Abstract
Interleukin 1 is a critical inflammatory mediator and involved in host defense to several pathogens. Oral T. gondii infection causes lethal ileitis in C57BL/6 (BL6) mice and serves to investigate the mechanisms of acute intestinal inflammation. Here we show that IL-1 is expressed upon oral T. gondii (76K strain) infection in the small intestine and mediates ileitis as IL-1R1 deficient mice have reduced neutrophil recruitment in the lamina propria, parasite invasion, inflammatory lesions and enhanced survival as compared to BL6 infected control mice. Protection in the absence of IL-1R1 signaling was associated with reduced IFN-γ expression and preserved Paneth cells, while these cells were eliminated in infected BL6 mice. Furthermore, blockade of IL-1 by IL-1β antibody attenuated inflammation in BL6 mice. In conclusion, IL-1 signaling contributes to the inflammatory response with increase IFN-γ expression and Paneth cell depletion upon oral T. gondii infection.
Keywords: Toxoplasma gondii, IL-1R1 receptor signaling, Paneth neutralizing antibody, inflammation, innate immunity
Introduction
Toxoplasma gondii is an opportunistic parasite with a worldwide distribution which triggers an innate immune response characterized by a rapid recruitment of neutrophils to the site of infection followed by a strong Th1 protective response associated with the production of proinflammatory cytokines, including IL-12 and TNF-α [1,2]. Neutrophils, dendritic cells and monocytes/macrophages are recruited and, the latter two populations are known to synthesize IL-12 early after the infection [3]. We reported previously that IL-17R signalling contributes to T. gondii induced fatal ileitis, since IL-17RA deficient mice are partially protected to T. gondii infection [4].
IL-1β is a potent mediator of acute inflammation and member of the IL-1 family consisting of IL-1α and β and the receptor antagonist, IL-1Ra, all the ligands bind to IL-1R1 which associates with IL-1Racp for cell activation [5]. IL-1β together with TGF-β induces IL-17A expression [6]. IL-1β has been shown to induce IL12 and IFN-γ in NK cells contributing to host resistance [3]. Using a mouse model of ileitis induced by oral infection with Toxoplasma gondii, it has been described that a crosstalk between IL-15 and IL-18 promoted intestinal recruitment of inflammatory monocytes, via their chemokine receptor CCR1, which was indispensable for their recruitment into the inflamed gut. These CD11b Ly6C monocytes produce copious amount of inflammatory cytokines such as IL-1, IL-6 and TNF-α [7].
In the present paper, we asked whether IL-1 contributes to T. gondii induced ileitis in mice. We report that T. gondii induced inflammatory changes and tissue damage in the ileum are diminished in IL-1R1-/- mice with enhanced survival as compared to BL6 mice, suggesting that IL-1 contributes to the pathology of T. gondii infection. Importantly, reduced IFN-γ production was associated with preserved Paneth cells in the absence of IL-1R signalling, which are depleted in infected BL6 mice. Moreover, IL-1β antibody blockade diminished T. gondii induced intestinal pathology in BL6 mice. Therefore, IL-1R1 signalling is involved in intestinal inflammation induced by T. gondii oral infection.
Materials and methods
Mice
C57BL/6 (BL6) wild type mice, IL-1R1-/- mice [8], IL-1α-/- and IL-1β-/- mice [9] were bred in our specific pathogen free animal facility at CNRS, Orleans, France. All Knockout (KO) mice were on the BL6 genetic background. Mice were maintained in a temperature-controlled (23°C) facility with a strict 12 h light/dark cycle and were given free access to food and water. The experiments were performed with gender-matched mice aged 8 - 10 weeks. All animal experimental protocols complied with the French ethical and animal experiments regulations (see Charte Nationale, Code Rural R 214-122, 214-124 and European Union Directive 86/609/EEC) and were approved by the “Ethics Committee for Animal Experimentation of CNRS Campus Orleans” (CCO), registered (N°3) by the French National Committee of Ethical Reflexion for Animal Experimentation (CLE CCO 2012-042).
