Abstract
Purpose of review
Myocardial remodeling driven by excess pressure and volume load is believed to be responsible for the vicious cycle of progressive myocardial dysfunction in chronic heart failure. Left ventricular assist devices (LVADs), by providing significant volume and pressure unloading, allow a reversal of stress-related compensatory responses of the overloaded myocardium. Herein, we summarize and integrate insights from studies which investigated how LVAD unloading influences the structure and function of the failing human heart.
Recent findings
Recent investigations have described the impact of LVAD unloading on key structural features of cardiac remodeling – cardiomyocyte hypertrophy, fibrosis, microvasculature changes, adrenergic pathways and sympathetic innervation. The effects of LVAD unloading on myocardial function, electrophysiologic properties and arrhythmias have also been generating significant interest. We also review information describing the extent and sustainability of the LVAD-induced myocardial recovery, the important advances in understanding of the pathophysiology of heart failure derived from such studies, and the implications of these findings for the development of new therapeutic strategies. Special emphasis is given to the great variety of fundamental questions at the basic, translational and clinical levels that remain unanswered and to specific investigational strategies aimed at advancing the field.
Summary
Structural and functional reverse remodeling associated with LVADs continues to inspire innovative research. The ultimate goal of these investigations is to achieve sustained recovery of the failing human heart.
Keywords: heart failure, left ventricular assist device, myocardial recovery, remodeling
Introduction
Myocardial remodeling driven by excess pressure and volume load is believed to be responsible for the vicious cycle of progressive myocardial dysfunction in chronic heart failure [1]. This mechanistic model led to the hypothesis that left ventricular assist device (LVAD) support would disrupt this cycle and, by providing profound volume and pressure unloading, allow a reversal of stress-related compensatory responses of the overloaded myocardium [2–4]. This in turn would lead to subsequent structural and functional ‘reverse remodeling’ at organ and tissue levels [2–4]. Improvement in myocardial function in patients on LVAD support was first shown by Frazier et al. [5]. Limited clinical data have suggested that LVAD therapy can occasionally reverse the complex process of chronic myocardial remodeling to the point when a subset of patients can be successfully weaned from LVAD ( bridge to recovery ) [6,7••–9••]. Achieving sustained myocardial recovery after LVAD weaning in a patient with chronic advanced heart failure is one of the most desirable goals in contemporary treatment of heart disease [10]. Consequently, the mechanisms which might facilitate LVAD unloading-induced myocardial reverse remodeling have become the subject of intensive research [2–4]. However, fundamental questions at the basic science, translational and clinical levels remain unanswered.
Experience from ‘bridge to recovery studies’
Most of the clinical outcome studies that addressed myocardial recovery during LVAD support were retrospective and the results, as far as success of LVAD weaning and of achieving sustained myocardial recovery, varied significantly (Table 1). These inconsistencies can be explained by numerous limitations in study design, such as absence of a prespecified protocol to monitor for functional myocardial recovery, absence of a prespecified protocol for the use of adjuvant pharmacotherapy with potential antiremodeling effects, variable duration of LVAD support, lack of standardized LVAD explantation criteria, and diversity of the populations studied in their propensity for recovery. The most effective approach aimed at recovery of myocardial function reported so far is that of the Harefield group [8••,9••,18••]. Reproducibility of these results in larger patient cohorts and in a randomized fashion is of great importance. A multicenter North American clinical trial (HARPS), which combined LVAD and the Harefield pharmacologic protocol, enrolled 40 patients and the results of this investigation will be presented in the Spring of 2011. A European study (Harefield Athens Recovery Program) using the same approach is currently ongoing (Table 1, [4,10]). With the exception of the recent study by Birks et al. [9••], the great majority of devices utilized in the bridge to recovery studies have so far included first-generation, pulsatile LVADs.
Table 1.
Bridge to recovery studies
| Study year | Design | N | Adjuvant antiremodeling drug protocol |
Protocol for monitoring cardiac function |
Unloading duration (m) |
Recovery overall [N (%)] |
Recovery nonischemic [N (%)] |
HF recurrence/follow-up |
|---|---|---|---|---|---|---|---|---|
| US multicenter, 2007 [6] | P | 67 | Not standardized | YES | 4.5 | 6 (9) | 5 (13.5) | Freedom from death or Tx 100%/6 months |
| Berlin Group, 2008 and 2010 [7••,11••] | R | 188 | Not standardized | YES | 4.3 | 35 (18.6) | 35 (18.6) | Freedom from recurrent HF 74 and 66%/3 and 5 years, respectively |
| Harefield Group, 2006 [8••] | P | 15 | Yes | YES | 10.6 | 11 (73) | 11 (73) | Freedom from recurrent HF 100 and 89%/1 and 4 years, respectively |
| Harefield Group, 2011 [9••] | P | 20 | Yes | YES | 9.5 | 12 (60) | 12 (60) | Freedom from recurrent HF 83.3%/3 years |
| University of Athens-Harefield Group, 2007 [4,10] | P | 8 | Yes | YES | 6–10 | 4a (50) | 4a (50) | Freedom from recurrent HF 100%/2 years |
| Gothenburg Group, 2006 [12] | P | 18 | Not standardized | YES | 6.7 | 3 (17) | 3 (20) | Freedom from recurrent HF or Tx 33%/8 years |
| Pittsburgh Group, 2003 [13] | R | 18 | Not standardized | YES | 7.8 | 6 (33) | 5 (38) | Freedom from recurrent HF 67%/1 year |
| Osaka Group, 2005 [14] | R | 11 | Not standardized | N/A | 15.1 | 5 (45) | 5 (45) | Freedom from recurrent HF 100%/8 to 29 months |
| Pittsburgh Group, 2010 [15] | R | 102 | N/A | N/A | 4.9 | 14 (13.7) | 14 (13.7) | Freedom from recurrent HF or death 71.4%/5 years |
| Multicenter, 2001 [16] | R | 271 | N/A | N/A | 1.9 | 22 (8.1) | 22 (8.1) | Freedom from recurrent HF or death 77%/3.2 years |
| Columbia Group, 1998 [17] | R | 111 | N/A | N/A | 6.2 | 5 (4.5) | 4 (8) | Freedom from recurrent HF or death 20%/15 months |
HF, heart failure; m, months; N, number; N/A, not applicable; P, prospective studies; R, retrospective studies; Tx, transplant.
A fifth patient fulfilled recovery criteria (5/8, 62.5%) but died of stroke just before LVAD explantation.
A review of the outcomes of studies listed in Table 1 suggests that better understanding of the effects of LVAD unloading on myocardial structure and function could lead to approaches that may deliver sustained recovery to more patients. It is our opinion that we need first to understand better the effects of LVAD unloading on myocardial remodeling and subsequently organize more bridge to recovery trials. The LVAD population provides a rare opportunity for in-depth investigations in human biology for the following reasons:
The pre-LVAD and post-LVAD myocardial tissue specimens provide the opportunity to correlate human structural findings with functional data. Correlation between structure and function is achievable in animal models, but it is very rare that this level of understanding can be achieved in humans.
The hemodynamic support provided by LVAD makes these patients more tolerant to arrhythmic or hemodynamic adverse events [19].
The numbers of potential study participants (i.e. LVAD patients) is rapidly increasing [20].
In-depth evaluations of aggressive investigational therapies combined with LVAD are possible. This strategy could evolve to become a useful step in the research development and evaluation of new investigational therapies (pharmacologic, cell-based therapies, gene-based delivery systems) aiming not necessarily at reverse remodeling, but at cardiac regeneration or targeting other beneficial myocardial effects.
