Abstract
Objectives
While global measures of cardiovascular (CV) risk are used to guide prevention and treatment decisions, these estimates fail to account for the considerable interindividual variability in pre-clinical risk status. This study investigated heterogeneity in CV risk factor profiles and its association with demographic, genetic, and cognitive variables.
Methods
A latent profile analysis was applied to data from 727 recently postmenopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Women were cognitively healthy, within three years of their last menstrual period, and free of current or past CV disease. Education level, apolipoprotein E ε4 allele (APOE4), ethnicity, and age were modeled as predictors of latent class membership. The association between class membership, characterizing CV risk profiles, and performance on five cognitive factors was examined. A supervised random forest algorithm with a 10-fold cross-validation estimator was used to test accuracy of CV risk classification.
Results
The best-fitting model generated two distinct phenotypic classes of CV risk 62% of women were “low-risk” and 38% “high-risk”. Women classified as low-risk outperformed high-risk women on language and mental flexibility tasks (p = 0.008) and a global measure of cognition (p = 0.029). Women with a college degree or above were more likely to be in the low-risk class (OR = 1.595, p = 0.044). Older age and a Hispanic ethnicity increased the probability of being at high-risk (OR = 1.140, p = 0.002; OR = 2.622, p = 0.012; respectively). The prevalence rate of APOE-ε4 was higher in the high-risk class compared with rates in the low-risk class.
Conclusion
Among recently menopausal women, significant heterogeneity in CV risk is associated with education level, age, ethnicity, and genetic indicators. The model-based latent classes were also associated with cognitive function. These differences may point to phenotypes for CV disease risk. Evaluating the evolution of phenotypes could in turn clarify preclinical disease, and screening and preventive strategies.
ClinicalTrials.gov NCT00154180
Introduction
Several known risk factors for the development of vascular disease have been linked to not only cardiovascular (CV) disease endpoints, but also to accelerated cognitive decline and prodromal Alzheimer’s Disease (AD) [1]–[5]. Despite public awareness campaigns and the availability of well-established preventive options, CV disease remains one of the leading causes of death for women in the United States [6]–[7]. Menopause-related changes in hormonal profile may potentiate the increased risk in CV disease. Postmenopausal women are at higher risk than age-matched men, possibly due to gonadal failure and reduced gonadal steroid production [8]. Estrogens play a key role in maintaining adequate levels of high-density lipoprotein cholesterol (HDL-C), a positive influential CV health factor and a significant independent predictor of nitric oxide-dependent coronary vasodilation (as measured by flow-mediated dilation) in healthy individuals [9]–[10]. In contrast, elevated plasma levels of low density lipoprotein cholesterol (LDL-C) contributes to endothelial dysfunction and progression of coronary heart disease [11]–[12]. Endothelial dysfunction, often characterized by decreases in production of nitric oxide, is mediated not only by lipid profile, but also by other putative CV risk factors such as elevated glucose and triglyceride levels and high-sensitivity C-reactive protein [13]–[14].
The association between these classical CV disease risk factors and the magnitude of reactive hyperemia in small arteries can be observed in individuals with no history of CV disease and normal lipid profile [15]. Findings further suggest that menopause and aging also are independent risk factors for endothelial dysfunction in normotensive women [16]. Moreover, predisposing vascular disease risk factors such as central obesity, unhealthy diet and physical inactivity may synergistically increase risk for vascular disease (for example, low serum HDL-C, diabetes, and elevated blood pressure). Lower education and income are socio-economic factors often linked to increased risk of vascular disease, especially in women [17]–[18]. Genetic characteristics such as the apolipoprotein E (APOE) gene polymorphism, particularly the presence of an ε4 allele (or APOE4 isoform), are frequently linked, not only to hyperlipoproteinemia, but also to AD and cognitive impairment in non-demented adults [8], [19].
The importance of a multifactorial approach to the evaluation of both traditional and newer markers of vascular risk has been shown to augment the predictive accuracy of global estimates of CV risk [1], [20]. Thus there is an important need for increased understanding of the prevalence of these risk factors as well as their covariation. However, the assessment of a multiplicity of risk factors often involves the use of laboratory values with no “natural” cut-off between “normal” and “abnormal” levels primarily because some of these measurements (e.g., lipid levels) are continuous. Moreover, the threshold or cut-point at which a potential risk factor in the continuum of risk exposure can be considered a “true” risk is a subject of debate [21]–[22]. For instance, specific thresholds for arterial hypertension and hyperlipidaemia are on an arbitrary dichotomy [23]. It is also possible that cut-off criteria for conventional vascular risk factors may in fact vary systematically by gender, race/ethnicity or socio-cultural background. Indeed, global measures of risk based on a graded summation of factors, such as Framingham-based risk scores, have required re-calibration to improve accurate risk estimation in older women [24] and in ethnic minorities [25]. Moreover, the interactive influence of these characteristics may give rise to complex differential effects on health outcomes. Even when the assessment of vascular disease risk yields results within clinically ‘acceptable’ ranges there can be considerable between- and within-individual heterogeneity in the measures considered in the global evaluation of “risk.”
Using a finite mixture modeling approach, [26] this study aimed at elucidating potential phenotypic heterogeneity in risk based on multiple measures of vascular disease risk obtained at baseline from a cohort of postmenopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) [27]. We hypothesized that 1) co-variation among multiple manifest vascular disease risk factors could be fully explained by a discrete latent variable (latent groups) capturing heterogeneity in the sample and 2) latent group or class membership will be associated with demographic, racial/ethnic, genetic, and cognitive function variables.
Methods
Sample Description and Setting
KEEPS and KEEPS-Cog studies were reviewed and approved by Institutional Review Boards at all nine enrollment sites and at the University of Wisconsin, the KEEPS Cognitive and Affective (KEEPS-Cog) coordinating site. IRB numbers for KEEPS institutions: The central KEEPS and Phoenix KEEPS (IRB protocol by the Western IRB): STUDY NUM: 1058663 and WIRB PRO NUM: 20040792KEEPS (main study & cognitive substudy) #10-02980 and MDBHAS #11-05383. Brigham and Women’s Hospital (Partners): #2004-P-002144 BWH. Mayo Clinic: 2241-04. Columbia: IRB#: AAAA-8062. Yale: 0409027022. University of Utah: 13257. Einstein/Montefiore: 04-08-213. University of Wisconsin, Madison: H-2005-0059. University of California, San Francisco (UCSF): KEEPS (main study & cognitive substudy) #10-02980. University of Washington: IRB #26702; VAPSHCS IRB #01048.
All participants provided written informed consent to participate in the main KEEPS study and in the KEEPS-cog ancillary study. The ethics committees approved the consent procedure utilized in the study. Enrollment occurred between August 2005 and July 2008 with final visits completed in 2012.
Data for this study were obtained from the multisite KEEPS and KEEPS-Cog substudy. The parent study, KEEPS, was a randomized, blinded, placebo-controlled clinical trial designed to compare the effect of 48 months of treatment with low-dose oral conjugated equine estrogen and transdermal estradiol to placebo on cardiovascular endpoints in recently menopausal women [27]–[28]. The KEEPS-Cog ancillary study aimed to evaluate the potential differential efficacy of the two forms of menopausal hormone therapy (MHT) on cognitive and mood function. Participants were recruited from nine sites across the nation. Exclusion criteria for the trial included the presence of past or current CV or cerebrovascular disease, uncontrolled hypertension, and use of lipid lowering medications. Determinations of “low risk” for CV disease were based on body mass index (BMI), blood pressure, fasting cholesterol and glucose values, tobacco use, and assessment of coronary artery calcification (CAC) measured by computerized tomography (CT). For a more detailed overview of the KEEPS study design, sample enrollment criteria, and randomization and data collection procedures, please refer to the comprehensive descriptions provided in Harman et al. [27] and Miller et al. [29].