T. gondii infection
T. gondii 76K stain cysts were prepared by homogenization, in PBS, of brain tissue extracted from infected CBA/J mice that had been orally infected with 100 cysts eight weeks earlier. Numeration of cysts was performed by counting 8 times 10 μL samples of this homogenate. The brain suspension containing cysts was diluted in order to contain 30 cysts for BL6 mice strain and 100 cysts for CBA/J mice strain per 200 μL and was administered intragastrically to each animal by gavage.
Infections of IL-1R1-/- mice and IL-1 antibody neutralization
BL6 and IL-1R1 deficient mice were orally infected with 30 cysts of the 76K strain as described above. Further, infected BL6 mice received an anti-IL-1β antibody (Dr H Gram, F Di Padova, Novartis Basel) administration (5 μg per mouse subcutaneously every days until the beginning of the infection).
The mice were analysed at day 7 for neutrophil recruitment in the ileum and morphological alterations of various organs.
RNA extraction and PCR in ileum
Ileum from control and infected BL6 mice was isolated and RNA was extracted. Total RNA were isolated from 100 mg of intestinal tissue previously snap-freezed in liquid nitrogen. We performed RNA extraction in two steps to obtain better quality. First, RNA was extracted with RNA TRIzol reagent (Sigma) and then purified using a commercial kit (RNeasy, Qiagen) following manufacturer’s instructions. The purified total RNA were used to generate first-strand cDNA by reverse transcription using 1 μg of total RNA, M-MuLV Reverse Transcriptase (MP Biomedicals) and a random hexamer. Semi-quantitative PCR were realised using 100 ng of cDNA, 20 μM of each forward and reverse primer, of dNTP 20 μM (MP Biomedicals) and Taq polymerase 1U (Amersham). HPRT expression was used to normalize the relative expression levels of IL-1α, IL-1β and IL-1Ra. The primers used in this study were obtained from Qiagen. Amplifications were performed as follows: IL-1α, IL-1β, IL-1Ra: 35 cycles, annealing temperature of 56°C; HPRT: 35 cycles, annealing temperature of 60°C.
Parasites in tissues were quantified by a semi quantitative PCR targeting a repetitive 529-bp cDNA fragment of T. gondii. Semi-quantitative PCR were realised using 100 ng of cDNA, 20 μM of each forward and reverse primer (TOX-9, 5′-AGGAGAGATATCAGGACTGTAG-3′; TOX-10, 5′-GCGTCGTCTCGTCTAGATCG-3′), of dNTP 20 μM (MP Biomedicals) and Taq polymerase 1U (Amersham). A standard curve was performed using 5000-5 pg T. gondii DNA to quantification of total cDNA of samples.
Cytokines and chemokines concentration measurement
The ileum (100 mg) was homogenized and supernatants were tested for IL-1α, IL-1β, IFN-γ, TNF-α and KC/CXCL1 using commercial ELISA kits (R&D systems) according to the manufacturer’s instructions.
Myeloperoxidase activity (MPO) in ileum
MPO activity was evaluated in tissues of the small intestines as previously described [4]. In brief, ileum was flush with saline to remove fecal content and frozen at -20°C until use. Ileum was homogenized in PBS by Ultra Turrax, centrifuged and the supernatant was discarded. The pellets were resuspended in 1 mL PBS containing 0.5% hexadecyltrimethyl ammonium bromide (HTAB) and 5 mM ethylene-diamine tetra-acetic acid (EDTA). Following centrifugation, 150 μL of supernatants were placed in test tubes with 200 μL PBS-HTAB-EDTA, 1 mL Hanks’ balanced salt solution (HBSS), 100 μL of o-dianisidine dihydrochloride (1.25 mg.mL-1), and 100 μL H2O2 0.05%. After 15 min of incubation at 37°C in an agitator, the reaction was stopped with 100 μL NaN3 1%. The MPO activity was determined as absorbance at 460 nm.
Microscopic investigations
The ileum was collected 7 days after the infection, fixed in 4% buffered formaldehyde and paraffin embedded under standard conditions. Tissue sections (3 μm) were stained with haematoxylin and eosin (HE). The inflammatory cell infiltrate, exudate, oedema and mucosal and epithelium destruction were assessed by a semi-quantitative score from 0 to 3 (with increasing extent). Phloxine tartrazine (PT) histological staining was performed and number and size of Paneth cells were assessed and scored from 0 to 3.
