Abstract
Systemic lupus erythematosus (SLE) is a chronic, disabling, progressive disease, with many associated comorbidities, affecting patients during prime working years resulting in a high economic burden on society, producing high direct, indirect and intangible costs.
In this article, our goals are two-fold. First, we review and discuss studies published in the period 2002–2012 concerning costs of SLE and point out gaps in the published literature. Second, we propose further research studies to advance our understanding of the economic perspective in SLE in the current area of new and emerging therapies.
The literature evaluating disease costs in SLE remains limited and to date has only included a small number of countries. Despite these limitations, available studies indicate that SLE has significant socio-economic ramifications. Future studies are needed, especially to assess novel biologic therapies which have been made available or currently under investigation for SLE. An interesting approach in these new economic evaluations in SLE may be represented by the selection of the targets of the treatment to include in the cost-effectiveness and cost-utility analyses. Future treat-to-target strategies will likely include evaluation of their pharmacoeconomic implications.
Keywords: systemic lupus erythematosus, costs, economic, burden of disease
Introduction
Systemic lupus erythematosus (SLE) is a chronic illness, affecting mainly young women during childbearing years (1). Over the past decades a marked increase in long term patient survival has been achieved (2). As patients live longer, attention to the damage resulting from the disease, and important co-morbidities of SLE and its therapies, such as cardiovascular disease, osteoporosis and cancer, has increased (3–7).
Recently, new drugs are being developed for the treatment of SLE to improve control of activity and quality of life, and reduce damage accrual and glucocorticoid usage (8). If these new therapies are shown to improve long-term patient outcomes, issues of cost-effectiveness are certain to arise, as has been seen with the introduction of biological agents in the treatment of rheumatoid arthritis (RA) and other rheumatic diseases (9–14).
In this article, our goals are two-fold. First, we review and discuss studies published in the period 2002–2012 concerning costs of SLE and point out gaps in the literature. Second, we propose further studies to advance understanding of the economic perspective in SLE in the current era of new and emerging therapies.
A systematic literature review of the cost dimension of SLE
The objective of this systematic literature review is to present data concerning the economic burden of this disease. The review includes papers published over the last decade and is designed in accordance with the recommendations of the Centre for Reviews and Dissemination (15) and of the Cochrane Collaboration (16), thereby using an established rigorous and reproducible methodology. A protocol was developed to define the review question.
Methods
Published studies in English language were searched using PubMed MEDLINE. The search was performed for the period June 2002–June 2012. This choice was made to obtain a modern perspective reflective of current disease outcomes, given advances in patient management as well as in treatment protocols over the last decade (17). The search strategy was as follows: (cost AND systemic lupus erythematosus) AND ((“2002/06/01”[PDat]:”2012/06/30”[PDat])AND Humans[Mesh] AND English[lang]).
Publications were assessed for inclusion by a three-step-process: i. titles and abstracts of all identified studies were assessed by one reviewer and re-evaluated by a second reviewer; ii. full texts of relevant articles were then obtained and inclusion criteria applied independently by two reviewers. Possible discordances between reviewers were resolved by consensus; iii. data were extracted by one reviewer and reevaluated by a second reviewer.
Inclusion criteria
Studies were included in the Systematic Literature Review based on the following inclusion criteria:
Studies: all reports on economic evaluation as cost analysis, cost minimisation analysis (CMA), cost effectiveness analysis (CEA), cost utility analysis (CUA) and cost benefit analysis (CBA).
Patients: all adult patients (≥18 years) regardless disease characteristic or organ manifestation.
Outcomes: direct costs, indirect costs, quality of life costs, and cost associated with flares.
Exclusion criteria
Studies not published in English, all publications before 2002, reviews, conferences proceedings, case reports, letters and commentaries were excluded. In addition, papers referring to the same cohorts of patients were excluded.