The mixture modeling analysis used baseline (pre-randomization) data from 727 postmenopausal women, between the ages of 42 and 58, who were within 3 years of their final menstrual period. Table 1 presents a summary of the sample characteristics at study entry. In terms of demographic characteristics, the sample was predominantly non-Hispanic white (80.5%), averaged 53 (SD = 2.6) years of age, and 73.5% reported having obtained a minimum of a college degree. Additionally, of those consenting to DNA testing (N = 596), 26% had at least one APOE ε4 allele; a genetic risk marker often associated with adverse changes in cognitive functioning occurring prematurely during the aging process, [30]–[31] CV disorders in middle age, [32]–[33] and shown to interact with female gender [34]–[35]. Most participants (87%) were entirely free of CAC at baseline. The remaining 13% of the sample had CAC volume scores ranging from 0.015 to 50.
Table 1. Selected Demographic and Clinical Characteristics of the Total Sample at Baseline.
| Characteristics (N, Mean ± SD, Range; unless otherwise noted) | |||
| Demographic | N | Mean ± SD) | Range |
| Age (years) | 727 | 52.68±2.60) | 42 to 58 |
| Years since menopause | 725 | 1.44±0.73) | 1 to 3 |
| Self-reported race/ethnicity ( N, % ) | (692) | ||
| Asian or Pacific Islander | 21(3.03) | – | |
| Black/African American | 54 (7.80) | – | |
| Non-Hispanic White/Caucasian | 557 (80.49) | – | |
| Hispanic | 53 (7.70) | ||
| Other | 7 (1.01) | – | |
| Education ( N, % ) | (717) | ||
| Grade school | 3 (0.42) | – | |
| Some high school | 3 (0.42) | – | |
| High school diploma or GED | 52 (7.25) | – | |
| Some college or vocational school | 132 (18.41) | – | |
| College graduate | 293 (40.86) | – | |
| Some graduate or professional school | 34 (4.74) | – | |
| Graduate or professional degree | 200 (27.89) | – | |
| Apolipoprotein E ε4 allele (APOE4) ( N, % ) | (596) | – | |
| 156 (26.2) | |||
| Vascular Disease Risk Factors | |||
| Body mass index (BMI) (kg/m2) | 727 | 26.19±4.31 | 16 to 35 |
| Waist Circumference (cm) | 716 | 83.2±15.20 | 57.2 to 256.5 |
| Total Cholesterol (mg/dL) | 727 | 208.10±33.7 | 122 to 315 |
| Mean systolic blood pressure (sBP) (mm/Hg) | 727 | 117.43±14.90 | 82 to 189 |
| Mean diastolic blood pressure (dBP) (mm/Hg) | 727 | 75.30±9.22 | 50 to 113 |
| Mean arterial blood pressure (MAP) [1] | 727 | 89.62±10.31 | 63.3 to 132.3 |
| Low density lipoprotein-cholesterol (LDL-C) (mg/dL) | 727 | 110.90±27.8- | 11 to 194 |
| High density lipoprotein-cholesterol (HDL-C) (mg/dL) | 727 | 72.0±14.60 | 24 to 129 |
| Triglycerides (mg/dL) | 727 | 87.00±55.90 | 7.0 to 374 |
| Fasting blood glucose (FBG) (mg/dL) | 727 | 79.60±10.00 | 55 to 126 |
| Total Framingham Point Score (FPS) | 727 | 4.00±3.19 | −5 to 14 |
| Current tobacco use (N, %) | (727) | – | |
| 50 (6.90) | |||
| Measures of Vascular Disease | |||
| Coronary artery calcification (CAC) volume score | 727 | 1.33±5.18 | 0 to 50.00 |
| Carotid artery intima-media thickness (CIMT) | 727 | 0.72±0.09 | 0.53 to 1.17 |
| Clinical - Cognitive Scores | |||
| Factor Scores | |||
| Global Cognition | 662 | 0.0±0.88 | −3.06 to 2.54 |
| Verbal Learning & Memory | 662 | 0.0±0.87 | −2.96 to 2.40 |
| Auditory Attention & Working Memory | 662 | 0.0±0.75 | −2.73 to 2.09 |
| Visual Attention & Executive Function | 662 | 0.0±0.73 | −2.15 to 2.01 |
| Speeded Language & Mental Flexibility | 662 | 0.0±0.79 | −2.50 to 2.50 |
| Mini Mental State Examination (MMSE) | 647 | 29.1±1.40 | 22 to 30 |
(1) MAP was estimated as 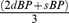 .
.
Selected anthropometric, clinical, serum, and behavioral indicators of vascular disease risk for the total sample at baseline are also summarized in Table 1 . The mean body mass index (BMI) was 26.19 kg/m2 (SD = 4.31) with close to 50% of the sample with a BMI ranging from 26 to 35 kg/m2, which is considered overweight or obese as defined by published standards [36]. Approximately 33% of the sample had a waist circumference above the cut-off score for female central/abdominal obesity (>88 cm) among Caucasians in the United States [37]. Waist circumference measures for the total sample ranged from 57 to 256.5 cm (M = 83.2, SD = 15.2). A relatively small percentage of women in the sample (6.9%) self-identified as current smokers. Although mean values for other vascular disease risk factors shown in Table 1 were within ‘normal’ reference standards, the lower (e.g., HDL-C) or upper (e.g., total cholesterol, triglycerides, LDL-C) limit for the range of measures, in most risk factors, were slightly beyond the boundaries of ‘clinically desirable levels.
Laboratory Analyses and Anthropometric Measurements
Seven vascular disease risk variables were used as surrogates for latent class membership in the analysis. These included six absolute measures (BMI, carotid artery intima-media thickness (CIMT), LDL-C, fasting blood glucose (FBG), HDL-C, and triglycerides) and a global average value of risk based on Framingham point scores (FPS) [38]. All participants underwent venous blood draws in the morning after at least 12 hours of fasting. Blood samples for lipid, glucose, and triglycerides levels measurements were sent to and analyzed by Kronos Science Laboratories (Phoenix, AZ). Blood pressure readings were taken in the morning at least 30 minutes before the blood draws or weight measurement. CIMT was measured by high-resolution B-mode ultrasound [39]. FPS were computed following standard procedures for points assignment and summation described in Wilson et al. [38] from the following six variables: 1) age, 2) systolic blood pressure, 3) diastolic blood pressure, 4) smoking, 5) HDL-C, and 6) total cholesterol. For each of these risk factors, points were assigned according to the level of associated risk.
Height (cm) and weight (kg) measurements were obtained as part of KEEPS health examination protocol and BMI was calculated as weight divided by height squared (kg/m2). Table 2 summarizes the zero-order correlations for the seven vascular disease risk variables at baseline. As expected, all variables were significantly correlated with at least one other variable in the set; with triglycerides and HDL-C having the highest inverse correlation (ρ = −0.486; p<0.002).
Table 2. Bivariate Correlations for the Vascular Disease Risk Variables at Baseline.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | BMI | 1 | ||||||
| 2 | CIMT | 0.057 | 1 | |||||
| 3 | LDL-C | 0.157 | 0.030 | 1 | ||||
| 4 | HDL-C | −0.336 | −0.046 | −0.129 | 1 | |||
| 5 | Triglycerides | 0.330 | 0.073 | 0.231 | −0.486 | 1 | ||
| 6 | Fasting Glucose | 0.276 | −0.018 | 0.068 | −0.200 | 0.214 | 1 | |
| 7 | FPS | 0.376 | 0.152 | 0.399 | −0.485 | 0.458 | 0.238 | 1 |
Correlations greater than the absolute value of ρ = 0.12 were significant using a per-test Sidak-adjusted.
p<0.002 and a family-wise alpha of 0.05.
BMI = Body mass index; CIMT = carotid artery intima-media thickness; LDL-C = Low-density lipoprotein cholesterol; HDL-C = High-density lipoprotein cholesterol; FPS = Framingham point scores.
APOE Genotyping
APOE genotype was determined from DNA extracted from venous blood samples obtained from subjects who gave informed consent for genetic analysis. Blood samples were collected in ethylenediaminetetraacetic (EDTA) tubes during participants’ health examination. DNA was amplified by polymerase chain reaction using specific primers for the APOE gene. The DNA was then sequenced and analyzed for genotype using the FinchTV program (Version 1.3; Geospiza, Inc). APOE4, as well as age, race/ethnicity, and education (as an indicator of socio-economic status-SES) were modeled as predictors of latent class membership.