Lamina propria mononuclear cells (LPMC) isolation and FACS analysis
The small bowel was flushed with PBS, Payer’s patches and mesentery were removed and it was opened longitudinally. It was cut into 1 cm pieces and incubated in PBS/EDTA 3 mM during 20 min at 37°C under magnetic agitation. Pieces were then cut into 1 mm pieces and incubated in RPMI containing 0.5 mg/mL type IV collagenase (Life technologies), 1 ng/mL DNase (DN25, Sigma), 5% FCS (Perbio) and incubated 15 min at 37°C under magnetic agitation. Tissue debris and cell aggregates were removed by passage several times over a 10 mL syringe. Cells were filtered on 70 μm cells strainers and centrifuged 7 min at 1700 rpm. Cells pellets were resuspended in 40% percoll faction, overlayed on the top of a 80% percoll fraction and centrifuged 20 min at 3000 rpm without brake. LPMCs are collected in a white ring at the interphase of the two different percoll solutions and washed by RPMI 1640. Cells were then suspended in RPMI 1640 for experiments and 105 cells/mouse were stained by anti-CD11b PerCP Cy5.5 (Clone M1/70, BD pharmingen) and anti-Ly6G PE-Cy7 (Clone RBL6-8C5, eBioscience) antibodies or by control isotypes in presence of Fc Block (anti-CD32/CD16) (Clone 24.G2, BD pharmingen). FACS staining was assessed on a BD CANTO II cytometer and analysed with FlowJo Software.
Statistical analysis
The parametric 1-way ANOVA test with multiple Bonferroni’s comparison test was performed using the graphpad software. Values are expressed as mean ± SEM. Statistical significance was defined at a p-value < 0.05.
Results
T. gondii infection upregulates intestinal IL-1 expression and reduces Th1 cytokines
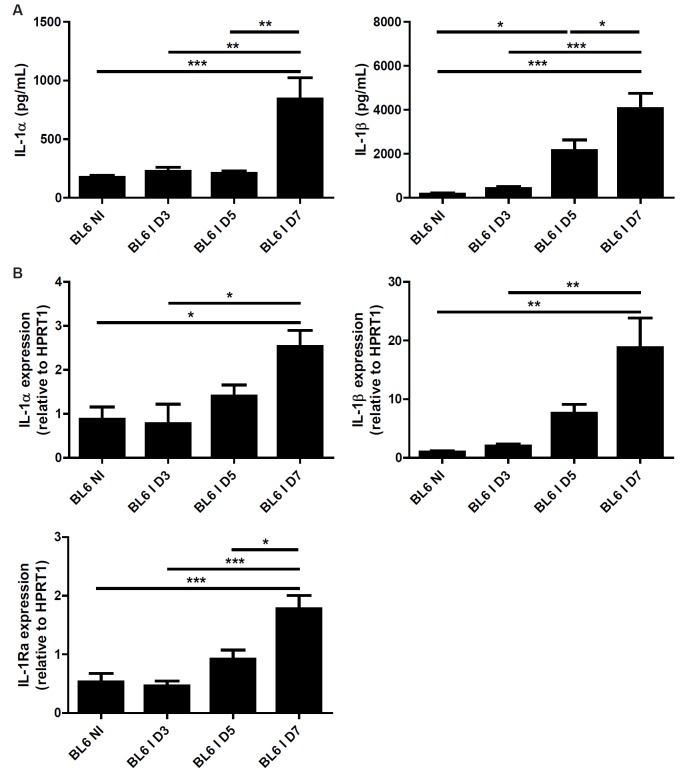
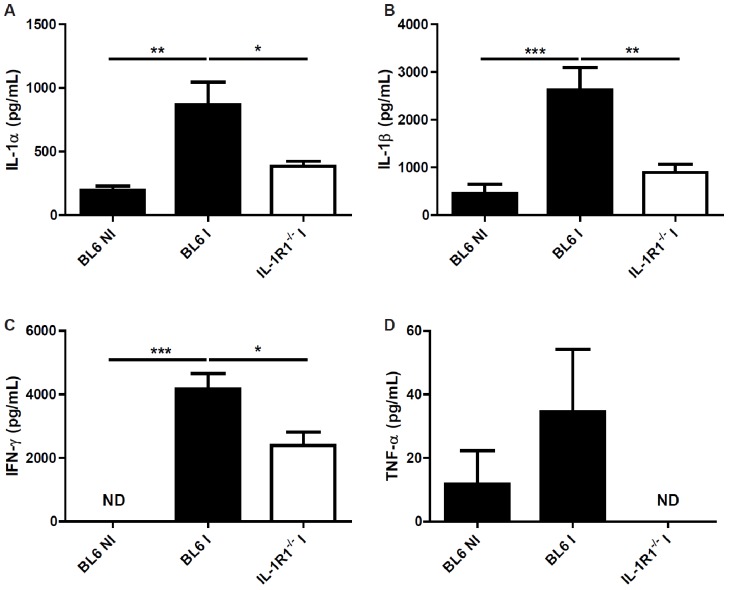