Papers included in the review
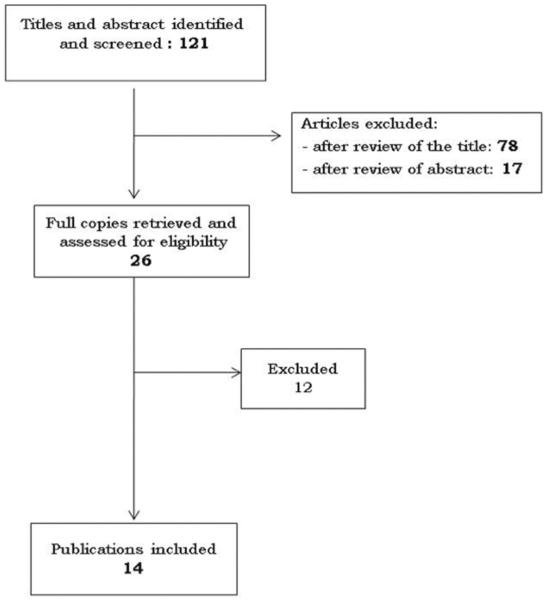
As of July 2012, 121 articles were extracted by the search procedure. After review of the titles, 43 relevant publications were identified. These were assessed for eligibility. After reading the abstract, 26 publications met inclusion criteria and were retrieved for full text review. Fourteen articles were included in the Systematic Literature Review (18–31). Twelve papers were excluded for the following reasons: reviews (32, 35–37, 39), did not provide economic data (33, 34, 38, 40–43).
None of the publications provided full economic evaluation (CMA, CEA, CUA and CBA). Among the studies, differences were present in terms of target population, study design, methodology, perspective and time horizon of the analysis.
The systematic literature review is based on 7 studies from the USA (20, 21, 23, 24, 27, 28, 30), 3 from Canada (19, 26, 27), 1 from Taiwan (18), 2 from Hong Kong (22, 25), and 1 from Germany (29).
Four included reports are retrospective (20–22, 25), 1 is cross-sectional (19), 3 are longitudinal studies (23, 26, 31), 1 is community based case-control (21), 1 is epidemiological (8) and 4 are not specified (18, 27, 29, 30).
Finally, 4 reports include only direct costs (18, 20, 23, 31), 7 analyse direct and indirect costs (19, 21, 22, 25–27, 29), 2 examine only indirect costs (24, 28), and 1 only hospitalisation costs (30).
Results
The examined publication only report a simple cost analysis as none includes data on cost-effectiveness, cost-benefit analysis or cost-utility analysis precluding a full economic evaluation. In addition, comparison of the results is made difficult by a large number of differences in the target population, severity of the disease studied, number of clinical manifestations and comorbidities considered, study design, perspective and time horizon of analysis, selection of costs/resources to be analysed, methodology used to monetise costs/resources, data sources (patient self-reported resource utilisation vs claims-based analyses; indemnity plans vs. Health maintenance organisation plans).
Although comparison between the studies is difficult, agreement exists on the fact that SLE patients consume more resources than healthy population.
Chiu et al. investigated the epidemiology and the medical costs of patients with SLE in Taiwan from January 2000 to December 2007. The authors report a mean expenditure for each outpatient service of US$71.5, and an expenditure for each hospitalisation of US$1,922.3. In 2007, the last year of analysis, the average of total yearly medical costs per patient was US$1,660 (18).
Carls et al. retrospectively estimate direct and indirect costs for SLE patients and a matched control group of patients who do not have SLE. The total direct medical expenditure in SLE group is $19,502 while in the control group it is $7,264. The indirect expenditure in terms of absenteeism costs in the 12 month study period is higher in the control group compared to SLE patients ($4,018 vs. $3,469) (21).
Zhu et al. retrospectively evaluated direct and indirect costs of SLE in patients with and without flares. Total costs for patients who did not have flares were $10,870 (direct costs: $6,034, indirect costs: $4,905), and for patients who had flares amounted to $22,580 (direct costs: $16,873, indirect costs: $5,756). The main costs involved inpatient care, accounting for 40% of the total direct costs in patients who did not have flares and 70% in patients with flares (22).
Huscher et al. evaluate the direct and indirect costs of illness in SLE patients and in other rheumatologic diseases. Data refer to 844 SLE patients enrolled in the national database of the German collaborative centres in 2002. Using different methods of assessment, indirect costs range between €6,518 and €14,411 and represent an important part of the total costs of SLE. Costs increase with increased disease duration ranging from €11,349 when the disease duration is <5 years to €17,695 when the disease duration is >10 years. No significant differences were observed between women and men (29).
Studies comparing costs between countries have shown that mean annual indirect costs in USA are $16,345, in Canada $11,101, and in UK $12,925. The mean 4-year cumulative indirect costs attributed to diminished labor market are $56,745 in USA, $38,642 in Canada, and $42,213 in UK. The indirect costs due to diminished non-labor market activity amount to $5,249, $5,455, and $8,572 respectively (21, 28).