Assessment of Cognitive Function
As part of the KEEPS-Cog substudy protocol, 662 participants were administered a comprehensive neuropsychological test battery by personnel trained in standardized assessment and scoring procedures. In order to efficiently analyze the cognitive functioning of study participants, a total of 25 test variables were first grouped into cognitive domains based on theoretical considerations. By summarizing our neuropsychological battery into cognitive domains, we limited capitalizing on chance associations in subsequent statistical analyses. These theoretical groupings were then tested iteratively using confirmatory factor analyses (CFA) [40]. We used multiple criteria and recommended thresholds for model selection [41]. These included: 1) comparative fit index (CFI) and Tucker-Lewis index (TLI) greater than 0.95, 2) root mean squared error of approximation (RMSEA) [42] less than 0.05, and 3) the smallest Bayesian information criterion (BIC) [43] value. All models were estimated using maximum likelihood (ML) estimation procedures with standard errors robust to non-normality of observations. The statistics software R, Version 2.15.1 (http://cran.r-project.org/) and the package lavaan, [44] Version 0.4–14, were used to fit the CFA models.
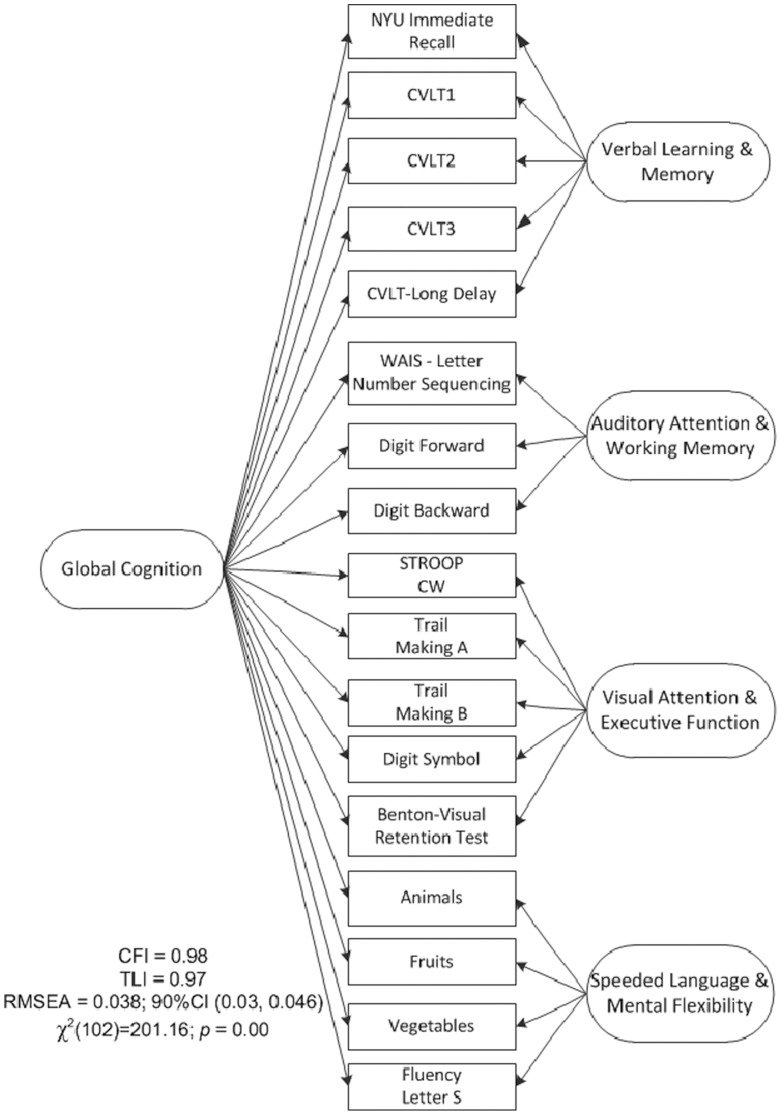
After testing a series of competing models, a bi-factor structure, [45] including a final set of 18 test variables sharing a common underlying construct, provided the best fit to the data. (For a detailed list of the tests included in the final model and an illustration of the bi-factor model, see Figure 1 and Table S1. Table S2 presents a summary of the bi-factor solution. The bi-factor model included a single broad, general construct or factor (labeled global cognition) and four specific and distinct factors uncorrelated with and varying independently of the general or global cognition factor. The four specific factors were labeled as 1) verbal learning & memory 2) auditory attention & working memory, 3) visual attention & executive function and 4) speeded language & mental flexibility. Scores on these five factors were modeled as outcomes conditioned upon latent class membership.
Figure 1. Bi-factor model for the cognitive baseline data.
Eighteen variables from nine tests were used to estimate the model with a global cognitive factor capturing covariation across all variables and four independent secondary factors explaining specific shared covariations beyond that shared with other variables.
Analytical Approach
To examine sample heterogeneity or clustering, we used multiple vascular disease risk indicators as responses in a finite mixture modeling approach. As mentioned above, the vascular disease indicators included: BMI, HDL-C, LDL-C, triglycerides, FBG, CIMT, and FPS. Since the components of finite mixture densities are modeled as latent classes, the analysis is also known in the literature as latent class cluster analysis or latent profile (LP) analysis [46]–[47]. Within this modeling framework, the clusters or “classes” are not predefined; they are estimated by the model. That is, class membership is unobservable and termed latent. It is reasonable to expect that these indicators will be statistically dependent; for instance, people with elevated triglyceride levels also tend to have low HDL-C levels and other conditions such as obesity and metabolic syndrome. An underlying assumption in the LP analysis is that indicators are associated because the study population is comprised of a mixture of subpopulations or classes [48]. A related underlying principle is that as the number of classes increases the indicators become more “homogeneous” or “locally independent” within class. That is, the mutually exclusive classes derived by the LP model maximize between-group variance and minimize within-group variance.
To determine sample heterogeneity as a function of vascular disease risk, we iteratively examined the plausibility of LP models with one, two, and three-latent class solutions. Models were compared by examining multiple fit criteria: [49] 1) a comparison of an c-class solution to an (c+1)-class using a Lo-Mendel Rubin likelihood-ratio tests (LRT) [50] with the choice of the most parsimonious model, 2) BIC, and 3) overall model interpretability. We also used relative entropy as a model selection criterion and the requirement of at least 5% of the sample in each class. Relative entropy is a measure of how well the observed indicators predict class membership with values ranging from 0 to 1 and higher numbers indicating better classification. The final decision on the number of classes needed to test the mixture model hypothesis not only took into account model fit indexes, but also an observed separation of classes showing structure and response patterns that were interpretable and meaningful from a theoretical and clinical perspective.
After rigorous model fit and selection procedures for unconditional models (no covariates) using the full pre-randomized sample (N = 727), we incorporated four predictors of class membership into the model, in the same step in which the measurement model was run, and re-assessed the composition of the classes. The predictors included: 1) age in years, 2) education level (dichotomized as college degree or higher versus below a college degree), 3) APOE4 status (carriers of the ε3/4 or the ε4/4 genotype were categorized as “1;” the absence of the ε4 allele was categorized as “0”), and 4) racial/ethnic background (categorized as non-Hispanic White, non-Hispanic Black, or Hispanic). Other races/ethnicities were excluded from the analysis performed at this stage, because the number of participants in these groups was not large enough to support meaningful comparisons and ensure some prevalence of the predictor level across classes.
Upon the final model selection, each participant was allocated to the most probable latent class, that is, the class with the highest posterior probability of membership. The posterior probability is a function of the parameters of the LP model, covariates, and the participant’s vascular risk profile.