Oral inoculation with 30 cysts of T. gondii (76K strain) caused intestinal pathology with a robust inflammatory response [4]. IL-1 is an essential proinflammatory cytokine and we asked whether IL-1α or IL-1β are expressed in the intestinal wall upon infection. We found increased Il1a and Il1b mRNA and protein expression in the ileum 7 days after the inoculation (Figure 1A and 1B). The natural antagonist, IL-1Ra, is also upregulated after 5 days (Figure 5B), suggesting a homeostatic control. Oral infection with T. gondii causes a Th1 dependent inflammation in the intestine. We then asked whether the typical pro-inflammatory Th1 cytokine response is altered in the absence of IL-1R signalling. We analysed pro-inflammatory cytokines in the ileum of infected BL6 and IL-1R1 deficient mice. We found diminished IFN-γ and TNF-α production in the ileum homogenate from T. gondii infected mice at 7 days (Figure 2C and 2D). In addition IL-1α and IL-1β are reduced in IL-1R1-/- mice as compared with BL6 mice suggesting an autocrine loop (Figure 2A and 2B).
Figure 1.
T. gondii infection induces IL-1α and β expression in the ileum. IL-1α and IL-1β protein (A) and Il1a, Il1b and Il1ra mRNA (B) expression in the ileum of BL6 mice upon infection. Groups of 5 mice were infected (I: Infected mice) or not (NI: Non-infected mice) by gavage with 30 T. gondii 76K strain cysts, and the ileum was analyzed for gene and protein expression before and at 3, 5 and 7 days. Mean values and SEM on one representative out of two independent studies are presented. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 5.
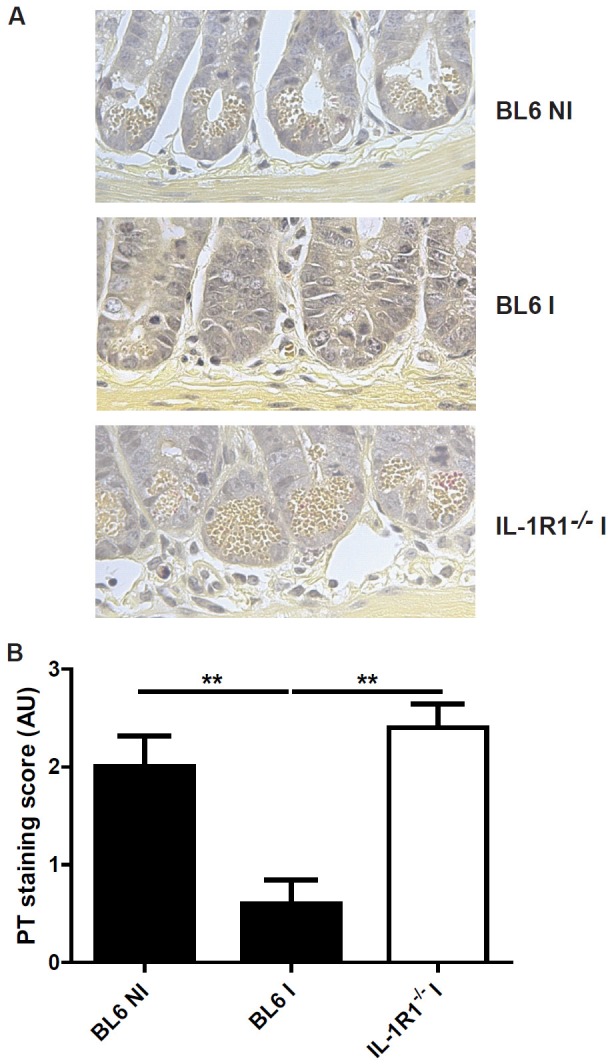
Reduction of Paneth cell depletion in the absence of IL-1R1 signaling. Paneth cell granules are visible in the crypt of non-infected (NI) and IL-1R1-/- infected (I) mice, but absent in BL6 infected mice (A). The difference was assessed with a semi-quantitative score (B). Groups of 5 mice were infected (I: Infected mice) or not (NI: Non-infected mice) by gavage with 30 T. gondii 76K strain cysts, and the ileum was analyzed at 7 days. Mean values and SEM on one representative out of three independent studies are presented. **P < 0.01.
