Abstract
Background
Human papillomavirus (HPV) infection, particularly with type 16, causes a growing fraction of oropharyngeal cancers, whose incidence is increasing, mainly in developed countries. In a double-blind controlled trial conducted to investigate vaccine efficacy (VE) of the bivalent HPV 16/18 vaccine against cervical infections and lesions, we estimated VE against prevalent oral HPV infections 4 years after vaccination.
Methods and Findings
A total of 7,466 women 18–25 years old were randomized (1∶1) to receive the HPV16/18 vaccine or hepatitis A vaccine as control. At the final blinded 4-year study visit, 5,840 participants provided oral specimens (91·9% of eligible women) to evaluate VE against oral infections. Our primary analysis evaluated prevalent oral HPV infection among all vaccinated women with oral and cervical HPV results. Corresponding VE against prevalent cervical HPV16/18 infection was calculated for comparison. Oral prevalence of identifiable mucosal HPV was relatively low (1·7%). Approximately four years after vaccination, there were 15 prevalent HPV16/18 infections in the control group and one in the vaccine group, for an estimated VE of 93·3% (95% CI = 63% to 100%). Corresponding efficacy against prevalent cervical HPV16/18 infection for the same cohort at the same visit was 72·0% (95% CI = 63% to 79%) (p versus oral VE = 0·04). There was no statistically significant protection against other oral HPV infections, though power was limited for these analyses.
Conclusions
HPV prevalence four years after vaccination with the ASO4-adjuvanted HPV16/18 vaccine was much lower among women in the vaccine arm compared to the control arm, suggesting that the vaccine affords strong protection against oral HPV16/18 infection, with potentially important implications for prevention of increasingly common HPV-associated oropharyngeal cancer.
ClinicalTrials.gov, Registry number NCT00128661
Introduction
A subset of oropharyngeal cancers (OPC) is caused by human papillomavirus (HPV) infection [1], with strong predominance of HPV16, which is detectable in about 90% of HPV-positive cases [2]. Evidence for the association between HPV and OPC has accumulated in recent years, and is based on extensive epidemiologic data and laboratory studies demonstrating molecular profiles indicative of high-risk HPV oncoprotein function [3].
HPV-positive OPC constitutes a distinct clinico-pathological entity with risk factors different from those for HPV-negative tumors. The incidence of OPC has increased significantly in the US [4], Australia [5], and several European countries [6]–[8], particularly in younger cohorts. In some areas, the increase in OPC has occurred despite declines in smoking and drinking, the main risk factors for HPV-negative OPC [4]. A recent study [9] showed that in the last 20 years, HPV detection in tumor specimens increased from 16% to 70% in the US. The authors estimated that in the next few decades, in the US, there will be more cases of HPV-positive OPC than of cervical cancer, where virtually all cases are attributable to HPV. In a report from Stockholm, Sweden [10], the incidence rate of HPV positive tonsillar cancers nearly doubled each decade between 1970 and 2007, while HPV negative tumors declined, leading the authors to suggest an epidemic of viral-induced carcinomas. The estimated number of new cases of OPC (including tonsils and base of tongue) is approximately 85 000 (ICD codes C01, C09-C10) per year in both sexes worldwide, with a male to female ratio of approximately 4∶1 [11].
Randomized trials have provided strong evidence for high efficacy of two virus-like particle (VLP) vaccines: the bivalent HPV16/18 vaccine (Cervarix®, GlaxoSmithKline Biologicals) [12], [13] and the quadrivalent HPV 6/11/16/18 vaccine (Gardasil™, Merck Sharp and Dohme) [14] against cervical [12], [14], vaginal and vulvar [14] infections and related diseases, and against anal HPV16/18 infections in women [15]. Among men, efficacy of the quadrivalent vaccine has been demonstrated against HPV-associated external genital lesions [16] and against anal HPV and intraepithelial neoplasia among men who have sex with men [17].
Oral anti-VLP antibodies are detectable in vaccinated subjects albeit at lower levels than those observed systemically [18], as is also true at the cervix [19]. Nonetheless, no studies have been reported on HPV vaccine efficacy (VE) in the oral cavity. Therefore, we evaluated efficacy of the bivalent vaccine to reduce oral HPV infection four-years following vaccination using data nested in our community-based double-blind randomized trial.
Methods
Ethics Statement
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.The trial was approved by institutional review boards of the National Cancer Institute in the US and the Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA) in Costa Rica, and all participants signed IRB-approved consent forms. The trial is registered at clinicaltrials.gov, identifier NCT00128661.
Study Procedures
This evaluation of VE against oral HPV infection was conducted in a randomized clinical trial initially designed to evaluate VE against persistent cervical HPV16/18 infection and precancerous lesions [13], [20], [21]. In 2004–2005, we invited a population sample of women aged 18–25 years from Guanacaste and Puntarenas, Costa Rica to participate. Women had to be good health, not pregnant or breastfeeding and using contraception during the vaccination period. 7 466 women were enrolled, representing 59·1% of eligible and 30·5% of women in the census [21].