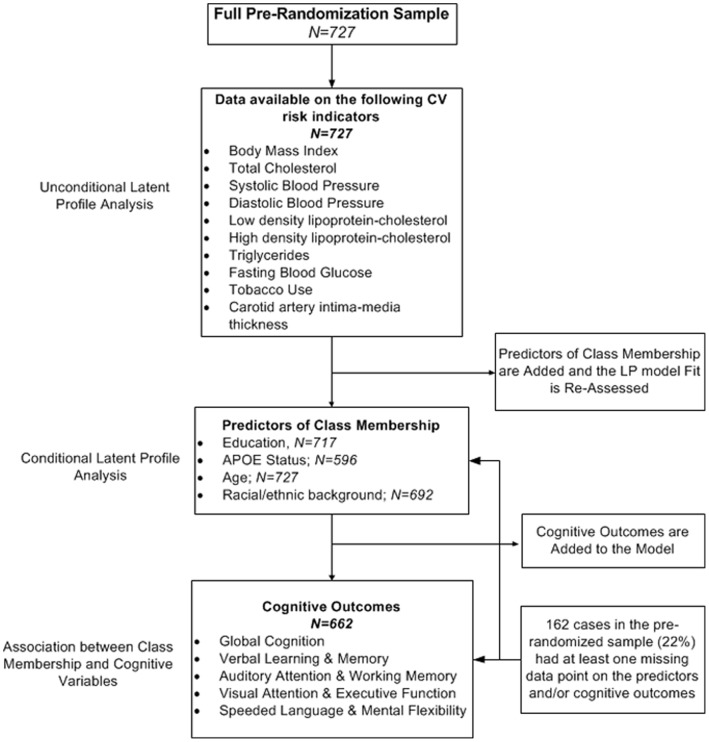
Finally, estimated latent classes were modeled as explanatory variables of cognition in separate analyses; one for each of the five independent cognitive outcomes. The Wald test, a chi-square analog of an F-statistic in analysis of variance, was used to assess the significance of the association between latent classes and cognitive function. (The full model is illustrated in Figure 2 .) A total of 162 cases in the pre-randomized sample (22%) had at least one missing data point on the predictors and/or cognitive outcomes. ( Figure 3 shows a schematic diagram of the steps in the analysis process.).
Figure 2. Diagram illustrating the latent profile model.
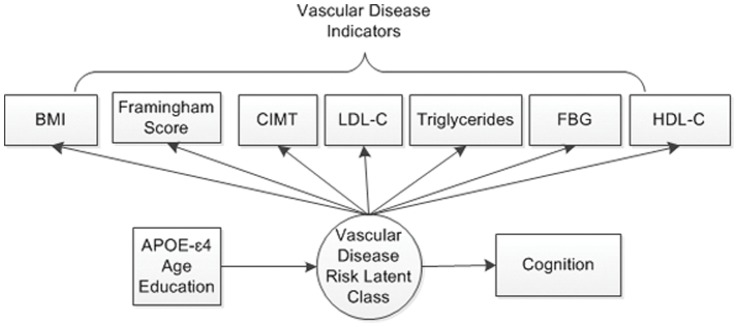
BMI = Body mass index; CIMT = Carotid artery intima-media thickness; LDL-C = Low density lipoprotein-cholesterol; HDL-C = High density lipoprotein-cholesterol; FSG = Fasting blood glucose.
Figure 3. Schematic diagram of the principal steps in the analysis.
Models were estimated via full information (direct) maximum likelihood algorithms using all available data.
The nature of each latent class or “phenotype” was examined by plotting the class-specific estimated mean values against each vascular disease risk variable across classes and inspecting the characteristics of the latent class in terms of CV disease risk profile. To examine the degree to which each class membership could be impacted by demographic and genetic covariates, we used multinomial logistic regression. Odds ratios (ORs) were reported comparing the association between covariates and latent class membership. All LP models were estimated in MPLUS, Version 6.10, using full information ML methods via the expectation-maximization (EM) algorithm to handle missing data [51]–[52]. ML estimation was performed under the assumption of missing at random (MAR). [52] We estimated robust standard errors to account for the non-normality of indicator variables. Results yielding a p-value less than 0.05 were deemed statistically significant.
Cross-validation Through Supervised Machine Learning
Bias due to over-fitting is a common criticism of “in-sample” model selection in latent class modeling. We used a supervised random-forest (RF) classification algorithm with a 10-fold cross-validation estimator [53] to assess overall classification accuracy (or error rate). Latent class membership was modeled as the outcome conditioned upon vascular risk variables. Details of the RF procedure are explained elsewhere [53]–[54]. Briefly, in an attempt to reduce the bias of a “single tree” prediction of classes, ntree bootstrap samples are drawn from the total data set and for each of the samples, a classification tree is grown. The split of each node in the tree is based on a random sample of predictors. New data are predicted by aggregating the ntree classification trees (i.e., the majority votes for the classification). The accuracy or “error rate” is estimated by predicting the data not in the bootstrap sample (generally 1/3 of the sample) using the classification tree obtained with the bootstrap sample (2/3 of the sample). All these predictions are aggregated to obtain an estimate of misclassification or error rate. In our analysis, we grew a total of ntree = 1,000 trees. As part of the algorithm, RF estimates variable importance measures for each tree through permutation of variable values. Variable importance is defined as the average increase in error over all the trees (mean decrease accuracy) grown in the classifier. The analyses were performed with the randomForest [55] package in R, Version 2.15.1 (http://cran.r-project.org/).
Results
Latent Profile Analysis
Results for the sequence of unconditional models fitted to the joint distribution of the seven CV disease risk factors indicated that a 2-class LP model adequately fit the data. As shown in Table 3 , the unconditional 2-class model had the highest classification accuracy or Entropy (0.802), the lowest BIC value, and a reasonable classification of vascular disease risk patterns. The classification accuracy was substantially lower (0.684) for the 3-class model. Despite the rejection of the Lo-Mendell-Rubin LRT test in favor of the 3-class model (marginal p-value = 0.04), the separation of classes was less interpretable. Including age, education level, race/ethnicity, and APOE4 as predictors of class membership (that is, the conditional model) improved overall model fit and did not change significantly the prevalence of risk in the two classes. BIC values were lower than those obtained in the unconditional model and the Entropy for the 2-class model increased to 0.811. Additionally, the LRT test indicated that a three-class solution did not represent a significant improvement over the two-class model (p = 0.054). Therefore, a 2-class model was chosen as the best fitting model.
Table 3. Fit of the Latent Class Profile Models.
| Model | No. of ParametersEstimated | Entropy | BIC | Lo-Mendell-RubinAdjusted LRT (p-value) |
| Unconditional Model | ||||
| 1-Class | 15 | – | 14,444 | – |
| 2-Class versus 1-Class | 28 | 0.802 | 13,821 | 0.000 |
| 3-Class versus 2-Class | 30 | 0.644 | 13,824 | 0.040 |
| Conditional Model | ||||
| 1-Class | 20 | – | 11,177 | – |
| 2-Class versus 1-Class | 33 | 0.811 | 10,719 | 0.000 |
| 3-Class versus 2-Class | 40 | 0.694 | 10,724 | 0.054 |
BIC = Bayesian information criterion; LRT = Likelihood ratio test.
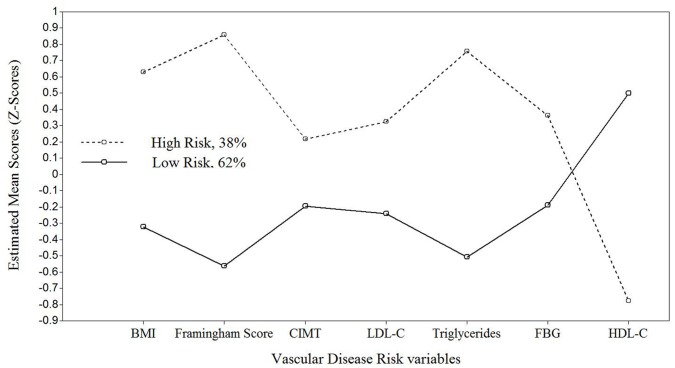
The patterns of estimated vascular disease risk measures and posterior probabilities assigned to each individual were used to label the latent classes. The first class was labeled “high-risk” because participants were more likely to have lower values on HDL-C and higher values on triglycerides, BMI, LDL-C, FBG, CIMT, and FPS (see Figure 4). The opposite was observed in the second class, labeled as “low-risk.” That is, participants tended to have higher HDL-C levels and lower triglycerides, LDL-C, FBG, CIMT, and FPS values. The prevalence in the “high-risk” and “low-risk” class was 38% and 62%, respectively. Table 4 shows the observed and model-estimated means for all the vascular disease risk variables by latent class. Using an independent samples t-test, mean differences between groups were highly significant for all vascular disease risk variables (p-values<0.001).
