SUMMARY
Heme-oxygenase-2 generates carbon monoxide in the enteric nervous system and in interstitial cells of Cajal in the canine, mouse and human jejunum. Carbon monoxide is considered a non-adrenergic and non-cholinergic inhibitory neurotransmitter and it establishes and maintains the resting membrane potential in the stomach and small intestine. The aim of this study was to determine the distribution of heme-oxygenase-2 in the enteric nervous system of the pig jejunum. Heme-oxygenase-2 immunoreactivity was found in neurons of myenteric ganglia and in nerve fibers in the circular and longitudinal muscle layers. These results suggest that carbon monoxide is produced in the enteric nervous system of the pig jejunum and might mediate inhibitory neural activity in myenteric ganglia and inhibitory neural input to smooth muscle cells in the circular and longitudinal muscle layers.
Keywords: enteric nervous system, heme-oxygenase-2, smooth muscle, non-adrenergic and non-cholinergic, pig jejunum
INTRODUCTION
Heme oxygenase (HO) generates carbon monoxide (CO), biliverdin and iron (Baranano and Snyder, 2001; Baranano et al., 2001). Three isoforms of HO - HO-1, HO-2 and HO-3 - have been identified. The constitutive isoform HO-2 appears to be responsible for CO synthesis in the gastrointestinal tract (Baranano and Snyder, 2001; Baranano et al., 2001) and its expression has been detected in enteric nerves in several species (reviewed in Gibbons and Szurszewski, 2004).
CO has been reported to hyperpolarize and relax circular smooth muscle of the small intestine in dog, human and mouse, internal anal sphincter circular smooth muscle in the opossum and circular smooth muscle of the lower esophageal sphincter and jejunum in pig by modulating a cGMP-dependent delayed rectifier K+ current (Colpaert et al., 2002; Farrugia et al., 1993; Farrugia et al., 1998; Farrugia et al., 2003; Rattan and Chakder, 2000; Zakhary et al., 1997).
CO has also been described in the mouse and opossum gastrointestinal tract as a non-adrenergic and non-cholinergic (NANC) inhibitory neurotransmitter (Farrugia et al., 1993; Rattan and Chakder, 1993) and in mouse, dog and human intestine as a hyperpolarizing agent that establishes and maintains the resting membrane potential (Farrugia et al., 2003; Sha et al., 2007).
The objective of the present study was to demonstrate by immunohistochemistry whether HO-2 is present in enteric nerves in pig jejunum using protein gene product (PGP) 9.5 as a marker of enteric neuronal network (Miller et al., 2001) and thus to determine whether CO might be a candidate gastrointestinal NANC transmitter in this species. Jejunum was chosen because it is often used to study the action of neurotransmitters in the gastrointestinal tract.
MATERIAL AND METHOD
Sample tissues were obtained from four adult pigs of either sex anesthetized with thiopental sodium following procedures approved by the Animal Care and Use Committee of the Mayo Clinic. After the abdomen was opened, a segment of 10 cm of jejunum ~20 cm distal to the ligament of Treitz was collected and placed in oxygenated Krebs solution at room temperature. A piece of jejunum measuring ~1 × 1 cm, containing the entire thickness of the intestinal wall was cut and immersed in freshly prepared 4% paraformaldehyde fixative overnight at 4 °C. The tissues were rinsed thoroughly in phosphate-buffered saline (PBS; 0.1 mol/L; pH 7.4), immersed overnight at 4 °C in PBS with 30% su crose, and frozen in isopentane at −40 to −50 °C. Cryostat sections, 12– 20 μm thick, were cut, thaw-mounted on gelatin–chrome alum-coated glass slides and air-dried. Approximately 20 sections were prepared from each tissue sample. Alternate sections were selected for immunolabelling. Methods and antibodies used for HO-2 and PGP 9.5 immunohistochemistry were as previously described by Farrugia et al., (1998) and Miller et al., (2001). Briefly, tissue sections were incubated in PBS containing 0.3% Triton X-100 and 10% normal donkey serum (NDS; Jackson Immuno Research Lab, Inc., West Grove, PA) in a humid chamber at room temperature for 60 min and then in rabbit polyclonal antiserum raised against HO-2 (StressGen Biochemicals, Victoria, Canada) or PGP 9.5 (Biogenesis, Kingston, NH) diluted 1:1000 in 5% NDS overnight at 4 °C. Sections were then incubated for 60-90 min at room temperature with CY3-labelled donkey anti-rabbit IgG (Jackson Immuno Research Lab, Inc.) diluted 1:100 in 2.5% NDS to visualize sites of HO-2 or PGP 9.5 immunoreactivity. Sections were washed thoroughly between each step using PBS. Sections were coverslipped in antifade mounting medium (InVitrogen Molecular Probes, Eugene, OR) and examined by epifluorescence (Zeiss Axiophot) or laser scanning confocal microscopy (Zeiss LSM 510; Zeiss, Thornwood, NY). In control experiments, no immunoreactivity was detected in sections incubated with only secondary antibody or when the primary antibodies were replaced with normal serum at the same dilution. The specificity of the HO-2 antibody has been previously demonstrated (Farrugia et al., 1998; Porcher et al., 1999).