There is opportunity to investigate the beneficial effects of removing a significant part of the excess load that drives the vicious cycle of myocardial remodeling [1].
These five translational research advantages that LVADs offer may ‘transform’ this patient population to a translational research vehicle for investigating new antiremodeling and regenerative therapies for heart failure. Yet, in order for these promises to be fulfilled, we must first understand the fundamental biological impact of LVAD-induced mechanical unloading on myocardial structure and function.
Effects of left ventricular assist device-induced unloading on myocardial structure
Key changes that have been described in the failing myocardium after LVAD unloading include alterations in cardiomyocyte hypertrophy, extracellular matrix, microvasculature and adrenergic pathways – sympathetic innervation, among others.
Cardiomyocyte hypertrophy
Hypertrophic growth of cardiomyocytes is a mechanism by which the heart reduces stress on the failing ventricular wall [21–24]. LVAD unloading has been shown to induce regression of cardiomyocyte hypertrophy [25,26]. This is in agreement with data showing that LVAD unloading reverses the altered cardiac production of natriuretic peptides along with parallel reductions in myocardial mass and myocyte size [27,28]. However, animal models of prolonged unloading of nonfailing myocardium by means of heterotopic transplantation [29], severing the chordae tendinae of papillary muscle [30], or LVAD [31] suggested that mechanical unloading could lead to cardiomyocyte atrophy. Whether this phenomenon applies not only to normal but also to hypertrophied, failing myocardium unloaded by LVADs is controversial. One animal study of failing, hypertrophied hearts indicated that unloading in a heterotopic transplantation model resulted in a decrease of cardiomyocyte size beyond normal values [32]. In two human studies, cardiomyocyte size decreased with LVAD unloading, but not beyond the size of normal cardiomyocytes [33•,34]. In the human study carried out by our group, the light microscopy findings were complemented by ultrastructural electron microscopy in which there was no evidence of cardiomyocyte atrophy or degeneration even in patients who underwent prolonged (>6 months) duration of LVAD unloading [33•]. In addition, given that myocardial atrophy has been associated with increased glycogen concentration [35,36], the lack of change of glycogen content after LVAD unloading observed in this study [33•] is not compatible with myocardial atrophy after LVAD unloading.
A recent study also examined cardiomyocyte DNA content, nuclear morphology and number of nuclei per cell, before and after LVAD support [37••]. After unloading, the number of polyploid cardiomyocytes and cardiomyocyte DNA content declined, whereas an increase in binucleated cardiomyocytes was observed. The authors hypothesized that the vast polyploidy of cardiomyocytes in the failing human heart was a result of hypertrophic growth associated with repeated rounds of DNA synthesis that, despite completion of DNA replication, did not result in cell division. The increase of binucleated cardiomyocytes could suggest that reduction of noxious hypertrophic stimuli through LVAD unloading may lead to beneficial cardiomyocyte duplication and regeneration. These findings suggest that there is a dynamic and plastic regulation of cardiomyocyte content in heart failure, which strengthens the notion that at least a proportion of cardiomyocytes are not terminally differentiated and could re-enter the cell cycle during regenerative processes [38]. Of course, this hypothesis requires further confirmation.
Extracellular matrix
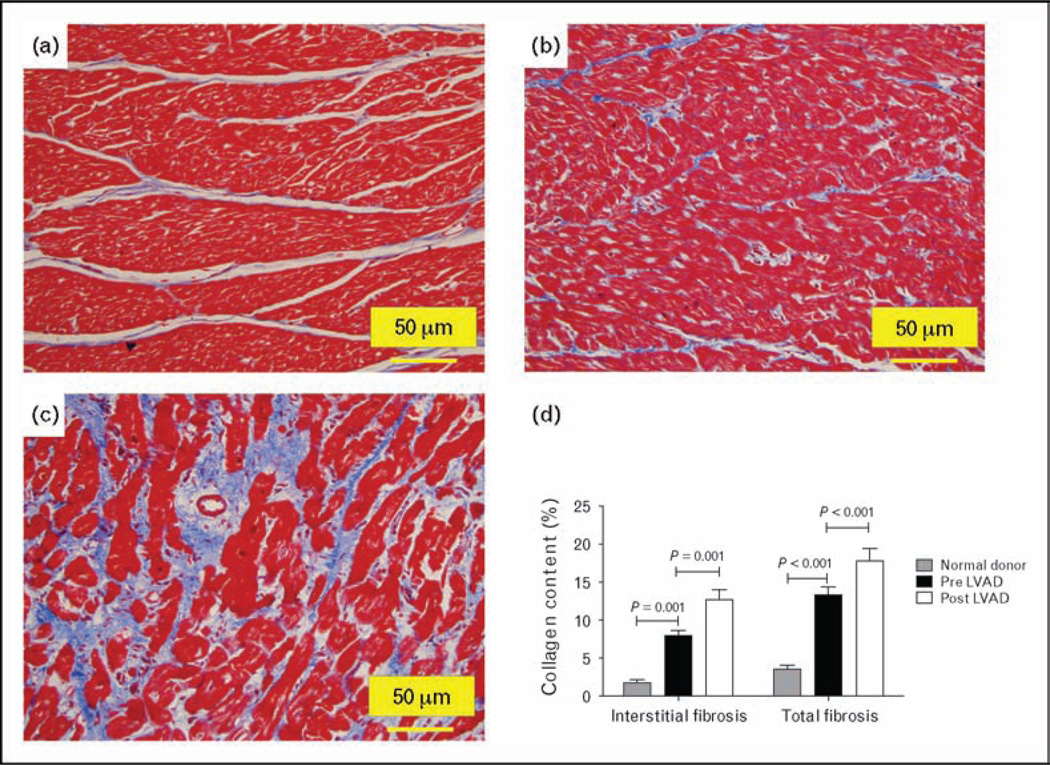
Remodeling of extracellular matrix, specifically an increase in fibrosis, is a hallmark feature of myocardial remodeling in chronic heart failure [39]. Altered collagen metabolism is responsible for ventricular dilatation and for changes in systolic and diastolic function [40,41]. Investigations of the effect of mechanical unloading on extracellular matrix have shown conflicting results: one group of investigators has reported decreased fibrosis [6,42–45], whereas others found increase in fibrosis [14,32,46–51] associated with increase in cross-linked collagen and myocardial stiffness [47]. The explanation for the contradictory observations is not clear, with some attributing the inconsistent results to differences in the methodology employed [2,3]. Applying recent advances in whole-field digital microscopy [52,53], we addressed this issue using digital histopathology and advanced image analysis techniques, an approach that reduces observer bias, markedly increases the amount of myocardial tissue analyzed and permits comprehensive endocardium-to-epicardium evaluation [33•]. This also eliminates the confounding effect of endocardial or epicardial sampling known to be associated with different degrees of fibrosis [54]. In failing hearts, interstitial and total fibrosis was higher compared with normal myocardium and the collagen content increased further after LVAD unloading (Fig. 1) [33•].
Figure 1. Increased fibrosis (Masson’s stain–collagen content stains blue) post LVAD-induced unloading.
Panel a (normal donor heart), panel b (pre-LVAD), panel c (post-LVAD); 200× magnification; LVAD, left ventricular assist device (modified with permission from ref. [31]).