At enrollment, a pelvic examination was performed on sexually experienced women, with collection of exfoliated cervical cells for liquid-based cytology and HPV DNA testing, and blood for HPV16/18 serology. Next, women were randomized in a blinded fashion to the bivalent vaccine or a hepatitis A vaccine (modified Havrix®, GSK Biologicals) as control. Both vaccines were formulated in three 0·5 ml doses and administered at enrollment, one and six months. Randomization was concealed for participants, clinical and laboratory staff and investigators throughout the study by using identical packaging and presentation of vaccines and coded labels, with vaccine allocation maintained by an independent Data Management Center. Additional details of this process have been previously reported [21]. Vaccines were assigned random identification numbers and each eligible participant was given the next available sequential number. Women not attending visits in allowable timeframes missed corresponding doses [21]. At annual follow-up visits, clinicians collected cervical cells for cytology and HPV testing from sexually active women, and those with abnormalities were referred for colposcopy and treatment as needed.
At the final blinded four-year study visit, after a new informed consent, a questionnaire was administered including oral and anal sexual behaviors and an oral specimen was collected by use of a 15-second rinse and 15-second gargle with 15 mL of commercially available alcohol-based mouth wash (Scope®, Procter and Gamble Company, Cincinnati, OH). This method of specimen collection [22] was chosen based on previous reports that a single mouthwash sample provides substantially larger amounts and higher molecular weight DNA than other methods of oral specimen collection [23], and that optimal specimen collection time is around 30 seconds after which point DNA recovery plateaus [24]. Specimens were kept between 2° and 8°Celsius until same-day processing at the local laboratory. The samples were concentrated by centrifugation (3000×g for 10 minutes) to obtain a pellet that was washed with 10 ml saline solution to remove residual mouthwash, re-centrifuged, and then resuspended in 1 ml of saline solution and frozen in liquid nitrogen tanks until testing.
For HPV DNA testing of oral and cervical specimens, DNA was extracted from each specimen via the MagNAPure LC DNA isolation procedure (Roche Diagnostics); 10 µl of extracted DNA were used for each PCR-reaction. All DNA samples were tested for the presence of HPV DNA by PCR amplification using the HPV SPF10 PCR-DEIA (DNA enzyme immunoassay)-LiPA25 (Line probe assay) version 1 system (Labo Biomedical Products, Rijswijk, The Netherlands). Briefly, this broad-spectrum PCR-based HPV DNA testing system uses SPF10 primers to amplify at least 57 HPV genotypes and the LiPA line detection system to genotype the following carcinogenic and non-carcinogenic HPVs [25], [26]: HPV 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. To increase the sensitivity of type-specific detection of HPV16 and 18 using the SPF10 system, all specimens that were SPF10 PCR/DEIA-positive were tested for the presence of HPV16 or 18 using type-specific primers detected by the TS16 and TS18 DEIA system [27].
The first 300 samples collected were tested using multiple volumes (200 µl, 400 µl, 800 µl) for DNA extraction as part of our laboratory optimization phase. The remaining specimens were tested in three batches of approximately the same size using 400 µl for DNA extraction. While we did observe batch-associated differences in the proportion of individuals positive by DEIA who were negative by LiPA (in other words, HPV infections of unknown type), the fraction of specimens positive for HPV16/18 was constant across batches (between 0·2% and 0·4%, excluding pilot), as was the fraction of specimens positive for an oncogenic or non-oncogenic HPV type. Thus, the variation between the batches was only for HPVs of unknown types and not for HPV 16/18 or the other types analyzed. As part of quality control, the final batch was retested with HPV16 or 18 type-specific primers using the same technique as in the primary testing, adding two HPV16 and one HPV18 infections. We decided that the limited potential yield of re-testing the other batches did not justify the extensive testing effort and associated cost. In our primary analysis, all infections detected were included in the analysis. We also conducted a sensitivity analysis excluding specimens positive in the second test (see results).
Serum collected at enrollment was used to determine HPV16 and HPV18 serological status using a VLP-based direct ELISA, for detection of polyclonal antibodies (GlaxoSmithKline Biologicals, Rixensart, Belgium), as described previously [28].
Statistical Analysis
Characteristics of women who accepted or declined the oral collection were compared using a chi-squared test for categorical variables. Among women who accepted, characteristics from the enrollment and four-year post-vaccination visits were compared by study arm. Median follow-up time was calculated and compared by arm using the Kruskal-Wallis test.