For double HO-2 and PGP 9.5 immunolabeling experiments, tissue sections were first incubated for 1 h in PBS containing 10% normal goat serum (NGS; Jackson Immuno Research Lab, Inc.) and 0.3% Triton X-100 and then for 12 h at 4 °C in a mixture of HO-2 and PGP 9.5 antisera (guinea pig anti-PGP 9.5, Novus Biological Inc., Littleton, CO and rabbit anti-human HO-2, kind gift from Dr. Snyder, Johns Hopkins University, Baltimore, MA) diluted 1:500 and 1:1000, respectively in PBS containing 0.3% Triton X-100 and 5% NGS. After several rinses with PBS for 1 hour, the sections were incubated for 3 h at room temperature in a mixture of secondary antibodies (CY3 conjugated goat anti-guinea pig and FITC conjugated goat anti-rabbit, Jackson Immuno Research Lab, Inc.), both diluted 1:800 with PBS containing 2.5% NGS and 0.3% Triton X-100. Tissue sections were finally coverslipped in antifade mounting medium and examined by epifluorescence microscopy.
RESULTS
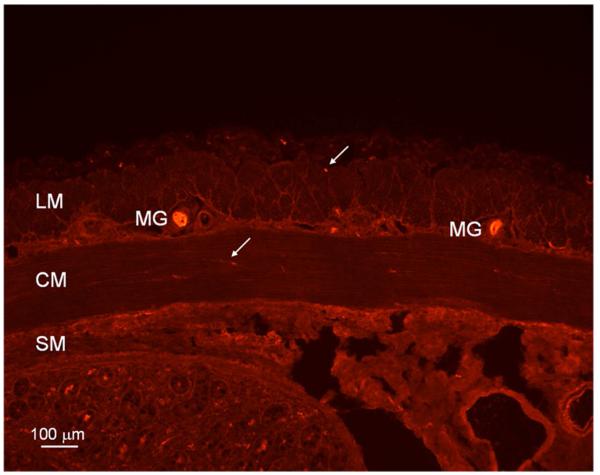
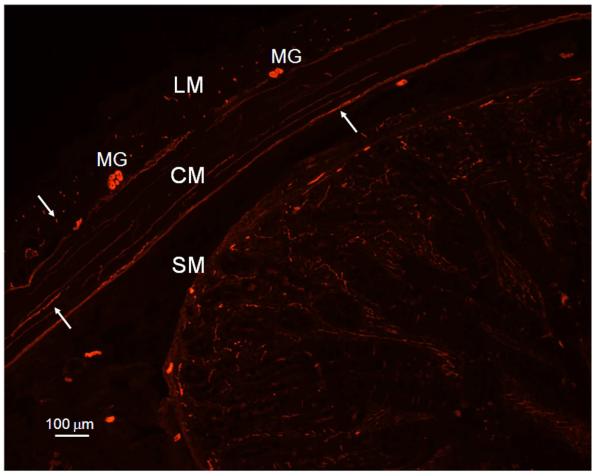
HO-2 immunoreactivity was localized in neuronal bodies in myenteric ganglia between the longitudinal and circular smooth muscle layers, in nerve-like fibers in the circular and longitudinal smooth muscle layers of pig jejunum (Figure 1). Figure 2 illustrates the distribution of PGP 9.5 immunopositivity in myenteric ganglia, long nerve fibers in the circular muscle layer and cross sections of nerve fibers in the longitudinal muscle layer similar to the distribution of HO-2 immunolabelling seen in Figure 1. Figure 3 illustrates that HO-2 and PGP 9.5 immunolabelling were co-localized in myenteric ganglion neurons.
Figure 1.