Figure 4. Estimated Mean Vascular Disease Risk for Each Latent Group.
BMI = Body mass index; CIMT = Carotid artery intima-media thickness; LDL-C = Low density lipoprotein-cholesterol; HDL-C = High density lipoprotein-cholesterol; FSG = Fasting blood glucose.
Table 4. Estimated and Observed Within-Class Means and Standard Errors for Vascular Disease Risk Variables From the Two-Class Model.
| Vascular Disease Risk Variables | Estimated Within-Class Means1 | Observed Within-Class Means | t | p-value | ||||||
| Class 1: Low Risk | Class 2: High Risk | Class 1: Low Risk | Class 2: High Risk | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| BMI | −0.324 | 0.057 | 0.630 | 0.073 | 24.80 | 3.88 | 28.97 | 3.73 | 12.57 | <0.001 |
| CIMT | −0.194 | 0.051 | 0.216 | 0.082 | 0.70 | 0.07 | 0.74 | 0.10 | 5.250 | <0.001 |
| LDL-C | −0.240 | 0.064 | 0.323 | 0.069 | 121.89 | 28.91 | 138.35 | 28.23 | 6.620 | <0.001 |
| HDL-C | 0.498 | 0.055 | −0.779 | 0.065 | 74.13 | 15.20 | 51.22 | 9.80 | −21.751 | <0.001 |
| Triglycerides | −0.510 | 0.051 | 0.755 | 0.086 | 66.73 | 25.74 | 129.26 | 60.24 | 14.402 | <0.001 |
| FSG | −0.188 | 0.049 | 0.361 | 0.083 | 87.31 | 8.46 | 92.73 | 9.78 | 6.718 | <0.001 |
| FPS | −0.564 | 0.059 | 0.857 | 0.063 | 2.16 | 2.25 | 6.89 | 2.14 | 25.926 | <0.001 |
| Latent Prevalence (Marginal Probability) | 62% | 38% | ||||||||
The estimated within-class means represent the mean difference between the vascular disease risk score of that particular class compared with the overall mean. Estimated means are based on standardized measures.
BMI = Body mass index; CIMT = Carotid artery intima-media thickness; LDL-C = Low density lipoprotein-cholesterol; HDL-C = High density lipoprotein-cholesterol; FSG = Fasting blood glucose; FPS = Framingham point scores.
Predictors of Class Membership
Age, education, and race/ethnicity were predictive of class membership (see Table 5 ). Older age and a Hispanic background increased the probability of being in the “high-risk” class (OR = 1.140, p = 0.002; OR = 2.621, p = 0.012; respectively). Women with a college degree or above were more likely to be in the “low-risk” class (OR = 0.63, p = 0.044). The prevalence rate of women with at least one APOE-ε4 allele was also higher in the “high-risk” class compared with rates in the “low-risk” class (OR = 1.52). However, APOE4 was not predictive of class membership (p = 0.073).
Table 5. Conditional Odds Ratios.
| Variable | Wald 95%Confidence Limits | ||||
| Odds Ratio | Lower | Upper | p-value | 95% CLR | |
| High Class on | |||||
| Education (college degree or higher relative to below college degree) | 0.627 | 0.398 | 0.987 | 0.044 | 2.5 |
| Hispanic (relative to non-Hispanic White) | 2.621 | 1.236 | 5.56 | 0.012 | 4.5 |
| Non- Hispanic Black (relative to non-Hispanic White) | 0.951 | 0.426 | 2.122 | 0.902 | 5.0 |
| Age | 1.140 | 1.05 | 1.238 | 0.002 | 1.2 |
| APOE4 | 1.521 | 0.961 | 2.406 | 0.073 | 2.5 |
Abbreviations: CLR = confidence limit ratio.
Association between Latent Classes and Cognitive Function
The Wald test of parameter constraints yielded a statistically significant association between latent classes and two cognitive factor scores obtained from the bi-factor solution: 1) speeded language & mental flexibility (χ2 (1df) = 6.995; p = 0.008) and 2) the general global cognition factor (χ2 (1df) = 4.786; p = 0.029). The estimated mean cognitive scores were significantly better in the “low-risk” class for speeded language & flexibility (M = 0.068) and global cognition (M = 0.772) compared to those obtained in the “high-risk” class (M = −0.139; M = −0.110, respectively). In a post-hoc analysis, we estimated the effect of posterior probabilities of class membership on cognitive performance across all domains after controlling for group differences in age and education. The relationship between the probability associated with membership in the “high” risk class and performance in speeded language and flexibility tasks remained highly significant (p = 0.001). That is, the higher the probability of being in the “high” risk class, the lower the score in speeded language and flexibility. However, differences in global cognition outcomes, as a function of class probabilities and age and education covariates, were attenuated (p = 0.06). In both analyses, latent classes were not associated with performance on three specific factors in the bi-factor model, namely, verbal learning & memory, auditory attention & working memory, and visual attention & executive function.
Cross-validation through Random Forests
We used the classifications obtained through LP analysis as a dependent variable conditioned upon vascular risk and predictor variables to assess the performance of a RF algorithm at predicting class membership. A 10-fold cross-validation estimator was used to assess overall error rate. The RF algorithm yielded an estimated classification accuracy of 96%. That is, the classifier allocated individuals into the “correct” or “LP-estimated class” with approximately 4% error rate. These results support the previously obtained solution and the observed pattern of vascular disease risk measures meaningfully separating individuals into two distinct groups or phenotypes. Interestingly, the weights assigned to each vascular disease risk variable ranking their “importance” as predictors in the RF classifier (see Figure 5 ) corresponded closely to the observed separation of classes. For example, the four variables with the highest importance weights (HDL-C, FPS, triglycerides, and BMI) also produced the highest separation between the latent classes illustrated in Figure 4 .
Figure 5. Variable Importance Measures Estimated by Mean Decreased Accuracy.
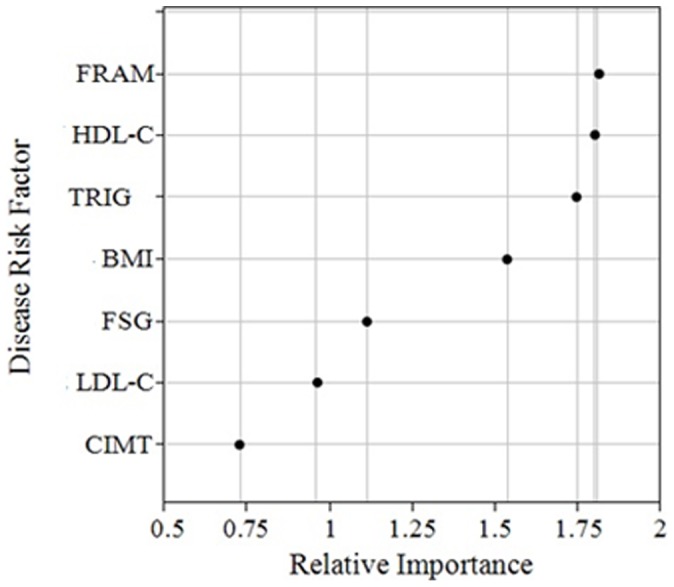
BMI = Body mass index; CIMT = Carotid artery intima-media thickness; LDL-C = Low density lipoprotein-cholesterol; HDL-C = High density lipoprotein-cholesterol; FSG = Fasting blood glucose; FRAM = Framingham Point scores; TRIG = Triglycerides.