In the tri-nation study assessing the health consumption and health status of SLE patients in Canada, USA and UK, 715 patients (Canada 231, USA 269, UK 215) followed in 6 tertiary care centres (two centres in each country) were evaluated. Mean cumulative direct costs per patient over 4 years were: $15,845 in Canada, $20,244 in USA and $17,647 in UK. Mean changes in SLICC/ACR DI were 0.49 in Canada, 0.63 in USA and 0.48 in UK. American patients incurred in higher healthcare expenditure, but did not experience superior health outcomes (31).
The impact and costs of hospitalisations has been evaluated by Krishnan who examined the hospitalisation and mortality outcomes of patients with SLE in the US from 1998 to 2002. The hospitalisation of patients with SLE includes 76,961 persons, of whom 8,710 (11% of the total population) have SLE as the main reason of admission; 3.1% of hospitalisations resulted in death. Patients with higher income and those with private insurance have better mortality outcomes than those with lower income or not having private insurance. The median hospital charge was $10,101, while the median charge for patients who died was $25,585 (30). As largely reported in the literature, SLE has an impact on patients quality of life and ability to work, this may determine high indirect costs. In this respect Campbell et al. analysed the impact of SLE in Carolina on employment, in particular work status and predictors of job loss, between 1997 in 1999. A follow up study was completed in 2001 (24). The study found that 26% of patients and 9% of subjects without SLE, who were working the year before the diagnosis or corresponding reference year had stopped working at follow-up. Ninety-two percent of patients compared to 40% of controls had stopped working due to their health status. The annual mean salary was higher in the control group compared to the patient group, $24,909 versus $21,540, respectively. The average salary loss for participants at risk of job loss was higher in patients compared to control subjects ($5,113 vs. $750, respectively). Among subjects who were working there were no differences in terms of median working hours per week and months per year; however, health-related absences from work in the previous year were higher among patients (median days: 10 vs. 6 in control group). Furthermore, 28% of patients compared to 7% of controls were unable to work for a period of 2 or more months.
Similarly Panopalis et al. estimate the healthcare costs associated with changes in work productivity from a societal perspective in 812 patients from the US. The rate of employment ranged from 76.8% in the year of diagnosis to 48.7% in the current year. Both direct healthcare costs and costs associated with changes in productivity are substantial and contribute significantly to the SLE total cost (27) (Table I).
Table I.
Annual direct healthcare costs.
| Author, year | Country | Results (Euro, 2012) |
|---|---|---|
| Chiu, 2010 (18) | Taiwan | Total study population |
| €1429 | ||
| Aghdassi, 2010 (19) | Canada | Patients LN vs. patients LNN |
| €10,646 vs. €8945 | ||
| Patients with active disease vs. patients without active disease (with lupus nephritis) | ||
| €12,020 vs. €7725 | ||
| Patients with active SLE vs. patients with inactive SLE (without lupus nephritis) | ||
| €10,703 vs. €6381 | ||
| Pellettier, 2009 (20) | USA | Patients with nephritis vs. patients without nephritis |
| €26,731 vs. €10,489 | ||
| Carls, 2009 (21) | USA | SLE group vs matched controls group |
| €18,749 vs. €6984 | ||
| Patients without nephritis vs. matched control group | ||
| €14,850 vs. €6556 | ||
| Patients with nephritis vs. matched control group | ||
| €56,136 vs. 11,082 | ||
| Zhu, 2009 (22) | Hong Kong | Patients with flares vs. patients without flares |
| €15,715 vs. €5666 | ||
| Li, 2009 (23) | USA | SLE patients vs. matched group vs. subgroup SLE with nephritis |
| €14,984 vs. €8622 vs. 25,578 | ||
| Zhu, Tam (25) | Hong Kong | SLE patients |
| €7628 | ||
| 0 NPSLE: €6218 | ||
| ≥1 NPSLE: €11,415 | ||
| With seizure: €20,316 | ||
| With CVD: €18,276 | ||
| With headache: €4613 | ||
| Clarke, 2008 (26) | Canada | Patients without renal damage vs. with renal damage |
| SLICC 0= €18,220 | ||
| SLICC 1= €24,969 | ||
| SLICC 2= €45,865 | ||
| SLICC 3= €89,187 | ||
| Panopalis, 2008 (27) | USA | Total study population |
| €11,775 | ||
| Huscher, 2006 (29) | Germany | SLE patients |
| €3413 | ||
| Clarke, 2004 (31) | Canada | Cumulative medical direct cost over 4 years |
| Canada: €15,661 | ||
| USA: €20,009 | ||
| UK: €17,443 |
SLE patients with active disease and lupus nephritis utilise more healthcare resources compared with patients with inactive disease and without lupus nephritis (19).
