Abstract
In November 2010, the iPrEx study reported that pre-exposure prophylaxis (PrEP) with daily tenofovir disoproxil fumarate/emtricitabine reduced HIV infections by 44% among men who have sex with men and subsequent trials corroborated efficacy among heterosexual men and women. During regularly scheduled follow-up visits from January-March 2011, participants in an ongoing phase 2b vaccine efficacy trial completed an anonymous web survey about PrEP. Among 376 respondents, 17% reported they were very likely to use PrEP in the next year. Non-white participants were more likely to use PrEP. Among those with some level of interest, intent to use PrEP was greatest if the drug were available through the clinical trial or health insurance. Most (91%) believed taking PrEP would not change their willingness to stay in the vaccine trial and few thought it would affect recruitment. As key stakeholders, currently enrolled trial participants can offer vital input about emerging prevention technologies that may affect the design of future HIV vaccine and non-vaccine prevention trials.
Keywords: clinical trials, HIV vaccines, pre-exposure prophylaxis, good participatory practice, HIV prevention
Introduction
Over the past few years, the HIV prevention landscape has rapidly changed, with several independent trials reporting the benefits of antiretroviral medications to reduce HIV acquisition and transmission.1-6 Guidelines for good participatory practice in biomedical HIV prevention trials highlight the need for protocol teams involved with ongoing studies, in close collaboration with community stakeholders, to assess new data and to determine how they may affect trial conduct.7 In late 2010, the global iPrEx trial reported that pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) reduced HIV infection by 44% in HIV-uninfected men who have sex with men (MSM) and transgender (TG) women at sexual risk for HIV acquisition.1 However, several questions remain concerning PrEP, including the regimen's long-term safety, and the lack of clear mechanisms for patients to access the drug and associated clinical monitoring in many settings. The HVTN 505 study is an ongoing, blinded, randomized and placebo-controlled phase 2b vaccine efficacy trial evaluating a DNA prime, adenovirus vector boost vaccine regimen in HIV-uninfected MSM and TG female participants from 21 clinical sites in the United States. After the iPrEx study results were released, the HVTN protocol team gathered input from several external stakeholder groups, and assessed the possible implications of these results on the vaccine trial's design. For example, if a substantial proportion of participants started taking PrEP, the observed HIV seroincidence in the enrolled cohort could decrease, requiring increases in sample size and/or longer follow-up to accrue the necessary number of incident infections to establish a reliable estimate of vaccine efficacy. While knowledge and use of PrEP in the community was limited before and immediately after the results were released, 8,9,10 awareness of and interest in taking PrEP by HVTN 505 participants were unknown. Thus, the HVTN 505 protocol team asked enrolled participants about their intention to use PrEP and assessed the potential impact of the iPrEx results on trial retention and recruitment.
Methods
From January through March 2011, enrolled HVTN 505 participants were invited to complete a short web-based survey during their regularly scheduled study visits. Given that the protocol requires visits every 3 months, a majority of enrolled participants had the opportunity to participate during this 3-month window. The brief survey assessed the perceived significance of the iPrEx results, personal intent to use PrEP, access to health care, and potential impact on study recruitment and retention. The supplemental survey was approved by the institutional review boards of participating research sites. To reinforce the anonymity of their responses, participants were informed that survey data were de-linked from other information collected as part of the HVTN 505 protocol, including questions about behavioral risks. Study staff offered a brief presentation of the iPrEx efficacy and safety results to participants prior to survey completion. Staff described the importance of daily adherence to PrEP and that HIV testing was required prior to PrEP initiation and at regular intervals during use; that a start-up syndrome including nausea was seen more commonly with FTC/TDF compared to placebo; that elevations in serum creatinine were observed, but improved with study drug discontinuation; and that longer-term side effects of PrEP, if any, were unknown. A four-point Likert scale was used to assess intent to use PrEP. Descriptive analyses were performed to assess participant intent to use and access to PrEP, perceived significance of the iPrEx study results, and their influence on continued participation or the participation of others in the HIV vaccine trial. Multiple logistic regression was performed to identify correlates of any intent to take PrEP including age, race/ethnicity, insurance status, and having a regular medical provider. Covariates were included in the model if significant on bivariate analysis with a p<0.1.
Results
Of the 693 active participants enrolled in HVTN 505 prior to January 1, 2011, 487 had a study visit during the 3 month survey period. Among those, 65 individuals were not offered the survey due to time constraints or omission, 41 provided incomplete survey data, and responses from the small number of self-identified transgender females (n=5) were excluded, giving a total of 376 (77%) for this analysis. The median age was 29 and a majority of respondents were white, non-Hispanic (73%); 51 respondents (14%) were African American, and 10% were Hispanic. The demographics of the respondents were comparable to the full HVTN 505 cohort at the time (data not shown). Three-fourths (74%) of respondents had health insurance, mostly through private insurers whereas only 6% of those insured had coverage through a public program. In this sample, 65% reported having a regular medical provider, and among those, only 4% had spoken to that provider about the PrEP results in the few months after they were released.