Before unblinding the data, we pre-specified our main objective for this analysis, which was an evaluation of VE against prevalent oral HPV16/18 infection approximately four years after the first vaccination among women with both oral and cervical HPV results available. Prevalence of oral HPV16/18 infections was the endpoint evaluated (defined as detection of either HPV16 or HPV18 or both in exfoliated cells from the oral cavity at the four-year study visit). VE against cervical HPV16/18 infections among the same women at the same time point are reported for comparison. Because this value-added component was introduced in response to the mounting evidence that HPV causes some oropharyngeal cancers, there was no pre-vaccination oral specimen obtained which would have allowed for exclusion from the analysis of women with prevalent oral HPV infection (as in a naïve cohort). To compensate, we pre-specified restricted cohorts in which to evaluate VE among women less likely to be exposed to HPV infection at vaccination, based on cervical HPV16/18 DNA or antibodies at enrollment. We also considered evaluating VE among women receiving fewer than three vaccine doses. However, given that only one subject in the vaccine arm had oral HPV16/18 detected 4 years after vaccination, these exploratory analyses became meaningless and were not formally conducted. We present in the results an estimate of VE among women who were HPV negative at the cervix.
Prevalences of oral and cervical HPV infections were expressed as number of infected women per 100 women vaccinated (stratified by vaccine arm). The complement of the ratios of the prevalence for the HPV and control arms constituted our VE estimates. We report asymptotic confidence intervals (95%CI) when cells had more than five events, and exact confidence limits otherwise [29], [30]. For analyses combining multiple HPV types, each woman was considered ‘positive’ if she harbored any of the types in question and ‘negative’ otherwise.
Oral and cervical VE estimates were compared using a GEE model [31] that accounts for correlation of oral/cervical infections within a woman. We also examined oral VE against other oncogenic HPV types and against HPV6/11, because of their association with laryngeal papillomatosis and the anatomical proximity of the larynx with the oral cavity.
At the time of this analysis, field work was on-going and individual information remained blinded. Thus, analyses were conducted by an external group, Information Management Systems (Rockville, MD), under the direction of the investigators. SAS 9.2 TS2M3 was used for analysis.
Results
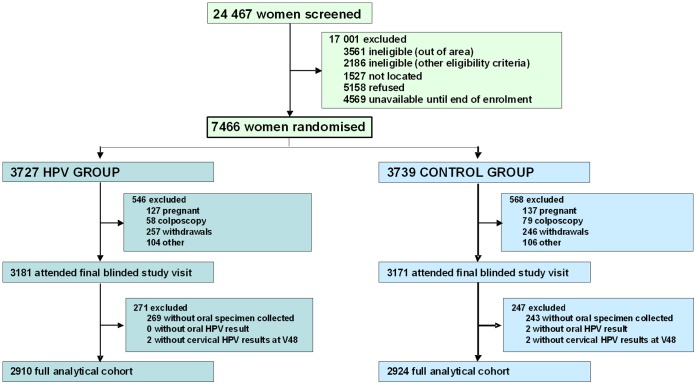
Of the 7 466 women randomized, 1 114 did not attend their four-year follow-up visit (Figure 1) and 6 352 attended the visit (3 181 HPV; 3 171 Control). 512 (269 HPV; 243 Control) women refused oral specimen collection, for an acceptance rate among eligible women of 91·9% (5 840 out of 6 352). After excluding two women with inadequate oral specimens and four women with unavailable cervical HPV results from the corresponding visit, the full analytic cohort comprised 5 834 women (2 910 HPV; 2 924 Control). The full cohort included all women vaccinated regardless of baseline cervical HPV DNA or serology results, treatment for cervical precancer or number of vaccine doses.
Figure 1. Consort diagram.
Percentages of women who accepted oral specimen collection were similar in both arms (91·5% vaccine and 92.3% control), although they were lower at one of the study clinics (Nicoya) (Table 1). Women with 4+ lifetime sexual partners, reporting oral and anal sex and positive for cervical HPV16/18 DNA at enrollment were significantly more likely to donate oral specimens.
Table 1. Proportion of women who accepted oral specimen collection among all women who attended the 4- year annual visit by selected characteristics.
| Characteristic | Number of women* | Percent who accepted oral collection | p value∫ | ||
| Age at Entry (in years) ◊ | 0.59 | ||||
| 18–19 | 1 859 | 91.6 | . | ||
| 20–21 | 1 437 | 91.8 | . | ||
| 22–23 | 1 340 | 92.8 | . | ||
| 24–25 | 1 204 | 91.8 | . | ||
| Study clinic | <0.001 | ||||
| Liberia | 1 661 | 92.6 | . | ||
| Nicoya | 1 620 | 85.6 | . | ||
| Cañas | 1 762 | 97.0 | . | ||
| Puntarenas | 797 | 93.7 | . | ||
| Lifetime number of vaginal sex partners at 4-year visit | <0.001 | ||||
| 0 | 324 | 84.4 | . | ||
| 1 | 1 489 | 90.5 | . | ||
| 2–3 | 2 007 | 92.4 | . | ||
| 4+ | 2 020 | 94.0 | . | ||
| Lifetime number of oral sex partners (reported at 4-year visit) | <0.001 | ||||
| 0 | 2 189 | 88.6 | . | ||
| 1 | 2 105 | 93.3 | . | ||
| 2+ | 1 516 | 95.3 | . | ||
| Age at oral sex debut in tertiles (age) | <0.001 | ||||
| Never Had Oral Sex | 2 189 | 88.6 | . | ||
| 1st tertile (11–19 years) | 1 328 | 95.0 | . | ||
| 2nd tertile (20–22 years ) | 1 216 | 93.3 | . | ||
| 3rd tertile (23–32 years ) | 984 | 94.1 | . | ||
| Lifetime number of anal sex partners (reported at 4-year visit) | <0.001 | ||||
| 0 | 4 768 | 90.9 | . | ||
| 1 | 878 | 97.0 | . | ||
| 2+ | 177 | 97.8 | . | ||
| Cervical HPV16/18 DNA at Enrollment | <0.001 | ||||
| Never had sex @ Enrollment | 1 137 | 87.1 | . | ||
| Negative | 4 184 | 92.8 | . | ||
| Positive | 511 | 96.2 | . | ||
| Vaccine Arm | 0.25 | ||||
| Control (Havrix) | 2 928 | 92.3 | . | ||
| HPV (Cervarix) | 2 912 | 91.5 | . | ||
Unknown excluded from calculation.