In a study comparing healthcare costs and the loss of productivity in 141 patients with SLE who had and did not have SLE nephritis, followed in rheumatology specialty centres in Canada between 2004 and 2009, the annual cost of patients with lupus nephritis was $12,597 versus $10,585 in those without lupus nephritis. In addition, a higher cost of $14,224 was observed for patients with active lupus nephritis versus $9,142 in those with inactive lupus nephritis (p<0,05). Additionally, a trend toward higher costs for patients with active disease but no nephritis with respect to patients with inactive disease was observed (12,666 versus 7,551). A higher loss of productivity was observed among caregivers of lupus nephritis patients (19).
Pellettier et al. compare healthcare resource utilisation and direct medical costs in patients with and without lupus nephritis over a period of 12 months identified from US health insurance between July 2006 and December 2008 (20). A total of 15,590 patients (1,068 patients with lupus nephritis and 14,522 without lupus nephritis) were included in the study. Total 12 month direct costs for all patients with SLE amount to more than US$207 million ($13,305 per patient). Patients with lupus nephritis consume more healthcare resources in terms of pharmacy services ($6,029 vs. $3,190), outpatient services ($15,267 vs. $6,202), and inpatient hospitalisation ($9,292 vs. $2,636, with a length of stay of 16.52 days vs. 9.69 days).
The longitudinal study of Li et al. assesses the long term medical costs for patients with SLE, a subset of patients with lupus nephritis and a matched reference group with the same number of persons without the disease. The mean annual medical expenditure in year 1 in SLE group is $16,089 versus $27,463 in lupus nephritis patients versus $9,258 in the group without the disease. The costs for SLE group decrease in year 2, and subsequently increase yearly at an average rate of 16% until year 5 ($23,860). Similarly, in SLE patients with LN, the cost decreases by 10% in year 2, followed by a yearly increase of 31%; at year 5 the annual medical cost amounts to $50,578 (23).
Furthermore, higher direct healthcare costs have been reported for patients with renal damage (25). In a subgroup analysis of the tri-nation study, Clarke et al. compare the costs and the quality of life (QoL) in SLE patients with and without renal damage (26). Seven hundred and fifteen patients were enrolled between July 1995 and February 1998 at 6 tertiary care SLE clinics in Canada (231), USA (269), and UK (215). The authors report the costs according to the renal item of the SLICC/ACR DI (Systemic Lupus International Collaborating Clinics/ACR Damage Index), which ranges from 0 to 3. The mean 4-year cumulative direct costs per patient ranges from $20,337 in SLICC/ACR DI = 0 to $99,544 in SLICC/ACR DI = 3. The mean 4-year cumulative indirect costs per patient ranges from $62,828 in group = 0 to $73,750 in group = 3. For patients with a renal subscale score of 3, the majority of total costs are absorbed by hospitalisation and dialysis.
Only one study assessed evaluated the direct and indirect costs of SLE and neuropsychiatric SLE (NPSLE) in 306 patients in Hong Kong, of these 26.8% had a total of 108 NPSLE events. Patients are divided in 5 groups: 0 NPSLE (n=223 patients), ≥1 NPSLE (n=83 patients), with seizure (n=12 patients), with CVD (n=11) and with headache (n=15 patients). Mean annual total costs in SLE patients amount to $13,307. Mean annual costs in 0 NPSLE are $11,124, in ≥1 NPSLE are $19,174, in patients with seizure are $28,560, in patients with CVD are $25,051 and in patients with headache are $7715. Mean direct costs are the following: in 0 NPSLE group $6,710, in ≥1 NPSLE $12,316, in patients with seizure are $21,920, in patients with CVD $19,719, and in patients with headache $4,977. From the study it emerges that patients with NPSLE incurred higher direct and indirect costs compared with patients without NPSLE (25).