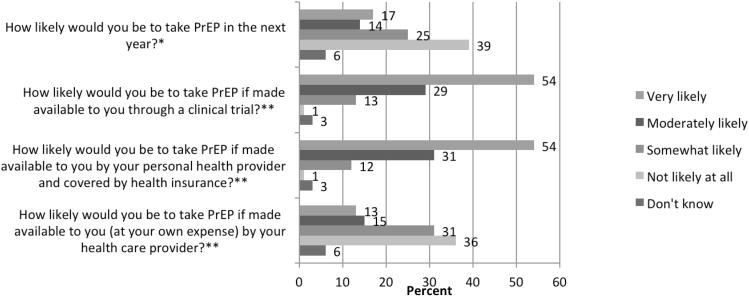
A majority of respondents (67%) reported that the PrEP results were either very (35%) or moderately (32%) important to them. Overall, 17% stated they were very likely to take PrEP in the next year, whereas 39% stated that they were not likely to take it at all (Figure). Among those who expressed any intent to take PrEP in the next year (n=231), 13% stated they would be very likely to take PrEP if required to pay out of pocket, whereas 54% would be very likely to take it if made available through the clinical trial, and 54% through a provider or health insurance. As seen in the table, in multiple logistic regression analysis, non-white identification was associated with any intent to use PrEP (Adjusted Odds Ratio 2.23, 95% CI 1.35-3.68, p=0.002). When asked “would taking PrEP change your willingness to stay in HVTN 505” almost all (91%) said it would not affect their participation in the trial. Moreover, only 16% felt that PrEP would affect others' willingness to enroll.
Figure 1. Likelihood of taking PrEP—overall and by method of PrEP access.
* Intent to use PrEP among all respondents (n=376)
** Respondents included those who were very, moderately, somewhat likely or didn't know if they intended to take PrEP in the next year (n=231)
Table. Correlates of intention to use PrEP* among HVTN 505 survey respondents (n=376).
| Characteristic | n | % | OR | 95%CI | p | Adj-OR | 95%CI | p |
|---|---|---|---|---|---|---|---|---|
| Age** | ||||||||
| 18 - 25 | 107 | 29 | Ref | Ref | ||||
| 26 - 34 | 152 | 41 | 1.11 | (0.68, 1.83) | 0.68 | 1.12 | (0.67, 1.87) | 0.67 |
| 35 - 50 | 115 | 31 | 1.37 | (0.80, 2.33) | 0.25 | 1.49 | (0.86, 2.58) | 0.15 |
| Race/Ethnicity** | ||||||||
| White, non-Hispanic | 274 | 73 | Ref | Ref | ||||
| Non-white | 101 | 27 | 2.33 | (1.43, 3.80) | <0.001 | 2.23 | (1.35, 3.68) | 0.002 |
| Type of Health Insurance | ||||||||
| Private | 254 | 68 | Ref | Ref | ||||
| Public | 24 | 6 | 2.32 | (0.93, 5.78) | 0.07 | 1.97 | (0.77, 5.06) | 0.16 |
| None | 98 | 26 | 1.69 | (1.04, 2.74) | 0.03 | 1.56 | (0.95, 2.56) | 0.08 |
| Has a Regular Medical Provider | ||||||||
| No | 132 | 35 | Ref | -- | -- | -- | ||
| Yes | 244 | 65 | 1.07 | (0.70, 1.65) | 0.74 | -- | -- | -- |
Any intent to use PrEP in the next year (somewhat, moderately, or very likely to use) compared to no intent.
Data were missing from respondents for age (n=2) and race/ethnicity (n=1). Non-white includes African American (n=51), Hispanic (n=37), American Indian or Alaskan Native (n=18), Asian (n=10), Native Hawaiian or Pacific Islander (n=1), or other race/ethnicity, not specified (n=19).
Discussion
We conducted a brief, web-based survey of MSM enrolled in an ongoing, blinded vaccine efficacy trial that offered a valuable snapshot of their interest in PrEP shortly after the iPrEx results were released. Our study showed that fewer than a fifth of respondents reported they would be very likely to take PrEP in the coming year. However, over half expressed strong interest in taking PrEP if it were provided through the trial or were available through their health care providers and covered by insurance. Notably, participants from communities of color were more likely to express intent to use PrEP. In addition, we found that PrEP would minimally influence enrollees' willingness to remain in HVTN 505 and that they believed it would be unlikely to hamper study recruitment.