P value for the comparison of women who did and did not accept oral specimen collections.
Two 17 yr olds are classified in the ‘18–19’ group and one 27 yr old is classified in the ‘24–25’ group.
Among women who agreed to oral specimen collection, there was balance in the HPV and control arms on enrollment characteristics: age at entry, number of clinic visits and self-reported vaginal, oral and anal sex, which were queried at the study visit corresponding to oral specimen collection, and on enrollment history of smoking and cervical HPV 16/18 DNA positivity (Table 2). Median follow-up time was 54·8 months for vaccine arm and 54·9 for control arm (p = 0·58).
Table 2. General characteristics of the analytic population by vaccination arm (N = 5834).
| HPV Arm | Control Arm | |||
| Characteristic | Number of women* | Percent (Column) | Number of women* | Percent (Column) |
| Age at Entry (in years) ∫ | ||||
| 18–19 | 907 | 31.2 | 949 | 32.5 |
| 20–21 | 739 | 25.4 | 696 | 23.8 |
| 22–23 | 647 | 22.2 | 693 | 23.7 |
| 24–25 | 617 | 21.2 | 586 | 20.0 |
| Total number of clinic visits attended | ||||
| 1–2 | 11 | 0.4 | 13 | 0.4 |
| 3–5 | 1.846 | 63.4 | 1.795 | 61.4 |
| 6–8 | 784 | 26.9 | 813 | 27.8 |
| 9+ | 269 | 9.2 | 303 | 10.4 |
| Lifetime number of vaginal sex partners at 4-year visit | ||||
| 0 | 168 | 5.8 | 156 | 5.3 |
| 1 | 715 | 24.6 | 771 | 26.4 |
| 2–3 | 1.007 | 34.6 | 999 | 34.2 |
| 4+ | 1.020 | 35.1 | 998 | 34.1 |
| Lifetime number of oral sex partners (reported at 4-year visit) | ||||
| 0 | 1.070 | 37.0 | 1.117 | 38.4 |
| 1 | 1.081 | 37.3 | 1.021 | 35.1 |
| 2+ | 744 | 25.7 | 771 | 26.5 |
| Age at oral sex debut in tertiles (age) | ||||
| Never Had Oral Sex | 1.070 | 37.6 | 1.117 | 39.0 |
| 1st tertile (11–19) | 660 | 23.2 | 667 | 23.3 |
| 2nd tertile (20–22) | 610 | 21.4 | 604 | 21.1 |
| 3rd tertile (23–32) | 505 | 17.8 | 478 | 16.7 |
| Lifetime number of anal sex partners (reported at 4-year visit) | ||||
| 0 | 2.356 | 81.2 | 2.407 | 82.5 |
| 1 | 463 | 16.0 | 414 | 14.2 |
| 2+ | 82 | 2.8 | 95 | 3.3 |
| Ever smoking (at enrolment) | ||||
| No | 405 | 13.9 | 400 | 13.7 |
| Yes | 2.504 | 86.1 | 2.519 | 86.3 |
| Study clinic ◊ | ||||
| Liberia | 789 | 27.1 | 871 | 29.8 |
| Nicoya | 855 | 29.4 | 764 | 26.1 |
| Cañas | 880 | 30.2 | 878 | 30.0 |
| Puntarenas | 386 | 13.3 | 411 | 14.1 |
| Cervical HPV16/18 DNA at Enrollment | ||||
| Never had sex @ Enrolment | 551 | 19.0 | 584 | 20.0 |
| Negative | 2.117 | 72.8 | 2.063 | 70.7 |
| Positive | 239 | 8.2 | 272 | 9.3 |
Unknown excluded from calculation.
Two 17 yr olds are classified in the ‘18–19’ group and one 27 yr old is classified in the ‘24–25’ group.
Chi square p value for difference by arm = 0.02.