Figure 2.
Reduced cytokine production in the absence of IL-1R1. IL-1α (A), IL-1β (B), IFN-γ (C) and TNF-α (D) protein level in the ileum of BL6 mice upon T. gondii infection. Groups of 5 mice were infected (I: Infected mice) or not (NI : Non-infected mice) by gavage with 30 76K cysts, and the ileum was analyzed for protein expression by ELISA at day 7. Mean values and SEM on one representative out of three independent studies are presented. *P < 0.05; **P < 0.01; ***P < 0.001.
Therefore, this reduced IFN-γ and TNF-α expression suggests an attenuated Th1 response in the absence of IL-1R1 signalling, in response to T. gondii.
Reduced T. gondii induced intestinal inflammation in IL-1R1 deficient mice
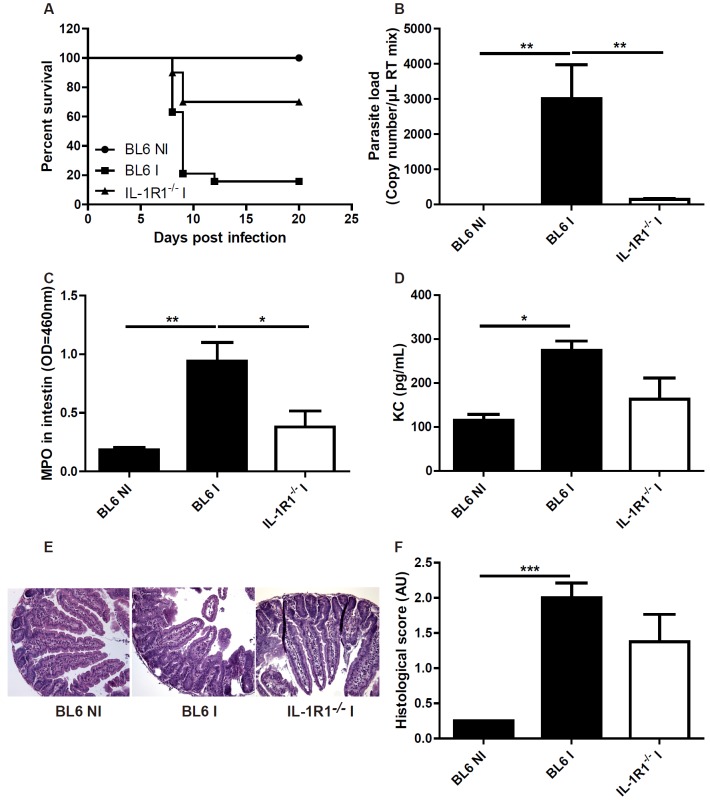
According to the diminished proinflammatory cytokine production in IL-1R1 deficient mice upon T. gondii infection, we expected a reduced intestinal inflammation in the absence of IL-1R1 signalling. BL6 mice infected orally with 30 cysts of T. gondii developed a severe ileitis with recruitment of neutrophils (MPO activity), increased expression of the chemokine KC/CXCL1, increased parasite load in the intestinal wall and rapid death (Figure 3A-D). Microscopy revealed acute ileitis in the ileum with oedema, erosions, ulcers and inflammatory cell infiltrate (Figure 3E, 3F).