Discussion
A latent profile (LP) analysis, using seven clinically-relevant variables for CV disease risk obtained at baseline from a cohort of recently menopausal women enrolled in the KEEPS study, revealed two distinct classes or phenotypes, depicting low versus high CV risk. The low CV risk group was, as expected, larger, with 62% of the respondents, while the high risk group comprised the remaining 38%. Our results supported the hypothesis that genetic and demographic variables were predictive of the model-identified classes or phenotypes. An interesting finding is that latent class membership was significantly associated with performance in cognitive tasks. That is, individuals in the low CV risk group, on average, obtained significantly higher scores particularly on executive function tasks measuring speeded language and mental flexibility compared to those in the high CV risk group. The speeded language and mental flexibility factor score was composed of tests of letter and category word list generation, which have been frequently used to investigate the semantic fluency deficits related to the progression of AD [56]–[57] and ischemic vascular dementia [58]–[59].
Midlife CV risk factors are well-known non-genetic risk factors for incident AD and cognitive decline. Published studies have also shown a relationship between higher systolic blood pressure or total cholesterol and LDL-C concentrations in midlife and increased risk of cognitive impairment or AD [60]–[62]. For example, Knopman et al. [60] found an association between hypertension and decline in processing speed tasks over a 6-year period. The relationship between BMI and cognitive function appears more complex, possibly varying depending on the location of adiposity. A recent study [63] using baseline data from the seminal Women’s Health Initiative (WHI) hormone trials cohort reported an inverse association between BMI and performance on the Modified Mini-Mental State examination (3MSE); a measure of global cognitive functioning. Interestingly, this association was stronger in women with smaller waist to hip ratio (WHR) (<0.78) and weaker with higher WHR and BMI measurements. These findings suggested a relationship between BMI and cognitive function conditioned upon abdominal obesity in cognitively normal older postmenopausal women. A second study using 4-year follow-up data from women enrolled in WHI Memory Study (WHIMS) [64] found significant interactions between BMI, WHR, and incident cognitive impairment and probable dementia. That is, in women with BMIs between 20 and 29.9 kg/m2, central adiposity (WHR≥0.80) was associated with an increased risk of cognitive impairment and probable dementia. Although the mechanisms underlying the complex associations between both indices of obesity and cognitive function are unclear, our study demonstrated the synergistic role of obesity, as measured by BMI, in identifying unobserved group heterogeneity.
The present study suggests that even within a relatively healthy sample of postmenopausal women at “low” CV risk, vascular disease risk factors exhibit important heterogeneity. The LP approach captured cross-sectional group differences in CV disease risk associated with demographic, genetic, and cognitive variables. To the best of our knowledge, this is the first study investigating whether model-based CV disease risk profiles or groups, based on multiple risk criteria, are associated with cognitive function in recently menopausal women. It is possible that the latent “at risk” group identified by this analysis is capturing women at increased risk for the vascular pathway to AD. The use of model-based analytical approaches to identify systematic heterogeneity and complex “within-class” inter-relationships among multiple biomarkers of risk may be more informative than using standard group-based approaches or “total” sample average scores of vascular risk variables. The accuracy and utility of single estimates of CV risk, such as FPS, can be greatly enhanced by considering additional factors that may help explain the considerable individual variability in risk that may exist in the larger population. Absolute risk in the Framingham population for a given set of factors may not be the same as that for all other populations with differing characteristics such as ethnicity. Therefore, the risk assigned by the FPS may miss a large number of individuals destined for CV events. Newer biomarkers such as CIMT and CAC scores, and both predisposing (e.g., BMI, physical inactivity, and abdominal obesity) and conditional (e.g., inflammatory markers and elevated serum triglycerides and lipoprotein) risk factors may potentially modify the magnitude of risk for individuals [65]–[66]. Our findings imply that a mixture-based approach can have potential to study the relatedness of multiple risk variables beyond single risk scores measures representing average values.
Our phenotypes portray a pattern consistent with a large number of studies showing the varying prevalence of risk factors and the underlying rates of CV disease events according to age, education level (as an indicator of SES), and race/ethnicity [67]–[72]. For example, in the Framingham study, women with less than 12 years of education had nearly a four-fold higher risk of developing CV disease than women with higher education level [69], [71]. The same study also reported a higher incidence of CV disease among postmenopausal women (up to the age of 55) than that found in younger pre-menopausal women. The Cardiovascular Health Study, a longitudinal study designed to examine risk factors for coronary artery disease in a large population of 5,201 men and women, reported that heavier weight at age 50 (i.e., a BMI ≥27) had a stronger association with prevalent CV disease in women than current weight at age 65 or older [72].
Present findings are also convergent with studies reporting a higher prevalence of vascular disease risk factors among Hispanics compared to non-Hispanic Whites [67], [73]–[76]. Risk factors observed in these studies included obesity, lower levels of physical activity, incidence of metabolic syndrome, and lipid abnormalities. The observed upward prevalence trends in APOE ε4 carriers among women in the “high risk” class in our study is also in agreement with findings from a large number of studies showing associations between APOE polymorphisms and cardiovascular risk and lipid profile phenotypes [8], [77]–[78]. Other studies have also demonstrated that a decrease in plasma estrogen levels after menopause and APOE may jointly affect lipid and triglyceride levels [79].
A limitation of the current study was the use of a selected number of CV risk factors in the LP analysis. However, these measures differentiated between distinct subclinical phenotypes and suggested a patterning of and unique co-variation in risk associated with cognitive function and demographic features. Further studies could explore the reproducibility of the results in ethnically-varied samples of postmenopausal women. Future longitudinal studies should also investigate the utility of the combination of CV disease risk variables used as surrogates for class membership in this study in predicting not only CV disease, but also cognitive impairment across multiple domains.
We acknowledge that questions remain regarding the complex nature of the interrelationships between vascular risk biomarkers included and not included in the present analysis and their dual prognostic utility for cognitive decline and CV events. Nonetheless, this study highlights the importance of a multifactorial approach to vascular disease risk. The use of an LP framework for the identification of empirically-derived qualitative phenotypes of risk based on a combination of both traditional and newer risk markers can be extremely useful in defining risk scoring systems with better prediction accuracy and clinical relevance for postmenopausal women and ethnic minority groups. Future work designed to evaluate the evolution of phenotypes could in turn contribute to the understanding of preclinical disease and the role of screening and preventive interventions.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
IRB numbers for KEEPS institutions: The central KEEPS and Phoenix KEEPS (IRB protocol by the Western IRB): STUDY NUM: 1058663 and WIRB PRO NUM: 20040792KEEPS (main study & cognitive substudy) #10-02980 and MDBHAS #11-05383. Brigham and Women’s Hospital (Partners): #2004-P-002144 BWH. Mayo Clinic: 2241-04. Columbia: IRB#: AAAA-8062. Yale: 0409027022. University of Utah: 13257. Einstein/Montefiore: 04-08-213. Univ of Wisconsin: H-2005-0059. UCSF: KEEPS (main study & cognitive substudy) #10-02980. University of Washington IRB #26702; VAPSHCS IRB #01048.