Prevalence of detectable oral HPV at the four-year study visit in the control group was 5·4% including identifiable (typeable) and untypeable types, 1·7% for infection with typeable HPV types, 1·3% for infections with oncogenic HPV types and 0·8% for non-oncogenic types. HPV16 was the most common type detected among controls (0·4%). Additional analyses of the uncharacterized oral HPV types detected are on-going. Oral HPV prevalence in the control group was significantly higher among women who were HPV-DNA positive at the cervix (3.5%) compared to those who were negative (1.0%). There was also a statistically significant association with single marital status and increasing numbers of lifetime vaginal sex partners, but there was no clear association with self-reported oral or anal sex [32].
In the full cohort (Table 3), estimated VE against oral HPV16/18 infection approximately four-years after first vaccination was 93·3% (one infection in vaccine arm, 15 in control, 95%CI = 62·5% to 99·7%). Type-specific VE was 91·6% against HPV16 (one and twelve women in vaccine and control arm, respectively, 95% CI = 51·7% to 99·6%) and 100% against HPV18 (0 and 4 women in the vaccine and control arm, 95%CI = −12·0% to 100·0%). The corresponding VE against prevalent cervical HPV16/18 infection for the same cohort of women at the same visit was 72·0% (95%CI = 63·0% to 79·1%) (p versus oral HPV VE = 0·04). The VE estimate against cervical HPV16 was similar to that against HPV18.
Table 3. Estimated vaccine efficacy against oral and cervical HPV16 and 18 infections 4 years after vaccination.
| Arm | Number of women | Number of women with infection* | Prevalence | 95%CI | Vaccine efficacy | 95%CI |
| Oral Infections | ||||||
| HPV16/18 ∫ | ||||||
| HPV | 2910 | 1 | 0.0 | 0.0∶0.2 | ||
| Control | 2924 | 15 | 0.5 | 0.3∶0.8 | 93.3% | 62.5% to 99.7% |
| HPV16 | ||||||
| HPV | 2910 | 1 | 0.0 | 0.0∶0.2 | ||
| Control | 2924 | 12 | 0.4 | 0.2∶0.7 | 91.6% | 51.7% to 99.6% |
| HPV18 | ||||||
| HPV | 2910 | 0 | 0.0 | 0.0∶0.1 | ||
| Control | 2924 | 4 | 0.1 | 0.0∶0.3 | 100% | −12.0% to 100% |
| Cervical Infections | ||||||
| HPV16/18 ∫ | ||||||
| HPV | 2910 | 61 | 2.1 | 1.6∶2.7 | ||
| Control | 2924 | 219 | 7.5 | 6.6∶8.5 | 72.0% | 63.0% to 79.1% |
| HPV16 | ||||||
| HPV | 2910 | 44 | 1.5 | 1.1∶2.0 | ||
| Control | 2924 | 151 | 5.2 | 4.4∶6.0 | 70.7% | 59.3% to 79.3% |
| HPV18 | ||||||
| HPV | 2910 | 18 | 0.6 | 0.4∶1.0 | ||
| Control | 2924 | 78 | 2.7 | 2.1∶3.3 | 76.8% | 61.9% to 86.5% |
There was one woman with a mixed infection with HPV 16 and 18.
P for arm* site interaction for VE against HPV 16/18 = 0.04.
The subject in the vaccine arm who had an oral HPV infection received only two vaccine doses, as did another 328 in the vaccine arm and 294 in the control arm. In addition, the HPV infection in this particular subject was only detected when her oral specimen was retested as part of quality control. Therefore, in the sensitivity analysis excluding HPV positive results from retested specimens, the VE against oral HPV16/18 infections was 100·0% (zero infection in the vaccine arm, thirteen in the control arm, 95% CI = 74·0% to 100·0%). When excluding women who were HPV 16/18 positive in the cervix at the enrolment visit, VE was 91.7% (95%CI = 52.3% to 99.6%).
There was no evidence of statistically significant protection against HPV31, 51, 52, 56, 39, or 6/11(Table 4). Estimated VE against HPV 31 (N = 8 total oral infections across both arms), the type for which cross-protection has been reported most consistently, was 39·7% (95% CI = −161·0 to 88·1%). Estimated VE against oncogenic types excluding HPV 16 and 18 was 13·2% (95% CI = −61·1, 53·6).and the VE against all oncogenic HPV types combined was 45·7% (95% CI = 6·9% to 69·0%).