Changes in neurohormonal milieu seen after LVAD implant support the above findings. Whereas older studies indicated that circulating levels of many neurohormones (plasma epinephrine, norepinephrine, arginine, vasopressin, renin and angiotensin II) decrease after LVAD implant [55], the effects of LVAD unloading on the myocardial tissue renin–angiotensin–aldosterone system (RAAS) components (including the profibrotic and prohypertrophic angiotensin II [56,57]) seem to be more complicated. Klotz et al. recently published results of the first study that systematically analyzed the different components of RAAS in paired myocardial tissue samples obtained before and after LVAD implantation [58••]. Renin levels in the pre-LVAD myocardium were the highest ever reported in human cardiac tissue (100× normal) and myocardial aldosterone level was also elevated (250× normal). After LVAD support, myocardial renin and aldosterone levels markedly decreased but, in contrast to this finding, myocardial angiotensin I and II levels increased five-fold to 10-fold [58••,59]. The authors hypothesized that the extremely high renin levels seen in the failing myocardium before LVAD implant result in depletion of myocardial angiotensinogen, and angiotensin I and II levels therefore become low instead of high. This phenomenon has also been noted in animals treated with high doses of angiotensin-converting enzyme inhibitor (ACE-I) [60]. Indeed, the myocardial angiotensinogen levels in the pre-LVAD hearts in the above study were only 5% of the normal serum angiotensinogen levels [58••]. It has therefore been proposed that at such high renin levels it is no longer possible to match the rapid metabolism of angiotensinogen in cardiac tissue either by increased uptake from blood or by local synthesis of angiotensinogen. Myocardial angiotensin generation can increase again only when renin levels decrease, as was the case post-LVAD in the same study. Whatever the explanation, the increase in myocardial level of angiotensin II during LVAD support was accompanied by a seven-fold rise in myocardial norepinephrine content [58••]. Increased norepinephrine levels are also known to lead to cardiac fibrosis. The same group of investigators reported an increase in the ratio of matrix metalloproteinases (MMPs) to tissue inhibitors of metalloproteinases (TIMP-1) in end-stage heart failure which normalized after LVAD unloading, favoring decreased collagen degradation and hence increased fibrosis [47]. Collectively, these post-LVAD myocardial biomarker alterations are compatible with the structural findings suggesting increased post-LVAD fibrosis.
Endothelium and microvasculature
Myocardial microvascular density is reduced in patients with heart failure [61]. LVAD unloading has been shown to lead to changes in expression of genes involved in the regulation of vascular organization and migration [62]. Moreover, experimental data of unloading by means of heterotopic transplantation resulted in increase in microvascular density [63]. In agreement with these experimental findings, our group demonstrated that pulsatile LVAD unloading resulted in increased microvascular density in failing human hearts [33•]. We also found strong evidence of endothelial cell activation both by immunohistochemistry [endothelial activation marker major histocompatibility complex class-II (MHC-II)] and by electron microscopy (ultrastructure analysis) [33•]. Animal models of angiogenesis in myocardial and skeletal muscle revealed that ultrastructural endothelial cell activation represents one of the early stages of capillary growth and arteriogenesis [64,65]. Therefore, the finding of endothelial activation after LVAD implant is consistent with the observed increase in microvascular density. The findings of post-LVAD increase in microvascular density and endothelial cell activation were also accompanied by increased interstitial and total myocardial fibrosis [33•]. This suggests that the recently described mechanistic link between the endothelium and cardiac fibrosis during the cardiac remodeling process – ‘endothelial to mesenchymal transition’ via pathways directly implicated in cardiomyocyte hypertrophy [66••,67] – might also apply to human myocardium. Obviously, direct proof for such a mechanism requires lineage tracing possible only in genetically manipulated animal models [66••,67]. Of note, work done in our laboratory has shown that endothelial proliferation and migration, hallmarks of angiogenesis, must be balanced by mechanisms that stabilize the endothelium, so that a functional vascular network may be established and maintained [68–70]. An imbalance in these competing signals after LVAD unloading may contribute to the increase in microvascular density, endothelial activation and cardiac fibrosis.
Adrenergic pathways and sympathetic innervation
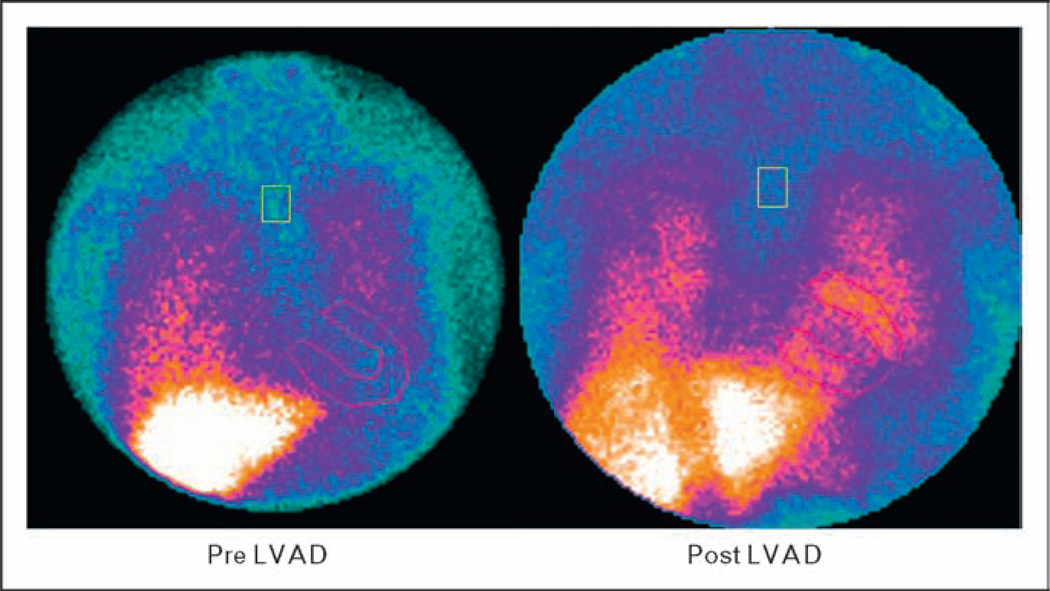
Signaling through β-adrenergic receptors regulates myocardial inotropy, chronotropy, dromotropy, and lusitropy [71]. Failing human heart demonstrates diminished contractile strength, down-regulation of β1-adrenergic receptors, impaired coupling with G protein and decreased responsiveness to β-adrenergic stimulation [72–75]. Long-term pulsatile LVAD support is associated with reverse adrenergic remodeling [76] which correlates with improved intrinsic myocardial contractile properties – greater magnitude of contraction, shorter time to peak contraction and reduced time to 50% relaxation [77]. Experiments on isolated myocytes and trabeculae from LVAD-unloaded myocardium have shown increased force and frequency of contractile response to β-adrenergic stimulation accompanied by an increase in β-adrenergic receptor density [78,79]. In a recent gene expression study, using a gene chip platform, significant changes were noted in genes of the β-adrenergic signaling pathway in hearts that recovered after LVAD unloading [80]. In another study using iodine 123-meta-iodobenzylguanidine (123I-MIBG) scintigraphy, pulsatile LVAD unloading resulted in improvements in sympathetic innervations in the failing heart which accompanied clinical, functional, and hemo-dynamic improvements (Fig. 2) [81•]. In addition to improved sympathetic innervation, pulsatile LVAD unloading also results in improved calcium (Ca2+) metabolism [79,82–86].
Figure 2. Effect of LVAD on H/M late MIBG image.
H/M (heart-to-mediastinum) uptake ratio in late image at baseline (pre-LVAD, zoom factor 1 ×) and after 3 months of LVAD support (post-LVAD, zoom factor 1, 2×). H/M, heart-to-mediastinum; LVAD, left ventricular assist device; MIBG, I-123 meta-iodobenzylguanidine (modified with permission from [75]).