To facilitate the comparison of the cost analysis of the studies included in the review, costs were converted to Euro-2012 (estimate at 17/07/2012). Costs in national currencies have been inflated to 2012 and currencies different from Euro were converted to Euros (Table I). The Consumer Price Index (http://www.bls.gov/bls/inflation.htm) and the Bank of Canada (http://www.bankofcanada.ca/rates/related/inflation-calculator/) were used respectively for inflating U.S. Dollars and Canadian dollars to 2012. Currency conversions from U.S. Dollars to Euro ($1=€0.81) and from Canadian Dollars to Euro ($CAN=0,80) were calculated as of 17/07/2012 (http://www.oanda.com/lang/it).
Gaps and limitations in the literature
SLE is a disease with a relevant economic burden on society, producing high direct, indirect and intangible costs. Direct costs of SLE include those related to professional fees, diagnostic procedures, and therapeutics provided in the emergency, hospital or outpatient setting. Indirect costs include changes in work productivity (absenteeism and presenteeism), unpaid work (work in the home), psychological impact and quality of life decrements. The detection and evaluation of these costs is not easy because SLE is a chronic disease, with periods of activity and periods of remission.
Although advances in therapy and management have occurred over the last decades, with a dramatic improvement of patient survival, SLE remains a morbid disease (43–46). Most patients accrue disease damage over time, and many have diminished quality of life (47). Poor outcomes are more common in racial/ethnic minorities and those with low socioeconomic status. Moreover, there are interesting studies reporting the social and work disability implications of SLE, measuring the number of working days lost. Between diagnosis and follow-up interview, the proportion employed declined from 74% to 54%. Over the same period, hours of work per year declined by 32.2% among all individuals with a work history, but by only 1% among those continuously employed (43). Until age 55 years, low rates of employment among persons with SLE may be due to lower rates of work entry rather than higher rates of work loss. Beyond age 55 years, both high rates of work loss and low rates of work entry contribute to low rates of employment (36). Rates of work loss were higher in those with cognitive impairment (28) and in those with incident thrombosis, musculoskeletal manifestations, and increased levels of disease activity (33).
Despite these facts, as we have shown, the literature evaluating disease costs remains limited and to date has only included a small number of countries. There are at least three main reasons – which are particularly important in public health care systems, where the government is the major payer – that may explain the paucity of data on economic implications: a) the low prevalence of the disease, compared to other chronic rheumatic diseases such as rheumatoid arthritis; b) the relatively modest medication costs for most drugs currently used for the condition; c) the high impact of indirect costs, that do not bear on the health care payer.
In particular, one of the difficulties in determining disease costs is that the results of cost of illness studies are dependent on how old the therapies are. In fact, with the exception of belimumab, all drugs used in SLE are old and off patent, this makes SLE seem cheaper than RA because RA has a lot of new, expensive drugs.
Moreover, most of the studies present too short a time horizon in respect to the chronic and progressive nature of SLE. Only a few articles consider a period longer than one year (23, 33). How does the progression of the disease affect resource utilisation and work disability over time? These questions require further analyses.
In spite of these limitations, available studies do illustrate that SLE has significant socio-economic ramifications. Future economic studies, especially those designed as long-term studies, including more detailed segmentation of SLE patients, more comprehensive sets of cost categories, and more sophisticated metrics for measuring QoL, are needed to provide important additional data (48).
Conclusion
SLE is a chronic, disabling, progressive disease, with many associated comorbidities, that affects patients during prime working years resulting in a high economic burden on society, producing high direct, indirect and intangible costs.
Few studies have addressed the socio-economic implications of SLE. Future studies are needed that take a more comprehensive approach to examine the socio-economic burden of SLE, one that carefully considers indirect costs as well as direct costs, and that adopts a longer time horizon, more coherent with the chronic and progressive nature of SLE. The need for more sophisticated economic analyses will continue to grow, especially in light of the number of novel biologic therapies that have entered the market or that are currently under investigation for SLE. As medication costs rise, payers, whether they are public or private entities, will be increasingly interested in evaluating the budget impact of new treatments and their cost-effectiveness in respect to standard therapies. What will be the impact of the new therapies on the other direct health care costs (visits, admissions, emergency accesses, costs of treating comorbidities, etc.), and on the indirect and intangible costs? How will these analyses affect reimbursement decisions? These are relevant questions for stakeholders.