Table 4. Estimated vaccine efficacy against oral infections with other HPV types.
| Arm | Number of women | Number of women with infection | Prevalence | 95%CI | Vaccine efficacy | 95%CI |
| HPV31 | ||||||
| HPV | 2910 | 3 | 0.1 | 0.0∶0.3 | ||
| Control | 2924 | 5 | 0.2 | 0.1∶0.4 | 39.7% | −161.0% to 88.1% |
| HPV51 | ||||||
| HPV | 2910 | 7 | 0.2 | 0.1∶0.5 | ||
| Control | 2924 | 10 | 0.3 | 0.2∶0.6 | 29.7% | −86.9% to 74.7% |
| HPV52 | ||||||
| HPV | 2910 | 3 | 0.1 | 0.0∶0.3 | ||
| Control | 2924 | 7 | 0.2 | 0.1∶0.5 | 56.9% | −63.9% to 91.0% |
| HPV56 | ||||||
| HPV | 2910 | 2 | 0.1 | 0.0∶0.2 | ||
| Control | 2924 | 4 | 0.1 | 0.0∶0.3 | 49.8% | −183.2% to 93.6% |
| HPV39 | ||||||
| HPV | 2910 | 3 | 0.1 | 0.0∶0.3 | ||
| Control | 2924 | 1 | 0.0 | 0.0∶0.2 | −201.4% | −7836.8% to 67.9% |
| HPV6/11 | ||||||
| HPV | 2910 | 4 | 0.1 | 0.0∶0.3 | ||
| Control | 2924 | 4 | 0.1 | 0.0∶0.3 | −0.5% | −345.5% to 77.3% |
| Other oncogenic | ||||||
| HPV | 2910 | 19 | 0.7 | 0.4∶1.0 | ||
| Control | 2924 | 22 | 0.8 | 0.5∶1.1 | 13.2% | −61.1% to 53.6% |
| All oncogenic | ||||||
| HPV | 2910 | 20 | 0.7 | 0.4∶1.0 | ||
| Control | 2924 | 37 | 1.3 | 0.9∶1.7 | 45.7% | 6.9% to 69.0% |
Discussion
In this first report evaluating efficacy of an HPV vaccine against oral infection, we observed, as part of a randomized trial of the bivalent vaccine among young women in Costa Rica, a 93·3% reduction of prevalent oral HPV 16/18 infection in the vaccine arm compared to the control arm approximately four years after vaccination.
Because our randomized trial was not specifically designed to evaluate VE against oral HPV infections, we had no baseline information on oral HPV status from study subjects, and we had to rely on HPV prevalence four years after vaccination rather than incidence of new infections. However, the VE estimate from a study restricted to HPV negative women at baseline would likely have been higher than the observed VE of 93%. Vaccination is known to be ineffective against established infections [20], and therefore inclusion of women already infected at baseline would tend to attenuate the VE estimates. For example, VE against prevalent cervical HPV16/18 infection at the same visit increased from 72.0% (95%CI = 63.0% to 79.1%, table 3) to 80.4% (95%CI = 72.4% to 86.4%) when excluding infections present at enrollment. Thus, although we recognize that the lack of insight into incident oral HPV infections is an important limitation of this analysis, we consider that the strong reduction in oral HPV 16/18 prevalence 4 years after vaccination is unlikely to be explained by this aspect of the study design.
There is limited knowledge about natural history of oral HPV infection, and the quantitative relationship between one-time detection of HPV in oral exfoliated cells and risk of future OPC is not established. In this context, our study does not constitute direct evidence that the vaccine prevents OPC. However, the high VE against oral HPV16/18 infection supports the possibility that vaccination may reduce risk of HPV-positive OPC, in particular HPV 16, the type most commonly associated with this cancer.
Although surrogate clinical endpoints, such as CIN2 or worse, were used to establish VE against cervical cancer, leading to licensing and mass vaccination programs, that approach is not possible with OPC because it lacks established precursor lesions. Direct evaluation of VE against OPC seems impractical, because given the relative rarity of both infection and OPC and the probably long interval between infection and the occurrence of cancer, such evaluation would require large studies and probably decades to complete. However, additional studies using virologic outcomes may further define the potential utility of HPV vaccines in prevention of these cancers in men and women.
We believe this study constitutes a valid randomized evaluation of VE against prevalent oral infections because the study and laboratory testing were blinded and there was balance by arm on demographic characteristics and risk factors for oral HPV acquisition. More than 90% of women agreed to donate oral specimens and valid HPV results were obtained on all but two of them. Although women who donated an oral specimen had evidence of more sexual activity than the relatively small number of women who did not, the balance by arm on all relevant characteristics is reassuring. In addition, the low prevalence of oral HPV16 infection in our control group (0·4%) was similar to its reported prevalence among healthy subjects in low-risk populations reported in a pooled analysis of 18 studies [33] and comparable to a prevalence of 0·3% reported among women 14–69 years old in a recent large survey in the US [34]. As is typically noted, the prevalence of cervical HPV16 detection in our control group was an order of magnitude higher (5·2%).
VE against prevalent oral HPV16/18 infections was significantly higher than against corresponding cervical HPV16/18 infections (p = 0·04) in the same cohort without excluding enrollment prevalent infections. This is consistent with the possibility that most oral infections were incident, with very few prevalent at enrollment and persisting for four years. Further, oral sex tended to start later than vaginal sex (data not shown), which would also result in a larger fraction of oral infections being acquired after vaccination compared to cervical infections. However, we did not see a clear association of oral HPV infection with oral sex.