The above findings suggest the potential importance of β-adrenergic receptor signaling in the reverse remodeling process that occurs in mechanically assisted failing hearts. It has also been shown that apoptotic cardiomyocyte death is selectively mediated by β1-adrenergic receptor, with β2-adrenergic receptor not being involved in this signaling [87]. Some investigators therefore suggested that reverse remodeling may be enhanced by combining ventricular unloading with augmentation of β2-adrenergic receptor signaling [18••,88]. This hypothesis was initially tested in an experimental study of a heterotopic transplantation model and a gene transfer technique to overexpress β2-adrenergic receptors [88]. In failing hearts treated with intracoronary adenoviral gene transfer of a β2-adrenergic receptor transgene, LV function improved significantly within the first week of unloading. The investigators concluded that adenoviral β2-adrenergic receptor gene therapy administered to the unloaded failing heart might represent a new form of ‘molecular assistance’ provided in synergy with mechanical assistance [88]. Sir Magdi H. Yacoub [18••] proposed a combination therapy of LVAD unloading and a selective β2 agonist, clenbuterol, to maximize the efficiency of LVAD as a bridge to recovery [8••,9••,18••]. This has been the most effective approach aimed at recovery of myocardial function using LVADs reported so far [4,8••,9••]; however, reproducibility of these results in a randomized larger-scale trial is yet to be established.
Effects of left ventricular assist device-induced unloading on myocardial function
Ventricular remodeling is associated with a rightward shift of the pressure–volume loop towards larger volumes [89], whereas pulsatile LVAD unloading results in a leftward shift in the direction of a normal physiological relationship [48]. Both continuous flow and pulsatile flow LVADs are associated with significant volume unloading and subsequent decrease in LV end-diastolic diameter [90]. However, pulsatile LVADs seem to have a more pronounced volume unloading compared with continuous-flow LVADs (Table 2) [91,93]. Continuous-flow LVADs, in addition to LV unloading, have also been associated with significant left atrial volume unloading and improved left atrial function at 3 and 6 months after LVAD implantation [99].
Table 2.
Effects of pulsatile vs. continuous-flow LVADs on the cardiovascular system
| Study | Design | Pulsatile vs. continuous-flow LVAD (N) | Effects |
|---|---|---|---|
| Klotz et al. [91] | R | 21 vs. 10 | Pressure unloading: similar |
| Volume unloading: Puls. superior | |||
| Zimpfer et al. [92] | P | 8 vs. 27 | PHT reversal: similar |
| Thohan et al. [42] | R | 12 vs. 8 | Pressure unloading: similar |
| Volume unloading: Puls. superior | |||
| LV mass regression: Puls. superior | |||
| Haft et al. [93] | P | 16 vs. 18 | Pressure unloading: similar |
| Volume unloading: Puls. superior | |||
| Exercise capacity at 3 months: similar | |||
| Garcia et al. [94] | P | 15 vs. 20 | Pressure unloading: similar |
| Volume unloading: similar | |||
| Patel et al. [95] | R | 43 vs. 34 | Incidence of RVF: similar |
| Welp et al. [96•] | P | 10 vs. 10 | Circulating RAAS components: greater reversal with Puls. |
| Vatta et al. [97] | P | 7 vs. 7 | Disrupted dystrophin: greater reversal with Puls. |
| Loebe et al. [98] | R | 6 vs. 6 | Increased inflammatory markers (IL-6 and C5a) with Contin. |
C5a, anaphylatoxin; Contin., continuous flow devices; f/u, follow-up; IL-6, interleukin-6; N, number; PHT, pulmonary hypertension; P, prospective; Puls., pulsatile flow devices; R, retrospective; RAAS, renin–angiotensin–aldosterone system; RVF, right ventricular failure.
LVAD unloading results in increased cardiac output and near normalization of pulmonary artery pressures and LV systolic pressures [91,93]. These changes are comparable regardless of cause of heart failure and are similar in magnitude in pulsatile and continuous-flow LVADs (Table 2). Etz et al. [100] reported that continuous-flow LVADs implanted in patients with medically refractory pulmonary hypertension resulted in significant reduction in mean pulmonary artery pressures and pulmonary vascular resistance, and these patients were subsequently successfully transplanted. Another study reported similar pressure unloading and normalization of pulmonary hemodynamics, which remained within normal range post transplantation [101•].
Left ventricular unloading by LVAD also has significant effects on the right ventricle (RV). LVAD unloading will typically result in decrease in RV afterload, improved RV geometry and systolic function [102,103]. However, higher-than-optimal continuous-flow LVAD unloading can result in a leftward shift of interventricular septum with resulting compromise in RV systolic function and RV failure [104].
Exercise intolerance in patients with chronic heart failure is associated with respiratory muscle weakness and poor outcomes [105,106]. In a study in which cardiopulmonary exercise testing was done before and after continuous-flow LVAD placement, we observed that all patients had a restrictive ventilatory pattern before LVAD implantation. This improved during the LVAD support and was associated with a significant increase in anaerobic threshold, peak work rate and exercise duration [107•], findings consistent with other investigations [93].
Effects of left ventricular assist device-induced unloading on electrophysiology and arrhythmias
Heart failure patients commonly have prolonged ventricular action potential and repolarization, as represented by prolonged QT interval and wide QRS complex. LVAD unloading has been shown to result in progressive shortening of QRS and QT intervals, findings consistent with reverse electrical remodeling of the failing heart [108,109]. We observed a strong correlation between corrected QT (QTc) shortening and increase in LV ejection fraction and decrease in LV filling pressures [110•]. The shortening of the action potential duration likely contributes to improved cellular contractile performance observed after sustained LVAD support.
The effect of LVAD therapy on the arrhythmogenicity of the heart remains controversial. Most published data are limited to retrospective studies which examined the incidence of clinically significant tachyarrhythmias using patient medical records. Whereas some investigations reported increase in monomorphic ventricular tachycardia after LVAD implant, other studies did not confirm this finding [93,111–115]. In a prospective study of selected LVAD patients with nonischemic cardiomyopathy, using 24-h Holter monitoring obtained before LVAD and at 2 months after LVAD implant, we found a significant decrease in premature ventricular contractions and ventricular couplets, but no change in the incidence of nonsustained or sustained ventricular tachycardia [110•].
Unresolved issues: future directions
The studies reviewed above provide important insights into the effects of LVAD unloading on myocardial structure and function. It is important to define, however, the limitations of these investigations as they relate to contemporary care:
Due to mainly engineering reasons, nonpulsatile, continuous-flow LVADs are now almost exclusively used. These LVADs produce a qualitatively different type of unloading compared with the pulsatile-flow LVADs examined in the majority of the studies reviewed above.
In most tissue studies, no functional myocardial recovery data were collected. Therefore, it cannot be distinguished which tissue, cellular and molecular changes would occur in all LVAD patients regardless of the presence of functional myocardial recovery (i.e. epiphenomena of the systemic hemodynamic improvement which occurs after LVAD implant), vs. which changes would occur only in the LVAD patients with signs of myocardial functional recovery. It is these latter changes that might be associated with true pathophysiologic mechanisms of reverse myocardial remodeling. In order to prove causality between structural changes and myocardial recovery, examination of tissue both from patients with evidence of LVAD-induced myocardial recovery and from patients without functional myocardial improvement is critical.
Many studies lacked prospectively designed protocols for myocardial tissue acquisition, preservation and analysis. In-depth investigation at the structural, ultrastructural and molecular levels is not possible by simply snap freezing the tissue in the operating room during the LVAD implant surgery, as was regularly done in many previous studies.