Figure 3.
Attenuated ileitis due to T. gondii infection in IL-1R1 deficient mice. Enhanced survival IL-1R1-/- mice (A), reduced parasite load in ileum wall (B), reduced MPO activity and KC/CXCL1 protein expression (C, D) and attenuated microscopic inflammatory lesions in the ileum without IL-1R1 and semiquantitative score of inflammation (E, F). Groups of 5 mice were infected (I: Infected mice) or not (NI: Non-infected mice) and analyzed at day 7. Mean values and SEM on one representative out of three independent studies are presented. *P < 0.05; **P < 0.01; ***P < 0.001.
By contrast, ileitis was dramatically reduced in IL-1R1 deficient mice with reduced neutrophil recruitment (MPO activity), KC/CXCL1 production, parasite load, intestinal lesions and a significant enhanced survival (Figure 3A-F). Reduced intestinal inflammation and mucosal lesions might explain the low parasite uptake, since the inflammatory milieu enhances the invasion of the parasite tissues.
To discriminate which ligand was involved in the IL-1R1-mediated response to T. gondii, we orally infected IL-1α−/− and IL-1β−/− mice [9] and found that IL-1β-/- mice were protected, while IL-1α-/- succumbed to acute infection similarly to BL6 control mice (data not shown). Therefore, these data suggested that IL-1β signalling through IL-1R1 is essential for T. gondii induced intestinal inflammatory response.
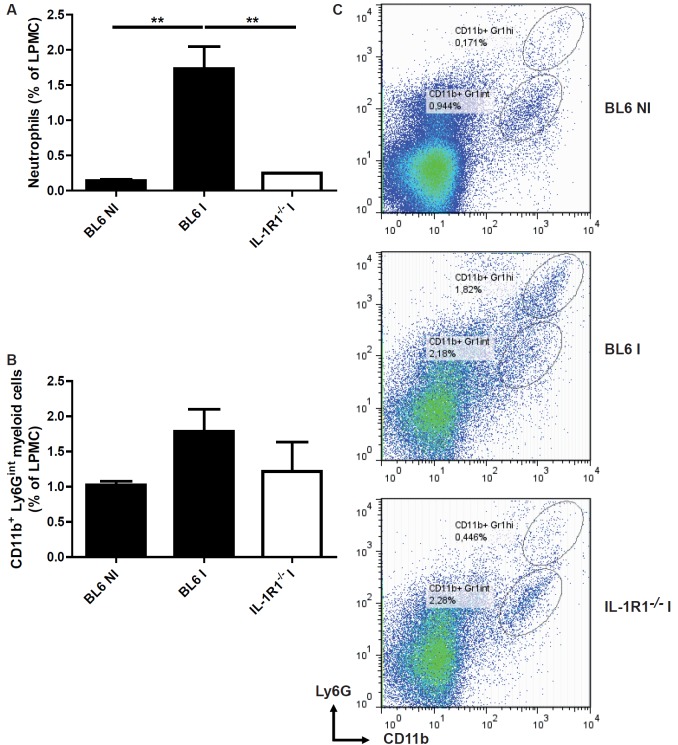
IL-1R1 dependent recruitment of neutrophils and inflammatory monocytes in the lamina propria
Since we demonstrated that the increased MPO activity and KC/CXCL1 expression upon T. gondii infection were dependent of IL-1R1 signaling pathway, we further characterize the inflammatory infiltrate in the ileum using flow cytometry. We observed that neutrophils (Figure 4A and 4C) and inflammatory CD11b+ Ly6Gint myeloid cells (Figure 4B, 4C) are recruited in the lamina propria at 8 days post infection in BL6 mice. We found a dramatic reduction of neutrophils (Figure 4B, 4C), but less of CD11b+ Ly6Gint myeloid cells (Figure 4B, 4C) in the absence of IL-1R1 signaling. Our data suggest that, in the absence of IL-1R1, reduced neutrophil recruitment may contribute to diminished ileitis.
Figure 4.