Until now, there have been no data on efficacy of any of the HPV vaccines for prevention of oral HPV infection, and this remains the case in men. However, it is likely that the protection we observed among women will also be present in men, as VE of both vaccines has been demonstrated against HPV infections among men and women at all mucosal sites evaluated. Our results suggest that administration of the HPV vaccine will guard against oral infection by the HPV types responsible for the vast majority of HPV-related OPC, and open the possibility of primary prevention of these increasingly common malignancies.
Supporting Information
CONSORT checklist.
(DOC)
Trial protocol.
(PDF)
Acknowledgments
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project, including Bernardo Blanco and his team (census), Ricardo Cerdas and Ana Hernández (blood processing), José Miguel González, Osman López, Johnny Matamoros, Manuel Sánchez, Rafael Thompson and Jorge Umaña (field activity coordinators), Su Yen Araya, Hazel Barquero, Hayleen Campos, Muriel Grijalba, Ana Cristina Monge, Ana Peraza, Diana Robles, María Fernanda Sáenz, Dorita Vargas, and Jessica Vindas (clinic coordinators), Paola Alvarez, Dinia Angulo, Ana Live Arias, Betzaida Barrantes, Marianela Bonilla, Mary José Calvo, Loretto Carvajal, Jessenia Chinchilla, Blanca Cruz, Marianela Herrera, Andrea Interiano, Fabiola Jiménez, Erick Lagos, Viviana Loría, Andrea Messeguer, Rebeca Ocampo, Silvia Padilla, Angie Ramírez, Libia Rivas, Daniela Romero, Byron Romero, Jessenia Ruiz, Daniela Ruiz, Genie Saborío, Sofía Ssoto, Malena Salas, Adriana Torrez, Natalia Ugalde, Ana Cristina Ugalde, Adriana Vallejos, Yesenia Vázquez, Maricela Villegas (clinicians), Marta Alvarado, Ana Cristina Arroyo, Gloriana Barrientos, Diana Díaz, Marlen Jara, Maureen Matarrita, María Ester Molina, Elida Ordóñez, Gina Sánchez, and Zihara Villegas (nurses), Arianne Castrillo and Vivian López (education and outreach effort coordinators), Karla Coronado (appointment coordinator), Ricardo Alfaro (quality control coordinator), Charles Sánchez and Livia Romero (document center coordinators), Cristian Montero (quality assurance, regulatory) and Carlos Avila and Eric Alpízar (IT coordinators). Special recognition is also extended to the staff of Fundación INCIENSA for their administrative support. In the United States we would like to extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Jean Cyr, Julie Buckland, Laurie Rich, Brian Befano at Information Management Services (IMS) and acknowledge the contributions made by individuals at Westat, Inc., who provided project development and/or monitoring support, including Kerry Grace Morrisey, Kirk Midkiff, Susan Truitt, Sonia Stoszek, Maribel Gomez, and Isabel Trejos. We acknowledge the assistance provided by Carla Chorley, Troy Moore, Kathi Shea, and Heather Siefers in the establishment of a specimen and vaccine repository for our trial and in their continued assistance with the handling and shipment of specimens. From GSK Biologicals, we would like to acknowledge the contributions of Gary Dubin, Anne Schuind, Frank Struyf, Kelechi Lawrence, Darrick Fu, and Bruce Innis for their contribution to discussions regarding trial conduct and Francis Dessy and Catherine Bougelet for HPV-16/18 antibody testing. We would like to thank members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Sarah Thomas and Raphael Viscidi). We thank Annet Westbroek and Yvonne Zomerdijk from DDL for their help in testing the oral specimens, and Sabrina Chen from IMS for help with the analysis.
Cervarix is a registered trade mark of the Glaxo Smith Kline Biologicals group of companies.
Protocol available at http://proyectoguanacaste.org
Names and Affiliations of investigators in the Costa Rica Vaccine Trial (CVT) group:
Prevention and Implementation Group, International Agency for Research on Cancer
Rolando Herrero (Co-Principal Investigator)
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytopathologist)
M. Concepción Bratti (co-Investigator)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Yenory Estrada (Pharmacist)
Paula González (co-Investigator)
Diego Guillén (Pathologist)
Silvia E. Jiménez (Co-Investigator, trial coordinator)
Jorge Morales (Colposcopist)
Luis Villegas (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Carolina Porras (co-Investigator)
Ana Cecilia Rodríguez (co-Investigator)
United States National Cancer Institute, Bethesda, MD, USA
Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer)
Aimée R. Kreimer (Investigator)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Medical Monitor & NCI co-Project Officer)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor & QC Pathologist)
Sholom Wacholder (Statistician)
University of Costa Rica, San José, Costa Rica
Enrique Freer (Director, HPV Diagnostics Laboratory)
José Bonilla (Head, HPV Immunology Laboratory)
Alfonso García-Piñeres (Immunologist)
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory)
Ivannia Atmella (Microbiologist, Immunology Laboratory)
Margarita Ramírez (Microbiologist, Immunology Laboratory)
SAIC, NCI-Frederick, Frederick, MD, USA
Ligia Pinto (Head, HPV Immunology Laboratory)
Troy Kemp (Scientist, HPV immunology Laboratory)
Women’s and Infants’ Hospital, Providence, RI, USA
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
Georgetown University, Washington DC
Mary Sidawy (Histopathologist)
DDL Diagnostic Laboratory, The Netherlands
Wim Quint (Virologist, HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
Linda Struijk (HPV DNA testing)
Funding Statement
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK), under a Clinical Trials Agreement with the NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. NCI and Costa Rica investigators were responsible for the study design, data collection, data management, data analysis, interpretation, and preparation of the report. The corresponding author had access to all summary-level data. The NCI and Costa Rica investigators had final responsibility for the decision to submit for publication. GSK had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. GSK had the right to review and comment on the report.