Various antiremodeling medications were routinely used in previous studies, but no randomization or standardization of their use was attempted. In most cases, no information regarding the patients’ concurrent drug therapy was reported. As a result, the drug-induced effects on remodeling cannot be separated from the effects of mechanical unloading alone in the majority of the studies.
Therefore, important questions remain unanswered:
-
What is the LVAD design that best promotes reverse remodeling – pulsatile, nonpulsatile, counterpulsatile?
The key known effects of pulsatile vs. continuous-flow LVADs on the cardiovascular system are summarized in Table 2. Whether the prospect of LVAD-induced reverse remodeling is better served by pulsatile [6,7••,8••,116], nonpulsatile [9••], or counterpulsation devices [117,118], and by full or partial unloading [119•], is unknown. More research is needed to better understand the properties of a ventricular assist device that would best promote reverse remodeling [120].
-
Which patients are most likely to achieve recovery?
The impact of the cause of heart failure on the potential for myocardial recovery needs to be further investigated. The likely candidates for reverse remodeling following mechanical unloading include patients with cardiomyopathy of different causes – hypertensive, valvular, peripartum, familial, and so on [120]. Ischemic cardiomyopathy patients who have suffered myocardial infarction and are characterized by large areas of noninfarcted myocardium that ‘remodeled’ over the years could also be considered candidates [4,10,120]. Furthermore, the impact of other factors such as duration of heart failure [11••], the concept of targeted adjuvant drug therapies [18••], the duration of LVAD unloading [32,48,51], advanced imaging protocols to monitor native heart’s function during unloading, the presence of RV failure [4,120] and the degree of the preunloading structural myocardial changes [121] also need to be elucidated.
-
Which investigational setting is more likely to advance our knowledge – ‘bridge to recovery’ or ‘bridge to transplant’?
The real need at this stage is a ‘bridge to knowledge’. From that perspective it seems that the ‘bridge to transplant’ study design offers more advantages compared with the ‘bridge to recovery’ study design. The bridge to transplant design offers the opportunity to study pre-LVAD and post-LVAD paired tissue specimens from both recovery responders and nonresponders along with the ability to study the effects of unloading at various time points. Large-scale and carefully designed ‘bridge to transplant’ studies, adequately powered to address many of the above unanswered questions, should come first. To follow, ‘bridge to recovery’ studies would aim to identify markers of sustained myocardial functional recovery and define reliable LVAD explantation criteria [9••,11••].
Conclusion
‘When you set out on your journey to Ithaca, you should hope the road to be long enough… full of obstacles and adventure, full of discovery…’ (from the poem ‘Ithaca’ by K. Kavafis, 1911, Alexandria, Egypt). This description of an unrelenting struggle and complete focus on fundamental targets also applies to the endeavor to use the opportunities presented by LVAD therapy as a springboard for future studies aiming at reverse remodeling and regeneration. Given the limited understanding of the effects of mechanical unloading alone, it may be preferable to delay combining LVADs with adjuvant ‘attractive’ therapeutic interventions (such as cell-based and gene-based therapies) and thus try to ignore the attractive sirens, as Odysseus managed to do during the original journey to Ithaca. Better understanding of the fundamental changes directly resulting from LVAD unloading should in turn allow identification and testing of specific therapeutic approaches that may eventually lead to myocardial recovery. We should always try to remember what Kavafis has been teaching us in the above referenced poem ‘Ithaca’: the grappling, the difficulties and the obstacles are necessary steps, given that the most important thing is the journey to Ithaca and not Ithaca per se.
Key points.
Limited clinical data suggest that left ventricular assist device (LVAD) unloading, possibly in combination with adjuvant therapies, could lead to myocardial recovery in a subset of patients with advanced heart failure.
Better understanding of the direct effects of LVAD unloading on the failing myocardium is needed and may lead to identification and testing of new approaches aimed at myocardial recovery and regeneration.
Increasing clinical use of LVADs presents a key opportunity for in-depth investigation of processes described above.
Acknowledgements
The work was funded by grants from: NHLBI, NIAID, Juvenile Diabetes Research Foundation, HA and Edna Benning Foundation, National Center for Research Resources Public Health Services research grant UL1-RR025764, and the Department of Defense (to D.Y.L.).
NIH 5R01HL089592-02 (to C.H.S.).
NIH NCRR grant that supports the CCTS UL1-RR025764 and C06-RR11234 (to S.G.D. and A.G.K.).
AHA #09CRP2050127 (to J.S.).
Deseret Foundation #00571 (to S.G.D. and A.G.K.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 273).
- 1.Katz AM. Maladaptive growth in the failing heart: the cardiomyopathy of overload. Cardiovasc Drugs Ther. 2002;16:245–249. doi: 10.1023/a:1020604623427. [DOI] [PubMed] [Google Scholar]
- 2.Soppa GK, Barton PJ, Terracciano CM, et al. Left ventricular assist device-induced molecular changes in the failing myocardium. Curr Opin Cardiol. 2008;23:206–218. doi: 10.1097/HCO.0b013e3282fc7010. [DOI] [PubMed] [Google Scholar]
- 3.Klotz S, Jan Danser AH, Burkhoff D. Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Prog Biophys Mol Biol. 2008;97:479–496. doi: 10.1016/j.pbiomolbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Drakos SG, Terrovitis JV, Anastasiou-Nana MI, et al. Reverse remodeling during long-term mechanical unloading of the left ventricle. J Mol Cell Cardiol. 2007;43:231–242. doi: 10.1016/j.yjmcc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Frazier OH, Radovancevic B, Abou-Awdi NL, et al. Ventricular remodeling after prolonged ventricular unloading ‘heartrest’ experience with the Heartmate left ventricular assist device [abstract] J Heart Lung Transplant. 1994;13:77. [Google Scholar]
- 6.Maybaum S, Mancini D, Xydas S, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 7. Dandel M, Weng Y, Siniawski H, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118:S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. •• This is so far the largest bridge to recovery study with longest follow-up. The study included exclusively nonischemic cardiomyopathy patients. The results, as far as likelihood of VAD explant and sustained recovery are concerned, are promising.
- 8. Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. •• Landmark prospective study establishing the concept of enhancement of LVAD-induced myocardial recovery by adding targeted adjuvant pharmacologic therapies. The recovery rates achieved in this study are the highest reported so far.
- 9. Birks EJ, George RS, Hedger M, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. •• First prospective LVAD bridge to recovery study using second-generation, continuous-flow LVADs. By combining continuous-flow LVADs with a specific adjuvant pharmacologic therapy, these investigators reproduced the high rates of recovery they achieved in the preceding LVAD era, using pulsatile LVADs and the same therapeutic strategy.
- 10.Drakos SG, Charitos EI, Nanas SN, et al. Ventricular-assist devices for the treatment of chronic heart failure. Expert Rev Cardiovasc Ther. 2007;5:571–584. doi: 10.1586/14779072.5.3.571. [DOI] [PubMed] [Google Scholar]
- 11. Dandel M, Weng Y, Siniawski H, et al. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq353. [Epub ahead of print] •• This important investigation described LVAD explantation criteria which predict sustained LVAD-induced myocardial recovery in the largest series of end-stage heart failure patients weaned from LVAD.