Future studies should focus on the cost-effectiveness and cost-utility analyses of different therapeutic approaches, including a comprehensive set of costs. An interesting issue in these new economic evaluations in SLE may be represented by the selection of the targets of the treatment to include in the cost-effectiveness and cost-utility analyses (47). Future treat-to-target strategies will likely be evaluated with respect to their economic implications.
The current debate over new drugs increases the urgency for the economic research in SLE to progress rapidly.
Fig. 1.
PRISMA flow diagram for cost studies in SLE.
Table II.
Annual indirect costs.
| Author, year | Country | Results (Euro, 2012) |
|---|---|---|
| Carls, 2009 (21) | USA | SLE group vs. matched control group |
| Absenteeism costs in 12-mo study period | ||
| €3335 vs. €3863 | ||
| Short term disability costs in 12-mo study period | ||
| €2157 vs. €1019 | ||
| Patients without nephritis vs. matched control group | ||
| €5492 vs. €3935 | ||
| Patients with nephritis vs. matched control group | ||
| €5582 vs. €4747 | ||
| Zhu, 2009 (22) | Hong Kong | Patients with flares vs. patients without flares |
| €4568 vs. €5361 | ||
| Campbell, 2009 (24) | USA | SLE patients vs. matched control group |
| Average salary loss for all participants at risk of job loss | ||
| €5398 vs. €791 | ||
| Zhu, Tarn (25) | Hong Kong | SLE patients |
| €4705 | ||
| 0 NPSLE: €4091 | ||
| ≥1 NPSLE: €6357 | ||
| With seizure: €6154 | ||
| With CVD: €5127 | ||
| With headache: €2523 | ||
| Clarke, 2008 (26) | Canada | Patients without renal damage vs. with renal damage |
| SLICC 0= €56,290 | ||
| SLICC 1= €63,234 | ||
| SLICC 2= €60,011 | ||
| SLICC 3= €66,076 | ||
| Panopalis, 2007 (28) | USA | SLE patients |
| USA: €17,059 | ||
| Canada: €11,586 | ||
| UK: €13,490 |
Footnotes
Competing interests: none declared.
References
- 1.AGMON-LEVIN N, MOSCA M, PETRI M, SHOENFELD Y. Systemic lupus erythematosus one disease or many? Autoimmun Rev. 2012;11:593–5. doi: 10.1016/j.autrev.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 2.MAK A, CHEUNG MWL, CHIEW HJ, LIU YL, CHUN.MAN HO R. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthitis Rheum. 2012;41:830–49. doi: 10.1016/j.semarthrit.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.MOSCA M, TANI C, ARINGER M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69:1269–74. doi: 10.1136/ard.2009.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GOVONI M, BOMBARDIERI S, BORTOLUZZI A, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a riskprofile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology. 2012;51:157–68. doi: 10.1093/rheumatology/ker310. [DOI] [PubMed] [Google Scholar]
- 5.TANI C, CARLI L, MOSCA M, et al. Adherence to cervical cancer screening in an Italian SLE cohort. Reumatismo. 2011;63:11–7. doi: 10.4081/reumatismo.2011.11. [DOI] [PubMed] [Google Scholar]
- 6.DI MUNNO O, MAZZANTINI M, DELLE SEDIE A, MOSCA M, BOMBARDIERI S. Risk factors for osteoporosis in female patients with systemic lupus erythematosus. Lupus. 2004;13:724–30. doi: 10.1191/0961203303lu1097oa. [DOI] [PubMed] [Google Scholar]
- 7.MOSCA M, TANI C, CARLI L, BOMBARDIERI S. Glucocorticoids in systemic lupus erythematosus. Clin Exp Rheumatol. 2011;29(Suppl. 68):126–9. [PubMed] [Google Scholar]
- 8.LATEEF A, PETRI M. Biologics in the treatment of systemic lupus erythematosus. Curr Opin Rheumatol. 2010;22:504–9. doi: 10.1097/BOR.0b013e32833b475e. [DOI] [PubMed] [Google Scholar]
- 9.TURCHETTI G, SCALONE L, DELLA CASA ALBERIGHI O, et al. The rationale of pharmacoeconomic analysis in rheumatologic indications. Clin Exp Rheumatol. 2012;30(Suppl. 73):S64–S71. [PubMed] [Google Scholar]
- 10.FURNERI G, MANTOVANI LG, BELISARI A, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(Suppl. 73):S72–S84. [PubMed] [Google Scholar]