To date, few studies nested within ongoing HIV prevention trials have assessed the perspectives of currently enrolled participants about emerging HIV prevention technologies, and as far as we are aware, none have produced results that directly influenced the design of the ongoing trial. In 2011, the sample size of HVTN 505 was expanded from 1350 to 2200 participants. This new enrollment target accounted for a conservative estimate of 20% PrEP uptake gleaned from our survey, and informed by the promising RV144 trial results,11 increased the statistical power to detect an impact on HIV acquisition as well as early viral load setpoint. Survey data supplemented the substantial input sought from community stakeholders that led to the decision to offer education about PrEP to study participants and to include active behavioral and biologic monitoring of PrEP use in the vaccine trial. In addition, the study team is working closely with trial sites to ensure community providers can accommodate referred participants interested in PrEP, and that HIV test results obtained at the site are readily shared with these providers to avoid misdiagnosis of HIV infection or unblinding to treatment assignment from vaccine-induced seropositivity (VISP).
While the stated willingness to use PrEP was modest in the months following the release of the iPrEx results, we recognize that demand for PrEP may evolve over time. This study and others10 have found that interest in PrEP use was affected by many contextual factors including perceptions about accessibility and cost. Since the survey went into the field, the US Centers for Disease Control and Prevention released interim guidance on PrEP 12 and new trial data were released in the summer of 2011 establishing the safety and efficacy of TDF/FTC in other populations including serodiscordant couples and at risk heterosexuals.3,4 On July 16, 2012, the U.S. Food and Drug Administration approved TDF/FTC to be taken daily as PrEP in combination with safer sex practices to reduce the risk of sexually-acquired HIV infection in adults at high risk.13 This new prevention indication is likely to spur both private and public insurers to cover the costs of PrEP.14 In addition, government-funded PrEP demonstration projects are being initiated or planned in several US cities which may increase access for some communities.
This study has some limitations. Participants represent a convenience sample of current HVTN 505 enrollees seen in the months following the release of the iPrEx results, which may not reflect the perspectives of the entire cohort, nor be generalizable to all MSM and TG communities at risk. This limitation is mitigated to a reasonable extent by the innovation of surveying participants shortly after the release of a major clinical research result and the desire for the study team to get valuable input from its enrollees which guided how HVTN 505 adapted to the iPrEx results. In addition, we may have underestimated the intent to take PrEP or to remain in the vaccine trial as some respondents may have been reluctant to disclose their interest in PrEP, believing it could threaten their ability to remain in the trial. We tried to reduce social desirability bias through the use of an anonymous, web-based survey and by reinforcing with our study volunteers that the decision to use PrEP would not in any way preclude continued involvement in the trial. Finally, several potential correlates of interest were not measured in this study, such as sexual risk behaviors, that have been shown to predict willingness to use PrEP.10 Planned analyses will consider these correlates in the assessment of actual PrEP use in HVTN 505.
These analyses reinforce the need for reliable mechanisms to access PrEP at low or no cost, particularly for minority American MSM.15-17 PrEP availability in these communities should be prioritized. While increased access may enhance PrEP uptake, providers have identified a number of challenges to PrEP implementation in clinical settings, including the need for additional training and definitive guidance from normative bodies.18 Furthermore, ongoing community concerns about adherence, risk compensation, viral resistance, and longer-term safety will likely influence the demand for PrEP as we await prospective data from open label studies.19,20 As our understanding of these issues continue to evolve, we have shown that it is feasible, and desirable, to gather input systematically from actively enrolled trial participants about new HIV prevention strategies. This input can directly inform changes to the trial's design and conduct. It is an exciting, and increasingly complex time in HIV prevention science as we determine how best to incorporate new tools to limit the spread of HIV. Future HIV vaccine trials, like prevention trials investigating other modalities, must thoughtfully consider these advances as researchers iteratively reevaluate the standards of prevention.21 Emerging data should be bolstered by effective and transparent engagement with key stakeholders, including trial participants.
Acknowledgments
We thank Dr. Albert Liu for his helpful comments on an early draft of this article. Additionally, we would like to gratefully acknowledge our study participants and the work and contributions of HVTN 505 trial site staff and community advisory boards.
Conflicts of Interest and Source of Funding: The HVTN is supported through a cooperative agreement with the National Institute of Allergy and Infectious Diseases (AI068614AI068635) which supports TM, DG, SK, MA, and GB. JF is supported under a Division of AIDS CTU award (UO1AI069496); MS and SH are supported under CTU and CTSA awards (UO1AI69470, UL1RR024156); KM is supported under CTU awards (UM1AI069480-05 and UO1AI069412) and unrestricted research grant support from Gilead Sciences, Inc. BK is supported under a CTU award (UO1AI69470).