- 12.Liden H, Karason K, Bergh CH, et al. The feasibility of left ventricular mechanical support as a bridge to cardiac recovery. Eur J Heart Fail. 2007;9:525–530. doi: 10.1016/j.ejheart.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Gorcsan J, 3rd, Severyn D, Murali S, et al. Noninvasive assessment of myocardial recovery on chronic left ventricular assist device: results associated with successful device removal. J Heart Lung Transplant. 2003;22:1304–1313. doi: 10.1016/s1053-2498(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 14.Matsumiya G, Monta O, Fukushima N, et al. Who would be a candidate for bridge to recovery during prolonged mechanical left ventricular support in idiopathic dilated cardiomyopathy? J Thorac Cardiovasc Surg. 2005;130:699–704. doi: 10.1016/j.jtcvs.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Simon MA, Primack BA, Teuteberg J, et al. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. J Card Fail. 2010;16:99–105. doi: 10.1016/j.cardfail.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002;21:516–521. doi: 10.1016/s1053-2498(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 17.Mancini DM, Beniaminovitz A, Levin H, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98:2383–2389. doi: 10.1161/01.cir.98.22.2383. [DOI] [PubMed] [Google Scholar]
- 18. Yacoub MH. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J. 2001;22:534–540. doi: 10.1053/euhj.2001.2613. •• First description of the concept of enhancement of LVAD-induced myocardial recovery by adding targeted adjuvant pharmacologic therapy.
- 19.Busch MC, Haap M, Kristen A, et al. Asymptomatic sustained ventricular fibrillation in a patient with left ventricular assist device. Ann Emerg Med. 2011;57:25–28. doi: 10.1016/j.annemergmed.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Fang JC. Rise of the machines: left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 21.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 22.Opie L, editor. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. Heart physiology. [Google Scholar]
- 23.Opie LH, Commerford PJ, Gersh BJ, et al. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 24.Gerdes AM. Cardiac myocyte remodeling in hypertrophy and progression to failure. J Card Fail. 2002;8:S264–S268. doi: 10.1054/jcaf.2002.129280. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani S, McCarthy PM, Kottke-Marchant K, et al. Left ventricular echocardiographic and histologic changes: impact of chronic unloading by an implantable ventricular assist device. J Am Coll Cardiol. 1996;27:894–901. doi: 10.1016/0735-1097(95)00555-2. [DOI] [PubMed] [Google Scholar]
- 26.Zafeiridis A, Jeevanandam V, Houser SR, et al. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998;98:656–662. doi: 10.1161/01.cir.98.7.656. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn M, Voss M, Mitko D, et al. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc Res. 2004;64:308–314. doi: 10.1016/j.cardiores.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Altemose GT, Gritsus V, Jeevanandam V, et al. Altered myocardial phenotype after mechanical support in human beings with advanced cardiomyopathy. J Heart Lung Transplant. 1997;16:765–773. [PubMed] [Google Scholar]
- 29.Razeghi P, Sharma S, Ying J, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–2541. doi: 10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- 30.Thompson EW, Marino TA, Uboh CE, et al. Atrophy reversal and cardiocyte redifferentiation in reloaded cat myocardium. Circ Res. 1984;54:367–377. doi: 10.1161/01.res.54.4.367. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita M, Takano H, Takaichi S, et al. Influence of prolonged ventricular assistance on myocardial histopathology in intact heart. Ann Thorac Surg. 1996;61:640–645. doi: 10.1016/0003-4975(95)01087-4. [DOI] [PubMed] [Google Scholar]
- 32.Oriyanhan W, Tsuneyoshi H, Nishina T, et al. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J Heart Lung Transplant. 2007;26:16–23. doi: 10.1016/j.healun.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 33. Drakos SG, Kfoury AG, Hammond EH, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. • This is the first study that examined the direct effects of LVAD unloading on myocardial endothelium and microvasculature. In addition, it correlated the impact of LVAD unloading on the recently mechanistically linked triad of cardiac remodeling: endothelium, fibrosis and hypertrophy.
- 34.Jacquet L, Zerbe T, Stein KL, et al. Evolution of human cardiac myocyte dimension during prolonged mechanical support. J Thorac Cardiovasc Surg. 1991;101:256–259. [PubMed] [Google Scholar]
- 35.McNulty PH, Liu WX, Luba MC, et al. Effect of nonworking heterotopic transplantation on rat heart glycogen metabolism. Am J Physiol. 1995;268:E48–E54. doi: 10.1152/ajpendo.1995.268.1.E48. [DOI] [PubMed] [Google Scholar]
- 36.Rajabi M, Kassiotis C, Razeghi P, et al. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 37. Wohlschlaeger J, Levkau B, Brockhoff G, et al. Hemodynamic support by left ventricular assist devices reduces cardiomyocyte DNA content in the failing human heart. Circulation. 2010;121:989–996. doi: 10.1161/CIRCULATIONAHA.108.808071. •• This interesting study suggested that there is a dynamic and plastic regulation of cardiomyocyte content in heart failure, which strengthens the notion that at least some cardiomyocytes are not terminally differentiated and might be able to re-enter the cell cycle with abrogation of hypertrophic stimuli by LVAD unloading.
- 38.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 40.Villari B, Campbell SE, Hess OM, et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–1484. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 41.Lorell BH, Grossman W. Cardiac hypertrophy: the consequences for diastole. J Am Coll Cardiol. 1987;9:1189–1193. doi: 10.1016/s0735-1097(87)80326-1. [DOI] [PubMed] [Google Scholar]
- 42.Thohan V, Stetson SJ, Nagueh SF, et al. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: a comparative analysis with pulsatile-type devices. J Heart Lung Transplant. 2005;24:566–575. doi: 10.1016/j.healun.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Bruckner BA, Stetson SJ, Perez-Verdia A, et al. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. J Heart Lung Transplant. 2001;20:457–464. doi: 10.1016/s1053-2498(00)00321-1. [DOI] [PubMed] [Google Scholar]
- 44.Akgul A, Skrabal CA, Thompson LO, et al. Role of mast cells and their mediators in failing myocardium under mechanical ventricular support. J Heart Lung Transplant. 2004;23:709–715. doi: 10.1016/j.healun.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Thompson LO, Skrabal CA, Loebe M, et al. Plasma neurohormone levels correlate with left ventricular functional and morphological improvement in LVAD patients. J Surg Res. 2005;123:25–32. doi: 10.1016/j.jss.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Liang H, Muller J, Weng YG, et al. Changes in myocardial collagen content before and after left ventricular assist device application in dilated cardiomyopathy. Chin Med J (Engl) 2004;117:401–407. [PubMed] [Google Scholar]
- 47.Klotz S, Foronjy RF, Dickstein ML, et al. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–374. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- 48.Madigan JD, Barbone A, Choudhri AF, et al. Time course of reverse remodeling of the left ventricle during support with a left ventricular assist device. J Thorac Cardiovasc Surg. 2001;121:902–908. doi: 10.1067/mtc.2001.112632. [DOI] [PubMed] [Google Scholar]
- 49.McGowan BS, Scott CB, Mu A, et al. Unloading-induced remodeling in the normal and hypertrophic left ventricle. Am J Physiol Heart Circ Physiol. 2003;284:H2061–H2068. doi: 10.1152/ajpheart.00873.2002. [DOI] [PubMed] [Google Scholar]
- 50.Li YY, Feng Y, McTiernan CF, et al. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation. 2001;104:1147–1152. doi: 10.1161/hc3501.095215. [DOI] [PubMed] [Google Scholar]
- 51.Bruggink AH, van Oosterhout MF, de Jonge N, et al. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant. 2006;25:1091–1098. doi: 10.1016/j.healun.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Ho J, Parwani AV, Jukic DM, et al. Use of whole slide imaging in surgical pathology quality assurance: design and pilot validation studies. Hum Pathol. 2006;37:322–331. doi: 10.1016/j.humpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Pots S. Angiogenesis measurement using digital pathology. Lab Medicine. 2008;39:265–271. [Google Scholar]
- 54.Virmani R, Burke A, Farb A, et al., editors. Cardiomyopathy. 2001 ed. Philadelphia, PA: Saunders Company; 2001. [Google Scholar]
- 55.James KB, McCarthy PM, Thomas JD, et al. Effect of the implantable left ventricular assist device on neuroendocrine activation in heart failure. Circulation. 1995;92:II191–II195. doi: 10.1161/01.cir.92.9.191. [DOI] [PubMed] [Google Scholar]
- 56.Danser AH, van Kesteren CA, Bax WA, et al. Prorenin, renin, angiotensinogen, and angiotensin-converting enzyme in normal and failing human hearts. Evidence for renin binding. Circulation. 1997;96:220–226. doi: 10.1161/01.cir.96.1.220. [DOI] [PubMed] [Google Scholar]
- 57.van Kesteren CA, van Heugten HA, Lamers JM, et al. Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J Mol Cell Cardiol. 1997;29:2147–2157. doi: 10.1006/jmcc.1997.0448. [DOI] [PubMed] [Google Scholar]
- 58. Klotz S, Burkhoff D, Garrelds IM, et al. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J. 2009;30:805–812. doi: 10.1093/eurheartj/ehp012. •• This excellent study systematically analyzed the different components of RAAS in paired myocardial tissue samples obtained before and after LVAD implantation. These important mechanistic insights may guide future pharmacologic interventions in an effort to enhance the LVAD-induced reverse remodeling.