References
- 1. Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, et al. (2005) Carcinogenicity of human papillomaviruses. Lancet Oncol 6: 204. [DOI] [PubMed] [Google Scholar]
- 2. Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, et al. (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772–1783. [DOI] [PubMed] [Google Scholar]
- 3. Gillison ML, Chaturvedi AK, Lowy DR (2008) HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 113: 3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown LM, Check DP, Devesa SS (2011) Oropharyngeal cancer incidence trends: diminishing racial disparities. Cancer Causes Control 22: 753–763. [DOI] [PubMed] [Google Scholar]
- 5. Hocking JS, Stein A, Conway EL, Regan D, Grulich A, et al. (2011) Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer 104: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddy VM, Cundall-Curry D, Bridger MW (2010) Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann R Coll Surg Engl 92: 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ligier K, Belot A, Launoy G, Velten M, Bossard N, et al. (2011) Descriptive epidemiology of upper aerodigestive tract cancers in France: incidence over 1980–2005 and projection to 2010. Oral Oncol 47: 302–307. [DOI] [PubMed] [Google Scholar]
- 8. Blomberg M, Nielsen A, Munk C, Kjaer SK (2011) Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer 129: 733–741. [DOI] [PubMed] [Google Scholar]
- 9. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, et al. (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29: 4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, et al. (2009) Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 125: 362–6. [DOI] [PubMed] [Google Scholar]
- 11. De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. (2012) The global burden of cancers attributable to infections in the year 2008: a review and synthetic analysis. Lancet Oncol 13: 607–615. [DOI] [PubMed] [Google Scholar]
- 12. Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, et al. (2012) Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13: 89–99. [DOI] [PubMed] [Google Scholar]
- 13. Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, et al. (2011) Prevention of persistent human papillomavirus (HPV) infection by a HPV 16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discovery 1: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. (2010) Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 102: 325–339. [DOI] [PubMed] [Google Scholar]
- 15. Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, et al. (2011) Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 12: 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, et al. (2011) Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 364: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, et al. (2011) HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 365: 1576–1585. [DOI] [PubMed] [Google Scholar]
- 18. Rowhani-Rahbar A, Carter JJ, Hawes SE, Hughes JP, Weiss NS, et al. (2009) Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. J Infect Dis 200: 1452–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, et al. (2003) Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J.Natl Cancer Inst 95: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 20. Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, et al. (2007) Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 298: 743–753. [DOI] [PubMed] [Google Scholar]
- 21. Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, et al. (2008) Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 26: 4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lum A, Le Marchand L (1998) A simple mouthwash method for obtaining genomic DNA in molecular epidemiological studies. Cancer Epidemiol Biomarkers Prev 7: 719–724. [PubMed] [Google Scholar]
- 23. Garcia-Closas M, Egan KM, Abruzzo J, Newcomb PA, Titus-Ernstoff L, et al. (2001) Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev 10: 687–696. [PubMed] [Google Scholar]
- 24. Walsh DJ, Corey AC, Cotton RW, Forman L, Herrin GL Jr, et al. (1992) Isolation of deoxyribonucleic acid (DNA) from saliva and forensic science samples containing saliva. J Forensic Sci 37: 387–395. [PubMed] [Google Scholar]
- 25. Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, et al. (1999) Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 37: 2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, et al. (1998) Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am.J.Pathol 153: 1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B (2006) Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol 44: 3292–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, et al. (2008) Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 4: 425–434. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Boice JD (1982) Epidemiologic analysis with a programmable calculator. Boston, MA, USA: Epidemiology Resources Inc.
- 30.Agresti A (2002) Categorical data analysis, 2nd ed. New York, NY: Wiley.
- 31. Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130. [PubMed] [Google Scholar]
- 32.Lang Kuhs KA, Gonzalez P, Quint W, Castro F, Hildesheim A, et al. (In Press) Prevalence of and Risk Factors for Oral Human Papillomavirus Infection Among Young Healthy Women in Costa Rica. J Infect Dis. [DOI] [PMC free article] [PubMed]
- 33. Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, et al. (2010) Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis 37: 386–391. [DOI] [PubMed] [Google Scholar]
- 34. Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, et al. (2012) Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 307: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist.
(DOC)
Trial protocol.
(PDF)