HVTN 505 Protocol Team and Clinical Research Site Investigators (in addition to named authors): Annandale, MD-- Donald Poretz. Atlanta, GA—Mark Mulligan, Sri Edupunganti. Bethesda, MD-- Barney Graham, Julie Ledgerwood. Birmingham, AL—Paul Goepfert. Boston, MA—Lindsey Baden, Doug Krakower. Chicago, IL—Richard Novak. Cleveland, OH—Michael Lederman, Benigno Rodriguez. Dallas, TX—Mamta Jain. Denver, CO—Thomas Campbell. Houston, TX—Hana El Sahly. Los Angeles, CA-- Stephen Brown. Nashville, TN—Spyros Kalams. New York, NY—Demetre Daskalakis. Orlando, FL—Edwin DeJesus. Philadelphia, PA – Ian Frank. Rochester, NY-- Michael Keefer. San Francisco, CA—Susan Buchbinder, Darpun Sachdev. Seattle, WA -- Juliana McElrath, Janine Maenza. HIV Vaccine Trials Network-- Soyon Im, Jim Maynard, Niles Eaton, Steve Wakefield, Shelly Mahilum, Peter Gilbert, Jim Kublin, Larry Corey. National Institute of Allergy and Infectious Diseases—Elizabeth Adams. Community representatives-- Rick Church, Richard Trevino, Jason Roberts, Coco Alinsug.
Footnotes
Preliminary analyses were presented at AIDS Vaccine 2011, Bangkok, Thailand
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. Erratum, Science 2011;333:524.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;363 doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AVAC/UNAIDS. Good participatory practice: Guidelines for biomedical HIV prevention trials. 2011 [Google Scholar]
- 8.Liu AY, Kittredge PV, Vittinghoff E, Raymond HF, Ahrens K, Matheson T, Hecht J, Klausner JD, Buchbinder SP. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):241–7. [PubMed] [Google Scholar]
- 9.Mimiaga MJ, Case P, Johnson CV, Safren SA, Mayer KH. Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):77–83. doi: 10.1097/QAI.0b013e31818d5a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, Mayer KH. Limited Awareness and Low Immediate Uptake of Pre-Exposure Prophylaxis among Men Who Have Sex with Men Using an Internet Social Networking Site. PLoS One. 2012;7(3):e33119. doi: 10.1371/journal.pone.0033119. Epub 2012 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–20. doi: 10.1056/NEJMoa0908492. Epub 2009 Oct 20. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Interim Guidance: Preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011 Jan 28;60:65–68. [PubMed] [Google Scholar]
- 13.FDA approves first drug for reducing the risk of sexually acquired HIV infection. [Accessed 16 July 12]; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 14.Underhill Kristen, Operario Don, et al. Implementation science of pre-exposure prophylaxis: preparing for public use. Curr HIV/AIDS Rep. 2010;7(4):210–219. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prejean J, An Q, Mermin J, et al. Esitmated HIV incidence in the United States, 2006-2009. PloS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koblin B, Mayer K, Eshelman S, et al. Correlates of HIV incidence among black men who have sex with men in 6 U.S. cities (HPTN 061). [MOAC0106]. Presented at: XIX International AIDS Conference; 2012; Washington DC. [Google Scholar]
- 17.Mayer K, Wang L, Koblin B, et al. An evolving concentrated epidemic: comparison of socioeconomic, behavioural and biological factors among newly diagnosed, previously diagnosed and HIV-negative black men who have sex with men in six US cities (HPTN 061) [MOAC0105]. Presented at: XIX International AIDS Conference; 2012; Washington DC. [Google Scholar]
- 18.Arnold EA, Hazelton P, Lane T, Christopoulos KA, Galindo GR, Steward WT, Morin SF. A Qualitative Study of Provider Thoughts on Implementing Pre-Exposure Prophylaxis (PrEP) in Clinical Settings to Prevent HIV Infection. PLoS One. 2012;7(7):e40603. doi: 10.1371/journal.pone.0040603. Epub 2012 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibowitz AA, Parker KB, Rotherram-Borus MJ. A US policy perspective on oral preexposure prophylaxis for HIV. Am J Public Health. 2011;101(6):982–5. doi: 10.2105/AJPH.2010.300066. Epub 2011 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krakower D, Mayer KH. What primary care providers need to know about preexposure prophylaxis for HIV prevention: A narrative review. Ann Intern Med. 2012 Jul 22; doi: 10.7326/0003-4819-157-7-201210020-00510. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen M, McCauley M, Sugarman J. Establishing HIV treatment as prevention in the HIV Prevention Trials Network 052 randomized trial: an ethical odyssey. Clinical Trials. 2012;9:340–347. doi: 10.1177/1740774512443594. [DOI] [PMC free article] [PubMed] [Google Scholar]