Additional KEEPS Investigators and Staff: Albert Einstein College of Medicine: Ruth Freeman, Hussein Amin, Barbara Isaac, Maureen Magnani, Rachel Wildman. Brigham and Women’s Hospital/Harvard Medical School: Maria Bueche, Marie Gerhard-Herman, Kate Kalan, Jan Lieson, Kathryn M. Rexrode, Barbara Richmond, Frank Rybicki, Brian Walsh. Columbia College of Physicians and Surgeons: Luz Sanabria, Maria Soto, Michelle P. Warren, Ralf C. Zimmerman. Kronos Longevity Research Institute: Mary Dunn, Panayiotis D.Tsitouras, Viola Zepeda. Mayo Clinic: Philip A. Araoz, Rebecca Beck, Dalene Bott-Kitslaar, Sharon L. Mulvagh, Lynne T. Shuster, Teresa G. Zais. University of California, Los Angeles, CAC Reading Center: Chris Dailing, Yanlin Gao, Angel Solano. University of California, San Francisco Medical Center: Nancy Jancar, Jean Perry, Rebecca S. Wong, Robyn Pearl, Judy Yee, Brett Elicker, Gretchen A.W. Gooding; UCSF Statistical Reading Center: Lisa Palermo. University of Southern California, Atherosclerosis Research Unit/Core Imaging and Reading Center: Yanjie Li, Mingzhu Yan. University of Utah School of Medicine: Paul N. Hopkins, M. Nazeem Nanjee, Kirtly Jones, Timothy Beals, Stacey Larrinaga-Shum. VA Puget Sound Health Care System and University of Washington School of Medicine: Pamela Asberry, SueAnn Brickle, Colleen Carney, Molly Carr, Monica Kletke, Lynna C. Smith. Yale University, School of Medicine: Kathryn Czarkowski, Lubna Pal, Linda McDonald, Mary Jane Minkin, Diane Wall, Erin Wolff (now at NIH/NICHD).
Additional Contributions: We gratefully acknowledge the dedicated efforts of all the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at KLRI, and the NIH Institutes supporting ancillary studies. Above all, we recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
Funding Statement
The KEEPS-cog project was supported by grants from the National Institutes of Health (NIH) R01 AG029624, P50AG033514, and R01AG031790. The parent KEEPS trial is funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, NIH HL90639 to VMM, Mayo CTSA1 UL1 RR024150, the Einstein College of Medicine CTSA UL1 RR025750, KL2 RR025749 and TL1 RR025748, the Mayo Foundation, the United States Department of Veterans Affairs Puget Sound Health Care System, Brigham and Women’s Hospital/Harvard Medical School CTSA, CTSA UL1 RR024139 and UCSF CTSA UL1 RR024131 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH and NIH Roadmap for Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Anderson KM, Odell PM, Wilson PWF, Kannel WB (1991) Cardiovascular disease risk profiles. Am Heart J 121: 293–298. [DOI] [PubMed] [Google Scholar]
- 2. Howard BV, Rodriguez BL, Bennett PH, Harris MI, Hamman R, et al. (2002) Prevention Conference VI: Diabetes and Cardiovascular disease: Writing Group I: epidemiology. Circulation 105: 132–137. [DOI] [PubMed] [Google Scholar]
- 3. McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, et al. (2003) Women’s early warning symptoms of acute myocardial infarction. Circulation 108: 2619–2623. [DOI] [PubMed] [Google Scholar]
- 4. Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra-Garcia M, et al. (2003) Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 61: 199–205. [DOI] [PubMed] [Google Scholar]
- 5. Sherwin BB (2003) Estrogen and cognitive functioning in women. Endocrine Reviews 24: 133–151. [DOI] [PubMed] [Google Scholar]
- 6. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, et al. (2011) Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation 123: 1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ (2010) Twelve-year follow-up of American women’s awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes 3: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, et al. (2002) Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137: 149–155. [DOI] [PubMed] [Google Scholar]
- 9. Chan N, Colhoun H, Vallance P (2001) Cardiovascular risk factors as determinants of endothelium-dependent and endothelium-independent vascular reactivity in the general population. J Am Coll Cardiol 38: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 10. Kuvin JT, Patel AR, Sidhu M, Rand WM, Sliney KA, et al. (2003) Relation between high-density lipoprotein cholesterol and peripheral vasomotor function. Am J Cardiol 92: 275–279. [DOI] [PubMed] [Google Scholar]
- 11. Schachinger V, Britten MB, Zeiher AM (2000) Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906. [DOI] [PubMed] [Google Scholar]
- 12. Biegelsen ES, Loscalzo J (1999) Endothelial function and atherosclerosis. Coron Artery Dis 10: 241–256. [PubMed] [Google Scholar]
- 13. Hirata K, Miki N, Kuroda Y, Sakoda T, Kawashima S, et al. (1995) Low concentration of oxidized low-density lipoprotein and lysophosphatidylcholine upregulate constitutive nitric oxide synthasemRNA expression in bovine aortic endothelial cells. Circ Res 76: 958–962. [DOI] [PubMed] [Google Scholar]
- 14. O’Keefe JH, Carter MD, Lavie CJ (2009) Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc 84: 741–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferre R, Aragones G, Plana N, Merino J, Heras M, et al. (2011) High-density lipoprotein cholesterol and apolipoprotein A1 levels strongly influence the reactivity of small peripheral arteries. Atherosclerosis 216: 115–119. [DOI] [PubMed] [Google Scholar]
- 16. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, et al. (1996) Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582. [DOI] [PubMed] [Google Scholar]
- 17. Christian A. Mochari H, Mosca LJ (2005) Coronary Heart Disease in Ethnically Diverse Women: Risk Perception and Communication Mayo Clin Proc. 80: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 18. Nyboe J, Jensen G, Appleyard M, Schnohr P (1989) Risk factors for acute myocardial infarction in Copenhagen. I: Hereditary, educational and socioeconomic factors. Copenhagen City Heart Study. Eur Heart J 10: 910–916. [DOI] [PubMed] [Google Scholar]
- 19. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261: 921–923. [DOI] [PubMed] [Google Scholar]
- 20. Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB (1991) Probability of stroke: a risk profile from the Framingham Study. Stroke 22: 312–318. [DOI] [PubMed] [Google Scholar]
- 21. Kahn R, Buse J, Ferrannini E, Stern M (2005) The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28: 2289–2304. [DOI] [PubMed] [Google Scholar]
- 22. Laakso M, Sarlund H, Mykkanen L (1990) Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis 10: 223–231. [DOI] [PubMed] [Google Scholar]
- 23. Erdine S, Ari O (2006) ESH–ESC guidelines for the management of hypertension. Herz 31: 331–338. [DOI] [PubMed] [Google Scholar]
- 24. Rodondi N, Locatelli I, Aujesky D, Butler J, Vittinghoff E, et al. (2012) Framingham Risk Score and Alternatives for Prediction of Coronary Heart Disease in Older Adults. PLoS ONE 7: e34287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Hong Y, D’Agostino RB Sr, Wu Z, Wang W, et al. (2004) Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-provincial Cohort Study. JAMA 291: 2591–2599. [DOI] [PubMed] [Google Scholar]
- 26.McLachlan GJ, Peel D (2000). Finite Mixture Models. New York: John Wiley & Sons.
- 27. Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, et al. (2005) KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8: 3–12. [DOI] [PubMed] [Google Scholar]
- 28. Harman SM, Brinton EA (2009) Biphasic effects of hormone treatment on risk of cardiovascular disease: Resolving the paradox in postmenopausal women. Menopausal Medicine 17: S1–S10. [Google Scholar]
- 29. Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, et al. (2009) Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res 2: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small BJ, Rosnick CB, Fratiglioni L, Backman L (2004) Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and Aging 19, 592–600. [DOI] [PubMed]
- 31. Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, et al. (2002) Cognitive change and the ApoE ε4 allele. Nature 418: 932. [DOI] [PubMed] [Google Scholar]
- 32. Kofler BM, Miles EA, Curtis P, Armah CK, Tricon S, et al. (2012) Apolipoprotein E genotype and the cardiovascular disease risk phenotype: impact of sex and adiposity (the FINGEN study).Atherosclerosis. 221: 467–470. [DOI] [PubMed] [Google Scholar]
- 33. Dupuy AM, Mas E, Ritchie K, Descomps B, Badiou S, et al. (2001) The relationship between apolipoprotein E4 and lipid metabolism is impaired in Alzheimer’s disease. Gerontology 47: 213–218. [DOI] [PubMed] [Google Scholar]
- 34. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, et al. (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: 1349–1356. [PubMed] [Google Scholar]
- 35. Yaffe K, Cauley J, Sands L, Browner W (1997) Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Archives of Neurology 54: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 36. National Institute of Health. National Heart Lung and Blood Institute (1998) Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report. Obesity Research 6: 51S–209S. [PubMed] [Google Scholar]
- 37. Jensen J, Nilas L, Christiansen C (1990) Influence of menopause on serum lipids and lipoproteins. Maturitas 12: 321–331. [DOI] [PubMed] [Google Scholar]
- 38. Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, et al. (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 39. Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, et al. (2001) Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial.Ann Intern Med 135: 939–953. [DOI] [PubMed] [Google Scholar]
- 40. Jöreskog KG (1969) A general approach to confirmatory maximum likelihood factor analysis. Psychometrika 34: 183–202. [Google Scholar]
- 41. Hu LT, Bentler PM (1999) Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria Versus New Alternatives, Structural Equation Modeling. 6: 1–55. [Google Scholar]
- 42. Steiger JH (1990) Structural model evaluation and modification, Multivariate Behavioral Research. 25: 214–212. [DOI] [PubMed] [Google Scholar]
- 43. Schwarz GE (1978) “Estimating the dimension of a model”. Ann Stat 6: 461–464. [Google Scholar]
- 44. Rosseel Y (2012) Lavaan: An R Package for Structural Equation Modeling. J Stat Softw 48: 1–36. [Google Scholar]
- 45. Gibbons RD, Hedeker DR (1992) Full-information item bi-factor analysis. Psychometrika 57: 423–436. [Google Scholar]
- 46.Lazarsfeld PF, Henry NW (1968) Latent Structure Analysis, Boston: Houghton Mifflin.