- 59.Klotz S, Danser AH, Foronjy RF, et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–1174. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 60.Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 61.Tsagalou EP, Anastasiou-Nana M, Agapitos E, et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–1398. doi: 10.1016/j.jacc.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 62.Hall JL, Grindle S, Han X, et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004;17:283–291. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 63.Rakusan K, Heron MI, Kolar F, et al. Transplantation-induced atrophy of normal and hypertrophic rat hearts: effect on cardiac myocytes and capillaries. J Mol Cell Cardiol. 1997;29:1045–1054. doi: 10.1006/jmcc.1996.0350. [DOI] [PubMed] [Google Scholar]
- 64.Zhou AL, Egginton S, Brown MD, et al. Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: an ultrastructural study. Anat Rec. 1998;252:49–63. doi: 10.1002/(SICI)1097-0185(199809)252:1<49::AID-AR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 65.Wolf C, Cai WJ, Vosschulte R, et al. Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J Mol Cell Cardiol. 1998;30:2291–2305. doi: 10.1006/jmcc.1998.0790. [DOI] [PubMed] [Google Scholar]
- 66. Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. •• This seminal study showed for the first time that ‘endothelial to mesenchymal transition’ through pathways implicated in cardiomyocyte hypertrophy could be an important mechanism of cardiac fibrosis during the myocardial remodeling process. These findings were widely perceived as the establishment of a significant pathophysiological link between endothelium, fibrosis and hypertrophy during the cardiac remodeling process.
- 67.Towbin JA. Scarring in the heart: a reversible phenomenon? N Engl J Med. 2007;357:1767–1768. doi: 10.1056/NEJMcibr075397. [DOI] [PubMed] [Google Scholar]
- 68.Whitehead KJ, Chan AC, Navankasattusas S, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson BD, Ii M, Park KW, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hajjar RJ, Muller FU, Schmitz W, et al. Molecular aspects of adrenergic signal transduction in cardiac failure. J Mol Med. 1998;76:747–755. doi: 10.1007/s001090050276. [DOI] [PubMed] [Google Scholar]
- 72.Feldman DS, Elton TS, Sun B, et al. Mechanisms of disease: detrimental adrenergic signaling in acute decompensated heart failure. Nat Clin Pract Cardiovasc Med. 2008;5:208–218. doi: 10.1038/ncpcardio1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brodde OE. Beta-adrenergic receptors in failing human myocardium. Basic Res Cardiol. 1996;91:35–40. doi: 10.1007/BF00795360. [DOI] [PubMed] [Google Scholar]
- 74.Bristow MR, Anderson FL, Port JD, et al. Differences in beta-adrenergic neuroeffector mechanisms in ischemic versus idiopathic dilated cardiomyopathy. Circulation. 1991;84:1024–1039. doi: 10.1161/01.cir.84.3.1024. [DOI] [PubMed] [Google Scholar]
- 75.Engelhardt S, Bohm M, Erdmann E, et al. Analysis of beta-adrenergic receptor mRNA levels in human ventricular biopsy specimens by quantitative polymerase chain reactions: progressive reduction of beta 1-adrenergic receptor mRNA in heart failure. J Am Coll Cardiol. 1996;27:146–154. doi: 10.1016/0735-1097(95)00425-4. [DOI] [PubMed] [Google Scholar]
- 76.Morawietz H, Szibor M, Goettsch W, et al. Deloading of the left ventricle by ventricular assist device normalizes increased expression of endothelin ET(A) receptors but not endothelin-converting enzyme-1 in patients with end-stage heart failure. Circulation. 2000;102:III188–III193. doi: 10.1161/01.cir.102.suppl_3.iii-188. [DOI] [PubMed] [Google Scholar]
- 77.Dipla K, Mattiello JA, Jeevanandam V, et al. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 78.Ogletree-Hughes ML, Stull LB, Sweet WE, et al. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 79.Heerdt PM, Holmes JW, Cai B, et al. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–2719. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 80.Hall JL, Birks EJ, Grindle S, et al. Molecular signature of recovery following combination left ventricular assist device (LVAD) support and pharmacologic therapy. Eur Heart J. 2007;28:613–627. doi: 10.1093/eurheartj/ehl365. [DOI] [PubMed] [Google Scholar]
- 81. Drakos SG, Athanasoulis T, Malliaras KG, et al. Myocardial sympathetic innervation and long-term left ventricular mechanical unloading. JACC Cardiovasc Imaging. 2010;3:64–70. doi: 10.1016/j.jcmg.2009.10.008. • This study evaluated effects of pulsatile LVAD unloading on myocardial sympathetic innervation and correlated it with clinical, functional, and hemodynamic improvements.