Heme-oxygenase-2 (HO-2) imunoreactivity in pig jejunum. HO-2 imunoreactivity is localized in neuronal bodies in myenteric ganglia (MG), in varicose nerves fibers (arrow) in circular muscle layer (CM) and cross section of nerve fibers (arrow) in longitudinal muscle layer (LM). Bar: 100 μm; SM: submucosa.
Figure 2.
Immunolocalization of PGP 9.5, a neuronal marker, reveals myenteric ganglia (MG), long nerve fibers (arrow) in the circular muscle layer (CM) and cross sections of nerve fibers (arrow) in the longitudinal muscle layer (LM) in the pig jejunum. Bar: 100 μm; SM: submucosa.
Figure 3.
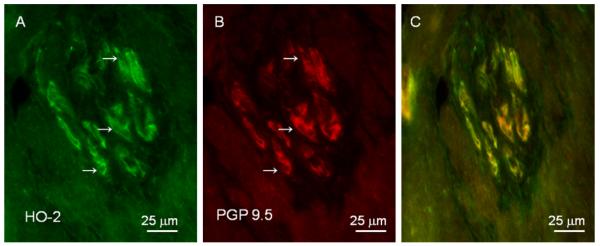
Double immunolabeling for HO-2 and PGP 9.5 in pig jejunum. HO-2 (A) and PGP 9.5 (B) are co-localized (C) in myenteric ganglion neurons (arrows). Bar: 25 μm.
DISCUSSION
In the gastrointestinal tract of several species including, mouse (Zakhary et al., 1996, 1997; Miller et al. 1998; Xue et al., 2000), rat (Donat et al., 1999), cat (Ny et al. 1996), dog (Farrugia et al., 1998), opossum (Battish et al., 2000), pig (Werkstrom et al., 1997; Colpaert et al. 2002) and humans (Porcher et al., 1999; Miller et al. 2001; Piotrowska et al., 2003) the CO producing enzyme, HO-2 is found in myenteric neurons and interstitial cells of Cajal. CO relaxes and hyperpolarizes gastrointestinal smooth muscle in different regions of the gastrointestinal tract, such as the lower esophageal sphincter, gastric fundus, jejunum and internal anal sphincter (Zahkary et al., 1997; Farrugia et al., 1998; Rattan and Chakder, 2000; Xue et al., 2000; Colpaert et al., 2002; Matsuda et al., 2004). CO plays a role in neurally-mediated nonadrenergic noncholinergic (NANC) relaxation of mouse ileum (Zakhary et al., 1997) and opossum internal anal sphincter (Rattan and Chakder, 2000) and in NANC hyperpolarization and relaxation of mouse jejunum (Xue et al., 2000). However, a previous study showed that CO was not a likely inhibitory mediator of neurotransmission in the pig jejunum (Matsuda et al., 2004).
Recently it has been suggested that endogenously generated CO, through the action of HO-2 in interstitial cells of Cajal, establishes and maintains the resting membrane potential gradient across the circular muscle layer in mouse, dog and human jejunum (Farrugia et al., 2003; Sha et al., 2007). Support for this concept has come from experiments using the HO-2 knockout mouse which showed that the gradient in resting membrane potential is abolished and that gastrointestinal motor activity is disordered (Xue et al., 2000; Zakhary et al., 1997) In humans, loss or damage to enteric neurons or interstitial cells of Cajal or both has been demonstrated in patients with diabetic gastrointestinal disorder, intestinal constipation and in Chagas’ disease of the colon (Adachi et al., 2008; Hagger et al., 2000; Lyford et al., 2002; Miller et al., 2008; Nakahara et al., 2002; Yu et al., 2002).
In summary, the present study shows that HO-2 is present in the enteric nervous system in the pig jejunum. Since CO does not appear to function as an inhibitory mediator of neural transmission in pig jejunum (Matsuda et al., 2004), we suggest that endogenously generated CO released from enteric neurons may help to establish and maintain a resting membrane potential gradient across the circular muscle layer as it does when it is released from the interstitial cells of Cajal in the dog stomach and in the dog, mouse and human jejunum. Further studies including direct measurement by intracellular recording of the resting membrane potential gradient across the circular muscle layer is necessary to test this hypothesis.