- 47. Magidson J, Vermunt JK (2001) Latent class factor and cluster models, bi-plots and related graphical displays. Sociological Methodology 31: 223–264. [Google Scholar]
- 48. Bandeen-Roche K, Miglioretti DL, Zeger SL, Rathouz PJ (1997) Latent variable regression for multiple discrete outcomes. JASA 92: 1375–1386. [Google Scholar]
- 49. Nylund KL, Asparouhov T, Muthén B (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling. A Monte Carlo simulation study. Structural Equation Modeling 14: 535–569. [Google Scholar]
- 50. Lo Y, Mendell NR, Rubin DB (2001) Testing the number of components in a normal mixture. Biometrika 88: 767–778. [Google Scholar]
- 51.Muthén B, Muthén L (2010) Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén.
- 52. Dempster AP, Laird NM, Rubin DM (1977) Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc B 39: 1–38. [Google Scholar]
- 53. Breiman L (2001) Random forests. Machine Learning 45: 5–32. [Google Scholar]
- 54. Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2–3: 18–22. [Google Scholar]
- 55.Breiman L, Cutler A, Liaw A, Wiener M (2012) Breiman and Cutler’s Random Forests for Classification and Regression. R package version 4.6–6.
- 56. Mickanin J, Grossman M, Onishi K, Auriacombe S, Clark C (1994) Verbal and non-verbal fluency in patients with probable Alzheimer’s disease. Neuropsychology 8: 385–394. [Google Scholar]
- 57. Weingartner HJ, Kawas C, Rawlings R, Shapiro M (1993) Changes in semantic memory in early stages of Alzheimer’s disease patients. The Gerontologist 33: 637–643. [DOI] [PubMed] [Google Scholar]
- 58. Carew TG, Lamar M, Cloud BS, Grossman M, Libon DJ (1997) Impairment in category fluency in ischemic vascular dementia. Neuropsychology 11: 400–412. [DOI] [PubMed] [Google Scholar]
- 59. Giovannetti T, Lamar M, Cloud BS, Swenson R, Fein D, et al. (2001) Different underlying mechanisms for deficits in concept formation in dementia. Archives of Clinical Neuropsychology 16: 547–560. [PubMed] [Google Scholar]
- 60. Knopman D, Boland LL, Mosley T, Howard G, Liao D, et al. (2001) Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 56: 42–48. [DOI] [PubMed] [Google Scholar]
- 61. Yaffe K, Barrett-Connor E, Lin F, Grady D (2002) Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 59: 378–384. [DOI] [PubMed] [Google Scholar]
- 62. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Alhainen K, et al. (2001) Midlife vascular risk factors and Alzheimer’s disease in late life: a longitudinal, population based study. BMJ 2 322: 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kerwin DR, Zhang Y, Kotchen JM, Epeland MA, Van Horn, L, et al (2010) The cross-sectional relationship between body mass index, waist-hip ratio, and cognitive performance in postmenopausal women enrolled in the Women’s Health Initiative. J Am Geriatr Soc. 58: 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kerwin DR, Gaussoin SA, Chlebowski RT, Kuller LH, Vitolins M, et al. (2011) Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: results from the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 59: 107–112. [DOI] [PubMed] [Google Scholar]
- 65. Grundy SM, Pasternak R, Greenland P, Smith S Jr, Fuster V (1999) AHA/ACC scientific statement: assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 34: 1348–1359. [DOI] [PubMed] [Google Scholar]
- 66. Naqvi TZ, Mendoza F, Rafii F, Gransar H, Guerra M, et al. (2010) High prevalence of ultrasound detected carotid atherosclerosis in subjects with low Framingham risk score: potential implications for screening for subclinical atherosclerosis. J Am Soc Echocardiogr 23: 809–815. [DOI] [PubMed] [Google Scholar]
- 67. Shetterly SM, Rewers M, Hamman RF, Marshall JA (1994) Patterns and predictors of hypertension incidence among Hispanics and non-Hispanic whites: the San Luis Valley Diabetes Study. J Hypertens 12: 1095–1102. [PubMed] [Google Scholar]
- 68. Luepker RV, Rosamond WD, Murphy R, Sprafka JM, Folsom AR, et al. (1993) Socioeconomic status and coronary heart disease risk factor trends. The Minnesota Heart Study. Circulation 88: 2172–2179. [DOI] [PubMed] [Google Scholar]
- 69. Eaker ED (1989) Psychological factors in the epidemiology of coronary heart disease in women. Psychiatric Clinics of North America 12: 167–174. [PubMed] [Google Scholar]
- 70. Tyroler HA (1989) Socioeconomic status in the epidemiology and treatment of hypertension. Hypertension 13: 194–197. [DOI] [PubMed] [Google Scholar]
- 71. Dannenberg AL, Garrison RJ, Kannel WB (1988) Incidence of hypertension in the Framingham Study. Am J Public Health 78: 676–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kraus JF, Borhani NO, Franti CE (1980) Socioeconomic status, ethnicity, and risk of coronary heart disease. Am J Epidemiol 111: 407–414. [DOI] [PubMed] [Google Scholar]
- 73. Derby CA, Wildman RP, McGinn AP, Green RR, Polotsky AJ, et al. (2010) Cardiovascular risk factor variation within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Ethn Dis. 20: 396–402. [PMC free article] [PubMed] [Google Scholar]
- 74. Harris TB, Savage PJ, Tell GS, Haan M, Kumanyika S, et al. (1997) Carrying the burden of cardiovascular risk in old age: associations of weight and weight change with prevalent cardiovascular disease, risk factors, and health status in the Cardiovascular Health Study. Am J Clin Nutr 66: 837–844. [DOI] [PubMed] [Google Scholar]
- 75. Burchfiel CM, Hamman RF, Marshall JA, Baxter J, Kahn LB, et al. (1990) Cardiovascular risk factors and impaired glucose tolerance: The San Luis Valley Diabetes Study. Am J Epidemiol 131: 57–70. [DOI] [PubMed] [Google Scholar]
- 76. Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK (1990) Risk factors for cardiovascular mortality in Mexican Americans and non-Hispanic whites: San Antonio Heart Study. Am J Epidemiol 131: 423–433. [DOI] [PubMed] [Google Scholar]
- 77. Reilly M, Rader DJ (2006) Apolipoprotein E and coronary disease: a puzzling paradox. PLoS Med 3: e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wilson PW, Schaefer EJ, Larson MG, Ordovas JM (1996) Apolipoprotein E alleles and risk of coronary disease: a meta-analysis. Arterioscler Thromb Vasc Biol 16: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 79. Schaefer EJ, Lamon-Fava S, Cohn SD, Schaefer MM, Ordovas JM, et al. (1994) Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study J Lipid Res. 35: 770–792. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)