Diminished neutrophils and myeloid cells recruitment in the lamina propria upon T. gondii infection in IL-1R1 deficient mice. Lamina propria cells were isolated at day 8 after T. gondii infection, stained with Ly6G and CD11b antibody and analyzed by flow cytometry (C): Neutrophils (A) and CD11b+ Ly6Gint myeloid cells (B). Groups of 5 mice were used and mean values and SEM from one representative out of two independent studies are presented. **P < 0.01.
Paneth cell integrity in T. gondii infected IL-1R1 deficient mice
Paneth cells are critical regulators of intestinal homeostasis and produce several mediators including antimicrobial peptides. A recent study established that T. gondii infection induces a disappearance of Paneth cells in an IFN-γ dependent manner [10]. In the present study, we found that T. gondii infection causes a massive depletion of Paneth cells (Figure 5A). Semi-quantitative assessment confirms a drastic elimination of Paneth cells in T. gondii infected BL6 mice (Figure 5B). By contrast, degranulation and depletion of Paneth cells is largely prevented in IL-1R1-/- mice as compared to infected BL6 control mice (Figure 5), suggesting that IL-1R1 was required for Paneth cell loss.
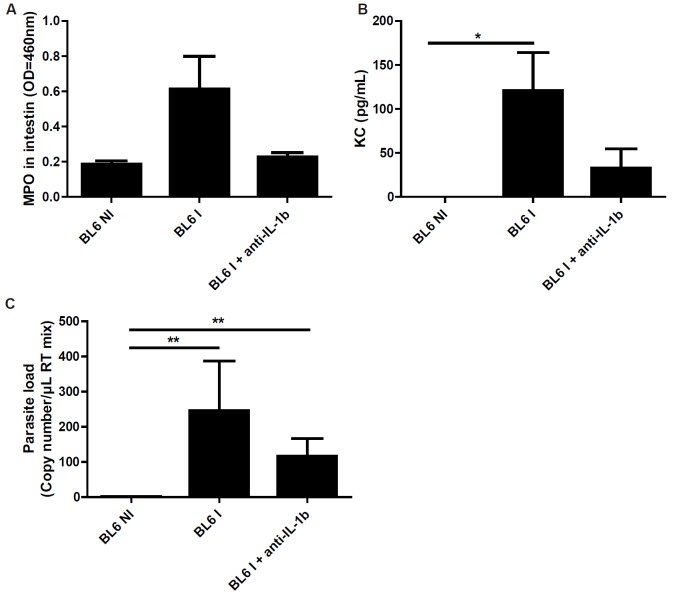
Neutralisation of IL-1β diminished T. gondii induced ileitis
Based on our results showing a dimished inflammatory response in IL-1R1-/- mice, we asked whether blockade of IL-1 through the use of neutralizing IL-1β antibody affects T. gondii inflammation in BL6 mice. We found that the administration of IL-1β antibody has a trend to reduced MPO and KC/CXCL1 production without altering parasite load (Figure 6A-D). By contrast, the administration of the IL-1R antagonist, Anakinra, had no effect, which is likely due to low bioavailability in vivo (data not shown). Therefore our data suggest that IL-1β neutralization diminishes T. gondii induced acute ileitis.
Figure 6.
Antibody neutralization of IL-1 attenuates ileitis in BL6 mice. IL-1β antibody reduces MPO activity (A), KC (B) in the ileum, and diminishes parasite load (C). Groups of 5 mice were infected (I: Infected mice) or not (NI: Non-infected mice) by gavage with 30 T. gondii 76K strain cysts, and the ileum was analyzed at 7 days. Mean values and SEM on one representative out of three independent studies are presented. *P < 0.05; **P < 0.01.
Discussion
The present study demonstrated that both IL-1α and IL-1β are upregulated and critically involved in the inflammatory response in the ileum upon oral T. gondii infection. Thus, the immunopathology of T. gondii ileitis shares key features of the inflammatory responses in inflammatory bowel disease (IBD) in humans and in models of experimental colitis in rodents [11]. Since IL-1 is upregulated in patients with Crohn’s disease [12], inhibitors of IL1 or neutralizing IL-1 antibodies might be beneficial in these patients.