- 11.CORTESI PA, SCALONE L, D'ANGIOLELLA L, et al. Systematic literature review on economic implications and pharmacoeconomic issues of psoriatic arthritis. Clin Exp Rheumatol. 2012;30(Suppl. 73):S126–S131. [PubMed] [Google Scholar]
- 12.TRIESTE L, PALLA I, FUSCO F, et al. The economic impact of gout: systematic literature review. Clin Exp Rheumatol. 2012;30(Suppl. 73):S145–S148. [PubMed] [Google Scholar]
- 13.PALLA I, TRIESTE L, TANI C, et al. A systematic literature review of the economic impact of ankylosing spondylitis. Clin Exp Rheumatol. 2012;30(Suppl. 73):S136–S141. [PubMed] [Google Scholar]
- 14.TRIESTE L, PALLA I, BALDINI C, et al. Systemic vasculitis: how little we know about their societal and economic burden. Clin Exp Rheumatol. 2012;30(Suppl. 73):S154–S156. [PubMed] [Google Scholar]
- 15.Centre For Reviews And Dissemination . CRD's guidance for undertaking reviews in health care. University of York; 2008. [Google Scholar]
- 16.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell; 2008. [Google Scholar]
- 17.HOUSSIAU FA, VASCONCELOS C, D'CRUZ D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–31. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 18.CHIU YM, LAI CH. Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus. 2010;19:1250–5. doi: 10.1177/0961203310373780. [DOI] [PubMed] [Google Scholar]
- 19.AGHDASSI E, ZHANG W, ST-PIERRE Y, et al. Healthcare cost and loss of productivity in a canadian population of patients with and without lupus nephritis. J Rheumatol. 2011;38:658–66. doi: 10.3899/jrheum.100482. [DOI] [PubMed] [Google Scholar]
- 20.PELLETIER E, OGALE S, YU E, BRUNETTA P, GARG J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a us claims database. Clinical Ther. 2009;31:2653–264. doi: 10.1016/j.clinthera.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 21.CARLS G, LI T, PANOPALIS P, et al. Direct and Indirect Costs to Employers of Patients With Systemic Lupus Erythematosus With and Without Nephritis. J Occup Environ Med. 2009;51:66–79. doi: 10.1097/JOM.0b013e31818a405a. [DOI] [PubMed] [Google Scholar]
- 22.ZHU T, TAM L, LEE VW, LEE KK, LI EK. The Impact of Flare on Disease Costs of Patients With Systemic Lupus Erythematosus. Arthritis Rheum. 2009;61:1159–67. doi: 10.1002/art.24725. [DOI] [PubMed] [Google Scholar]
- 23.LI T, CARLS G, PANOPALIS P, WANG S, GIBSON TB, GOETZEL RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large medicaid population. Arthritis Rheum. 2009;61:755–63. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 24.CAMPBELL R, COOPER G, GILKESON GS. The impact of Systemic Lupus Erythematosus on employment. J Rheumatol. 2009;36:2470–5. doi: 10.3899/jrheum.080586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ZHU T, TAM L LEE VW, LEE KK, LI EK. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost-of-illness study in Hong Kong. Rheumatology. 2009;48:564–8. doi: 10.1093/rheumatology/kep031. [DOI] [PubMed] [Google Scholar]
- 26.CLARKE A, PANOPALIS P, PETRI M, et al. SLE patients with renal damage incur higher health care costs. Rheumatology. 2008;47:329–33. doi: 10.1093/rheumatology/kem373. [DOI] [PubMed] [Google Scholar]
- 27.PANOPALIS P, YAZDANY J, GILLIS JZ, et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 2008;59:1788–95. doi: 10.1002/art.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PANOPALIS P, PETRI M, MANZI S, et al. The systemic lupus erythematosus tri-nation study: cumulative indirect costs. Arthritis Rheum. 2007;57:64–70. doi: 10.1002/art.22470. [DOI] [PubMed] [Google Scholar]
- 29.HUSCHER D, MERKESDAL S, THIELE K, et al. Cost of illness in rheumatoid arthritis, anky-losing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65:1175–83. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.KRISHNAN E. Hospitalization and mortality of patients with systemic lupus erythematosus. J Rheumatol. 2006;33:1770–4. [PubMed] [Google Scholar]