ACKNOWLEDGMENT
The authors thank Dr. Lei Sha for providing the image showing co-localization of HO-2 and PGP 9.5 in myenteric ganglia of pig jejunum. This work is supported by a grant from Fundação de Amparo a Pesquisas do Estado de São Paulo (FAPESP 2006/50084-2), by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 474531/2008-2) and by a grant from NIH (DK 17238).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi Y, Ishii Y, Yoshimoto M, Yoshida Y, Endo T, Yamamoto H, Akashi H, Imai K, Shinomura Y, Kato Y. Phenotypic alteration of interstitial cells of Cajal in idiopathic sigmoid megacolon. J Gastroenterol. 2008;43:626–631. doi: 10.1007/s00535-008-2207-4. [DOI] [PubMed] [Google Scholar]
- Baranano DE, Snyder SH. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci USA. 2001;98:10996–11002. doi: 10.1073/pnas.191351298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Battish R, Cao G-Y, Lynn RB, Chakder S, Rattan S. Heme oxygenase-2 distrubition in anorectum: co-localization with neuronal nitric oxide synthase. Am J Physiol. 2000;278:G148–G155. doi: 10.1152/ajpgi.2000.278.1.G148. [DOI] [PubMed] [Google Scholar]
- Colpaert EE, Timmermans JP, Lefebvre RA. Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus. Free Radic Biol Med. 2002;32:630–637. doi: 10.1016/s0891-5849(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Donat ME, Wong K, Staines WA, Krantis A. Heme oxygenase immunoreactive neurons in the rat intestine and their relationship to nitrergic neurons. J Auton Nerv Sys. 1999;77:4–12. doi: 10.1016/s0165-1838(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Irons WA, Rae JL, Sarr MG, Szurszewski JH. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993;264:G1184–1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol. 1998;274:G350–358. doi: 10.1152/ajpgi.1998.274.2.G350. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci USA. 2003;100:8567–8570. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol (Lond) 2004;556:325–336. doi: 10.1113/jphysiol.2003.056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger R, Finlayson C, Kahn F, De Oliveira R, Chimelli L, Kumar D. A deficiency of interstitial cells of Cajal in Chagasic megacolon. J Auton Nerv Syst. 2000;80:108–111. doi: 10.1016/s0165-1838(00)00076-x. [DOI] [PubMed] [Google Scholar]
- Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda NM, Miller SM, Sha L, Farrugia G, Szurszewski JH. Mediators of non-adrenergic non-cholinergic inhibitory neurotransmission in porcine jejunum. Neurogastroenterol Motil. 2004;16:605–612. doi: 10.1111/j.1365-2982.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology. 1998;114:239–244. doi: 10.1016/s0016-5085(98)70473-1. [DOI] [PubMed] [Google Scholar]
- Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121–131. doi: 10.1046/j.1365-2982.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- Miller SM, Narasimhan RA, Schmalz PF, Soffer EE, Walsh RM, Krishnamurthi V, Pasricha PJ, Szurszewski JH, Farrugia G. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol Motil. 2008;20:349–357. doi: 10.1111/j.1365-2982.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T, Shinomura Y, Matsuzawa Y. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–670. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- Piotrowska AP, Sokeri V, de Caluwe D, Puri P. Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J Ped Surg. 2003;38:73–77. doi: 10.1053/jpsu.2003.50014. [DOI] [PubMed] [Google Scholar]
- Porcher C, Orsoni P, Berdah S, Monges G, Mazet B. Distribution of heme oxygenase 2 in nerves and c-kit (+) interstitial cells in human stomach. Histochem Cell Biol. 1999;112:317–22. doi: 10.1007/s004180050453. [DOI] [PubMed] [Google Scholar]
- Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:G799–804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- Rattan S, Chakder S. Influence of heme oxygenase inhibitors in basal tissue enzymatic activity and smooth muscle relaxation of internal anal sphincter. J Pharmacol Exp Ther. 2000;294:1009–1016. [PubMed] [Google Scholar]
- Sha L, Farrugia G, Harmsen WS, Szurszewski JH. Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G438–445. doi: 10.1152/ajpgi.00037.2007. [DOI] [PubMed] [Google Scholar]
- Werkstrom V, Ny L, Persson K, Andersson K-E. Carbon-monoxide-induced relaxation and distribution of haem oxygenase isoenzymes in the pig urethra and lower oesophagogastric junction. Br J Pharmacol. 1997;120:312–318. doi: 10.1038/sj.bjp.0700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CS, Kim HC, Hong HK, Chung DH, Kim HJ, Kang GH, Kim JC. Evaluation of myenteric ganglion cells and interstitial cells of Cajal in patients with chronic idiopathic constipation. Int J Colorectal Dis. 2002;17:253–258. doi: 10.1007/s00384-001-0380-5. [DOI] [PubMed] [Google Scholar]
- Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U.S.A. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA. 1997;94:14848–14953. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]