We reported that inflammation, parasite invasion in the intestinal wall and recruitment of neutrophils in the ileum are dramatically reduced in the absence of IL-1R1 signalling. Furthermore, neutralization of IL-1β upon oral T. gondii infection attenuated the inflammatory pathology.
In the absence of IL-1R1 signalling, the Th1 response is attenuated with reduced IFN-γ and TNF-α production. In addition, we observed reduced KC/CXCL1 expression which could account for the diminished neutrophil recruitment resulting in partial protection of IL-1R1 deficient mice.
Recent reports suggested a critical role of inflammatory monocytes as effector cells of T. gondii-induced inflammation [13], although neutrophils contribute to the inflammatory pathology [14]. Here we confirmed increased monocytes/macrophages recruitment, but also neutrophils in accordance with previous reports showing that neutrophils depletion allowed controlling parasite replication and increasing survival of BL6 mice to acute infection induced by T. gondii. [1,14] Futhermore this neutrophils recruitment was reduced in the absence of IL-1R1 signalling.
Paneth cells play a critical role in intestinal homeostasis, especially through the production of antimicrobial peptides, and intestinal inflammation encountered in patients with inflammatory bowel disease (IBD) may deplete them [15]. The most striking finding of this study is that Paneth cells integrity is preserved in the absence of IL-1R1, while they are eliminated in infected BL6 mice. Recently it was reported that Paneth cells elimination upon T. gondii infection was dependent of IFN-γ production [10]. In view of the diminished IFN-γ expression observed in our study, we postulated that this IFN-γ dependent mechanism of Paneth cell ablation is abrogated in IL-R1 deficient mice.
Paneth cells are critical component of protective mucosal immunity with the production of antimicrobial peptides and several proinflammatory mediators including TNF and IL-17 [16]. However we were unable to detect those mediators in the infected intestines probably due to low sensitivity of the ELISA protein assays.
TLR9 and TLR11 mediate acute ileal inflammation in T. gondii model [10,17] and our results suggest that IL-1R1 can also be a key mediator for innate response to parasite by MyD88 signalling pathway.
In conclusion, our study established that IL-1 is upregulated upon oral T. gondii (76K strain) infection and contributes to ileitis based on diminished acute ileitis in IL-1R1 deficient mice. Infection mediated Paneth cell deletion was abrogated in the absence of IL-1R1 signaling and may be due to reduced IFN-γ expression.
Financial support
Région Centre (BR), EC FP6 programme EUMPODIC (VQ) and Fondation pour la Recherche Médicale (FRM to BR).
Conflict of interest statement
None declared.
References
- 1.Dunay IR, Sibley LD. Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr Opin Immunol. 2010;22:461–466. doi: 10.1016/j.coi.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011;27:388–393. doi: 10.1016/j.pt.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter CA, Chizzonite R, Remington JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 4.Guiton R, Vasseur V, Charron S, Arias MT, Van Langendonck N, Buzoni-Gatel D, Ryffel B, Dimier-Poisson I. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis. 2010;202:427–435. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 6.Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012;4:3–10. doi: 10.1093/jmcb/mjr042. [DOI] [PubMed] [Google Scholar]
- 7.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, Combadiere C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity. 2012;37:108–121. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 9.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, Gilpin CJ, Hooper LV, Yarovinsky F. Parasite-induced T(H)1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat Immunol. 2013;14:136–142. doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185(Suppl 1):S96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 12.Mahida YR, Lamming CE, Gallagher A, Hawthorne AB, Hawkey CJ. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991;32:50–54. doi: 10.1136/gut.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, Combadiere C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. Interleukin-15-Dependent NKp46(+) Innate Lymphoid Cells Control Intestinal Inflammation by Recruiting Inflammatory Monocytes. Immunity. 2012 Jul 27;37:108–21. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SF, Kwon JH, Boone DL. Novel players in inflammatory bowel disease pathogenesis. Curr Gastroenterol Rep. 2012;14:146–152. doi: 10.1007/s11894-012-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSorley SJ, Bevins CL. Paneth cells: targets of friendly fire. Nat Immunol. 2013;14:114–116. doi: 10.1038/ni.2519. [DOI] [PubMed] [Google Scholar]
- 17.Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]