- 31.CLARKE AE, PETRI M, MANZI S, et al. The systemic lupus erythematosus Tri-Nation Study: absence of a link between health resource use and health outcome. Rheumatology. 2004;43:1016–24. doi: 10.1093/rheumatology/keh229. [DOI] [PubMed] [Google Scholar]
- 32.ZHU TY, TAM LS, LI EK. The socioeconomic burden of systemic lupus erythematosus: state of art and prospects. Expert Rev Pharmacoecon Outcomes Res. 2012;12:53–69. doi: 10.1586/erp.11.92. [DOI] [PubMed] [Google Scholar]
- 33.YELIN E, TONNER C, TRUPIN L, et al. Longitudinal study of the impact of incident organ manifestations and increased disease activity on work loss among persons with systemic lupus erythematosus. Arthritis Care Res. 2012;64:169–75. doi: 10.1002/acr.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WALDRON N, BROWN S, HEWLETT S, ELLIOTT B, MCHUGH M, MCCABE C. `It's more scary not to know': a qualitative study exploring the information needs of patients with systemic lupus erythematosus at the time of diagnosis. Musculoskeletal Care. 2011;9:228–38. doi: 10.1002/msc.221. [DOI] [PubMed] [Google Scholar]
- 35.SLAWSLY KA, FERNANDES AW, FUSFELD L, MANZI S, GOSS TF. A structured literature review of the direct costs of adult systemic lupus erythematosus in the US. Arthritis Care Res. 2011;63:1224–32. doi: 10.1002/acr.20502. [DOI] [PubMed] [Google Scholar]
- 36.ZHU TY, TAM LS, LI EK. Cost of illness studies in systemic lupus erythematosus: a systematic review. Arthritis Care Res. 2011;63:751–60. doi: 10.1002/acr.20410. [DOI] [PubMed] [Google Scholar]
- 37.MAK A. The economic burden of systemic lupus erythematosus in Asia: the current state. Lupus. 2010;19:1442–6. doi: 10.1177/0961203310374308. [DOI] [PubMed] [Google Scholar]
- 38.WOLFE F, MICHAUD K, LI T, KATZ RS. EQ-5D and SF-36 Quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, non inflammatory rheumatic disorders, and fibromyalgia. J Rheumatol. 2010;37:296–304. doi: 10.3899/jrheum.090778. [DOI] [PubMed] [Google Scholar]
- 39.LAU CS, MAK A. The socioeconomic burden of SLE. Nat Rev Rheumatol. 2009;5:400–4. doi: 10.1038/nrrheum.2009.106. [DOI] [PubMed] [Google Scholar]
- 40.YELIN E, TONNER C, TRUPIN L, et al. Work loss and work entry among persons with systemic lupus erythematosus: comparisons with a national matched sample. Arthritis Rheum. 2009;61:247–58. doi: 10.1002/art.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CAMPBELL R, JR., COOPER GS, GILKESON GS. Two aspects of the clinical and humanistic burden of systemic lupus erythematosus, mortality risk and quality of life early in the course of disease. Arthritis Rheum. 2008;59:458–64. doi: 10.1002/art.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.COOPER GS, TREADWELL EL, ST CLAIR EW, GILKESON GS, DOOLEY MA. Sociodemographic associations with early disease damage in patients with systemic lupus erythematosus. Arthritis Rheum. 2007;57:993–9. doi: 10.1002/art.22894. [DOI] [PubMed] [Google Scholar]
- 43.YELIN E, TRUPIN L, KATZ P, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NOSSENT J, KISS E, ROZMAN B, et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus. 2010;19:949–56. doi: 10.1177/0961203310366572. [DOI] [PubMed] [Google Scholar]
- 45.MOSCA M, TANI C, ARINGER M, et al. Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice. Autoimmun Rev. 2011;10:383–8. doi: 10.1016/j.autrev.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ARINGER M, STRAND V. Endpoints for randomised controlled trials in systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:147–51. [PubMed] [Google Scholar]
- 47.MOSCA M, BOUMPAS D, BRUCE IN, et al. Treat-to-target in systemic lupus erythematosus: where are we today? Clin Exp Rheumatol. 2012;30(Suppl. 73):S112–S115. [PubMed] [Google Scholar]
- 48.TURCHETTI G, SPADONI E, GEISLER E. Health technology assessment. Evaluation of biomedical innovative technologies. IEEE Engineering in Medicine and Biology Magazine. 2010;29:70–6. doi: 10.1109/MEMB.2010.936553. [DOI] [PubMed] [Google Scholar]