [PubMed] [Google Scholar]
- 18.Bruns H, Meinken C, Schauenberg P, Härter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarty SD, Zhu G, Tsai MC, Mohan VP, Marino S, Kirschner DE, Huang L, Flynn J, Chan J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun. 2008;76:916–926. doi: 10.1128/IAI.01011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 22.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–268. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, Vary JC, Hawn TR, Dunstan SJ, Farrar JJ, Thwaites GE, King MC, Serhan CN, Ramakrishnan L. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, Ducar C, Millard M, Mayanja-Kizza H, Whalen C, Okwera A. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18:257–264. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 26.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old LJ. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171:3110–3118. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- 29.Olleros ML, Vesin D, Lambou AF, Janssens JP, Ryffel B, Rose S, Frémond C, Quesniaux VF, Szymkowski DE, Garcia I. Dominant-negative tumor necrosis factor protects from Mycobacterium bovis Bacillus Calmette Guerin (BCG) and endotoxin-induced liver injury without compromising host immunity to BCG and Mycobacterium tuberculosis. J Infect Dis. 2009;199:1053–1063. doi: 10.1086/597204. [DOI] [PubMed] [Google Scholar]
- 30.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 31.Dormans J, Burger M, Aguilar D, Hernandez-Pando R, Kremer K, Roholl P, Arend SM, van Soolingen D. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol. 2004;137:460–468. doi: 10.1111/j.1365-2249.2004.02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olleros ML, Guler R, Vesin D, Parapanov R, Marchal G, Martinez-Soria E, Corazza N, Pache JC, Mueller C, Garcia I. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–1120. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.du Toit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: current drug delivery approaches. Respir Res. 2006;7:118. doi: 10.1186/1465-9921-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, Milner EC, Miller CH, Dutcher PO, Hadley JA, Sanz I. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180:688–692. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Reino JJ, Carmona L, Angel Descalzo M. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum. 2007;57:756–761. doi: 10.1002/art.22768. [DOI] [PubMed] [Google Scholar]
- 36.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 37.Skerry C, Harper J, Klunk M, Bishai WR, Jain SK. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS One. 2012;7:e39680. doi: 10.1371/journal.pone.0039680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 1997;27:3182–3190. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 39.Guler R, Olleros ML, Vesin D, Parapanov R, Garcia I. Differential effects of total and partial neutralization of tumor necrosis factor on cell-mediated immunity to Mycobacterium bovis BCG infection. Infect Immun. 2005;73:3668–3676. doi: 10.1128/IAI.73.6.3668-3676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dambuza I, Keeton R, Allie N, Hsu NJ, Randall P, Sebesho B, Fick L, Quesniaux VJ, Jacobs M. Reactivation of M. tuberculosis infection in trans-membrane tumour necrosis factor mice. PLoS One. 2011;6:e25121. doi: 10.1371/journal.pone.0025121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ. Multiscale computational modeling reveals a critical role for TNF-alpha receptor 1 dynamics in tuberculosis granuloma formation. J Immunol. 2011;186:3472–3483. doi: 10.4049/jimmunol.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallis RS, Ehlers S. Tumor necrosis factor and granuloma biology: explaining the differential infection risk of etanercept and infliximab. Semin Arthritis Rheum. 2005;34:34–38. doi: 10.1016/j.semarthrit.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Wallis RS. Reactivation of latent tuberculosis by TNF blockade: the role of interferon gamma. J Investig Dermatol Symp Proc. 2007;12:16–21. doi: 10.1038/sj.jidsymp.5650031. [DOI] [PubMed] [Google Scholar]
- 45.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, Wallis RS, Sotgiu G, Schölvinck EH, Goletti D, Zellweger JP, Diel R, Carmona L, Bartalesi F, Ravn P, Bossink A, Duarte R, Erkens C, Clark J, Migliori GB, Lange C. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 46.Olleros ML, Vesin D, Fotio AL, Santiago-Raber ML, Tauzin S, Szymkowski DE, Garcia I. Soluble TNF, but not membrane TNF, is critical in LPS-induced hepatitis. J Hepatol. 2010;53:1059–1068. doi: 10.1016/j.jhep.2010.05.029. [DOI] [PubMed] [Google Scholar]