- 82.Takeishi Y, Jalili T, Hoit BD, et al. Alterations in Ca2+ cycling proteins and G alpha q signaling after left ventricular assist device support in failing human hearts. Cardiovasc Res. 2000;45:883–888. doi: 10.1016/s0008-6363(99)00415-0. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigue-Way A, Burkhoff D, Geesaman BJ, et al. Sarcomeric genes involved in reverse remodeling of the heart during left ventricular assist device support. J Heart Lung Transplant. 2005;24:73–80. doi: 10.1016/j.healun.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 84.Takaseya T, Ishimatsu M, Tayama E, et al. Mechanical unloading improves intracellular Ca2+ regulation in rats with doxorubicin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2239–2246. doi: 10.1016/j.jacc.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 85.Klotz S, Barbone A, Reiken S, et al. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 86.Terracciano CM, Harding SE, Adamson D, et al. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J. 2003;24:1329–1339. doi: 10.1016/s0195-668x(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 87.Zaugg M, Xu W, Lucchinetti E, et al. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 88.Tevaearai HT, Eckhart AD, Walton GB, et al. Myocardial gene transfer and overexpression of beta2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation. 2002;106:124–129. doi: 10.1161/01.cir.0000020220.79105.fd. [DOI] [PubMed] [Google Scholar]
- 89.Burkhoff D, Flaherty JT, Yue DT, et al. In vitro studies of isolated supported human hearts. Heart Vessels. 1988;4:185–196. doi: 10.1007/BF02058586. [DOI] [PubMed] [Google Scholar]
- 90.Weiss RMKW. The impact of prolonged rotary ventricular assist device support upon ventricular geometry and flow kinetics. J Am Soc Echocardiogr. 2010;24:149–156. doi: 10.1016/j.echo.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Klotz S, Deng MC, Stypmann J, et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg. 2004;77:143–149. doi: 10.1016/s0003-4975(03)01336-5. discussion 149–150. [DOI] [PubMed] [Google Scholar]
- 92.Zimpfer D, Zrunek P, Roethy W, et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg. 2007;133:689–695. doi: 10.1016/j.jtcvs.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 93.Haft J, Armstrong W, Dyke DB, et al. Hemodynamic and exercise performance with pulsatile and continuous-flow left ventricular assist devices. Circulation. 2007;116:I8–I15. doi: 10.1161/CIRCULATIONAHA.106.677898. [DOI] [PubMed] [Google Scholar]
- 94.Garcia S, Kandar F, Boyle A, et al. Effects of pulsatile- and continuous-flow left ventricular assist devices on left ventricular unloading. J Heart Lung Transplant. 2008;27:261–267. doi: 10.1016/j.healun.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Patel ND, Weiss ES, Schaffer J, et al. Right heart dysfunction after left ventricular assist device implantation: a comparison of the pulsatile Heart-Mate I and axial-flow HeartMate II devices. Ann Thorac Surg. 2008;86:832–840. doi: 10.1016/j.athoracsur.2008.05.016. discussion 832–840. [DOI] [PubMed] [Google Scholar]
- 96. Welp H, Rukosujew A, Tjan TD, et al. Effect of pulsatile and nonpulsatile left ventricular assist devices on the renin-angiotensin system in patients with end-stage heart failure. Thorac Cardiovasc Surg. 2010;58(Suppl 2):S185–S188. doi: 10.1055/s-0029-1240709. • This study suggested that pulsatile-flow LVADs, as compared with continuous-flow LVADs, have a greater impact on reversing changes in plasma renin activity and plasma aldosterone levels seen in heart failure.
- 97.Vatta M, Stetson SJ, Jimenez S, et al. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol. 2004;43:811–817. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 98.Loebe M, Koster A, Sanger S, et al. Inflammatory response after implantation of a left ventricular assist device: comparison between the axial flow Micro-Med DeBakey VAD and the pulsatile Novacor device. ASAIO J. 2001;47:272–274. doi: 10.1097/00002480-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 99.Drakos SG, Verma DR, Stehlik J, et al. Mechanical unloading with continuous-flow left ventricular assist devices: effects on left atrial remodeling. ISHLT 31st Annual Meeting & Scientific Session [abstract] 2011 [Google Scholar]
- 100.Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg. 2007;83:1697–1705. doi: 10.1016/j.athoracsur.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 101. John R, Liao K, Kamdar F, et al. Effects on pre and posttransplant pulmonary hemodynamics in patients with continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2010;140:447–452. doi: 10.1016/j.jtcvs.2010.03.006. • This study evaluated the effect of LVAD unloading on pulmonary pressures in patients with pulmonary hypertension who were otherwise good transplant candidates.
- 102.Santamore WP, Gray LA., Jr Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61:350–356. doi: 10.1016/0003-4975(95)01056-4. [DOI] [PubMed] [Google Scholar]
- 103.Kukucka M, Potapov E, Stepanenko A, et al. Acute impact of left ventricular unloading by left ventricular assist device on the right ventricle geometry and function: effect of nitric oxide inhalation. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 104.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–S39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Nanas S, Nanas J, Kassiotis C, et al. Respiratory muscles performance is related to oxygen kinetics during maximal exercise and early recovery in patients with congestive heart failure. Circulation. 1999;100:503–508. doi: 10.1161/01.cir.100.5.503. [DOI] [PubMed] [Google Scholar]
- 106.Meyer FJ, Borst MM, Zugck C, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103:2153–2158. doi: 10.1161/01.cir.103.17.2153. [DOI] [PubMed] [Google Scholar]
- 107. Dimopoulos SK, Drakos SG, Terrovitis JV, et al. Improvement in respiratory muscle dysfunction with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2010;29:906–908. doi: 10.1016/j.healun.2010.03.013. • This study demonstrated improved respiratory muscle function as a mechanism for improved exercise tolerance in continuous-flow LVAD patients.
- 108.Xydas S, Rosen RS, Ng C, et al. Mechanical unloading leads to echocardiographic, electrocardiographic, neurohormonal, and histologic recovery. J Heart Lung Transplant. 2006;25:7–15. doi: 10.1016/j.healun.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Harding JD, Piacentino V, 3rd, Gaughan JP, et al. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation. 2001;104:1241–1247. doi: 10.1161/hc3601.095718. [DOI] [PubMed] [Google Scholar]
- 110. Drakos SG, Terrovitis JV, Nanas JN, et al. Reverse electrophysiologic remodeling after cardiac mechanical unloading for end-stage non-ischemic cardiomyopathy. Ann Thorac Surg. 2011;91:764–769. doi: 10.1016/j.athoracsur.2010.10.091. • This is the first study that investigated the impact of LVAD support on the arrhythmogenicity of the failing human heart, a highly controversial topic, in a prospective manner.
- 111.Ziv O, Dizon J, Thosani A, et al. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 112.Bedi M, Kormos R, Winowich S, et al. Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol. 2007;99:1151–1153. doi: 10.1016/j.amjcard.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 113.Oz MC, Rose EA, Slater J, et al. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J Am Coll Cardiol. 1994;24:1688–1691. doi: 10.1016/0735-1097(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 114.Arai H, Swartz MT, Pennington DG, et al. Importance of ventricular arrhythmias in bridge patients with ventricular assist devices. ASAIO Trans. 1991;37:M427–M428. [PubMed] [Google Scholar]
- 115.Refaat M, Chemaly E, Lebeche D, et al. Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol. 2008;31:1246–1252. doi: 10.1111/j.1540-8159.2008.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maybaum S, Williams M, Barbone A, et al. Assessment of synchrony relationships between the native left ventricle and the HeartMate left ventricular assist device. J Heart Lung Transplant. 2002;21:509–515. doi: 10.1016/s1053-2498(01)00410-7. [DOI] [PubMed] [Google Scholar]
- 117.Drakos SG, Charitos CE, Ntalianis A, et al. Comparison of pulsatile with nonpulsatile mechanical support in a porcine model of profound cardiogenic shock. ASAIO J. 2005;51:26–29. doi: 10.1097/01.mat.0000150323.62708.35. [DOI] [PubMed] [Google Scholar]
- 118.Terrovitis JV, Charitos CE, Tsolakis EJ, et al. Superior performance of a paraaortic counterpulsation device compared to the intraaortic balloon pump. World J Surg. 2003;27:1311–1316. doi: 10.1007/s00268-003-6928-5. [DOI] [PubMed] [Google Scholar]
- 119. Meyns B, Klotz S, Simon A, et al. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J Am Coll Cardiol. 2009;54:79–86. doi: 10.1016/j.jacc.2009.04.023. • This important study evaluated partial support through a minimally invasive VAD as a means for reversing hemodynamic deterioration in advanced heart failure patients.
- 120.Malliaras KG, Terrovitis JV, Drakos SG, et al. Reverse cardiac remodeling enabled by mechanical unloading of the left ventricle. J Cardiovasc Transl Res. 2009;2:114–125. doi: 10.1007/s12265-008-9057-6. [DOI] [PubMed] [Google Scholar]
- 121.Bruckner BA, Razeghi P, Stetson S, et al. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J Heart Lung Transplant. 2004;23:36–42. doi: 10.1016/s1053-2498(03)00103-7. [DOI] [PubMed] [Google Scholar]