Abstract
Patients with idiopathic Parkinson’s disease exhibit impairments in executive processes, including planning and set-shifting, even at the early stages of the disease. We have recently developed a new card-sorting task to study the specific role of the caudate nucleus in such executive processes and have shown, using functional magnetic resonance imaging (fMRI) in young healthy adults, that the caudate nucleus is specifically required when a set-shift must be planned. Here the same fMRI protocol was used to compare the patterns of activation in a group of early-stage Parkinson’s disease patients (seven right-handed patients at Hoehn and Yahr stages 1 and 2; mean age 62 years, range 56–70) and matched control subjects. Increased cortical activation was observed in the patients compared with the control group in the condition not specifically requiring the caudate nucleus. On the other hand, decreased cortical activation was observed in the patient group in the condition significantly involving the caudate nucleus. This event-related fMRI study showed a pattern of cortical activation in Parkinson’s disease characterized by either reduced or increased activation depending on whether the caudate nucleus was involved or not in the task. This activation pattern included not only the prefrontal regions but also posterior cortical areas in the parietal and prestriate cortex. These findings are not in agreement with the traditional model, which proposes that the nigrostriatal dopamine depletion results in decreased cortical activity. These observations provide further evidence in favour of the hypothesis that not only the nigrostriatal and but also the mesocortical dopaminergic substrate may play a significant role in the cognitive deficits observed in Parkinson’s disease.
Keywords: executive functions, fMRI, Parkinson’s disease, set-shifting, striatum
Introduction
Parkinson’s disease is a movement disorder arising from the loss of dopamine (DA) neurons in the substantia nigra that project to the striatum (which includes the caudate nucleus and the putamen; Kish et al., 1988). The most documented symptoms are motor deficits that include tremor, rigidity and bradykinesia. However, neuropsychological studies have revealed deficits in a range of cognitive functions even at the early stages of the disease (Taylor et al., 1986; Taylor and Saint-Cyr, 1995; Dubois and Pillon, 1997). A major deficit lies in executive functions that include planning and set-shifting (Brown and Marsden, 1988; Morris et al., 1988; Saint-Cyr et al., 1988; Grossman et al., 1992; Owen et al., 1992). The initial functional brain imaging studies of Parkinson’s disease showed a reduction in movement-induced increases in cortical cerebral blood flow (Playford et al., 1992). Based on the model of Albin, Young and Penney (1989), the proposed explanation was that excessive inhibitory outflow from basal ganglia to cortex via the thalamus was responsible for both bradykinesia and cortical hypo-activation. A similar explanation, however, could not account for certain cognitive deficits associated with this disease, as PET studies had demonstrated increased pre-frontal cortex (PFC) activation in patients during performance of cognitive tasks sensitive to PFC dysfunction (Owen et al., 1992; Dagher et al., 2001). While the hallmark of Parkinson’s disease is dopaminergic nigrostriatal neuronal loss (Bernheimer et al., 1973; Kish et al., 1988), several postmortem studies (Javoy-Agid and Agid, 1980; Scatton et al., 1983) and PET investigations in patients (Ouchi et al., 1999) provided evidence that the mesocortical dopaminergic system is also damaged. Such a reduction of mesocortical DA in the PFC of patients may be responsible for these increases in cortical activity (Cools et al., 2002; Mattay et al., 2002), since it has been proposed that DA is important for focusing neural activity in the cortex (Swagushi, 2001). In a previous experiment, we scanned with functional magnetic resonance imaging (fMRI) early Parkinson’s disease patients and matched control subjects during the performance of the Wisconsin Card-Sorting Task (Monchi et al., 2004a). The results showed decreased activation in the patient group in those areas whose activity, in healthy controls, was linked with the striatum, namely the ventrolateral PFC when receiving negative feedback and the posterior PFC when matching following negative feedback (Monchi et al., 2004a). By contrast, greater activation was found in the patient group in areas that were not co-activated with the striatum in healthy controls, such as the posterior and dorsolateral PFC when receiving positive or negative feedback (Monchi et al., 2004a). Based on those results, we proposed that a breakdown in both nigrostriatal and mesocortical DA may play a role in executive deficits in Parkinson’s disease, depending on the extent to which the striatum is involved in the task at hand. However, it should also be noted here, that tasks requiring significant involvement of the striatum may be harder for patients to solve than those that do not, and that the difference in task demands may also be responsible for the different patterns of activity observed in their PFC. Indeed, previous functional neuroimaging studies on cognitive functions had reported increased PFC activity in conditions that probably did not require significant striatal involvement, in patients OFF versus ON L-dopa medication, even though no significant difference in task performance was observed (Cools et al., 2002; Mattay et al., 2002).
In order to test further the hypothesis that PFC activation in Parkinson’s disease depends on striatal engagement, we recently developed a new card-sorting task, the Montreal Card-Sorting Task (MCST), which allows teasing out the role of the ventrolateral PFC and caudate nucleus. We scanned with fMRI a group of young healthy adults during performance of this new task (Monchi et al., 2004b, 2006). The results showed significant activation in the caudate nucleus only in those conditions in which cognitive planning was required to perform a set-shift and not in those conditions when the new rule to perform the set-shift was implicitly given by the task or when no set-shift was required. In the present study, we scanned a group of early-stage Parkinson’s disease patients after 12–18 h of medication withdrawal and a group of matched controls during performance of the MCST. Based on our previous study (Monchi et al., 2004a) we predicted reduced PFC activity in the patient group in those conditions that specifically require the involvement of the caudate nucleus during planning a set-shift (i.e. retrieval with shift). In these situations, the nigrostriatal DA in patients may be critically responsible for the reduced prefrontal function. On the other hand, we predicted greater PFC activity in the patient group than in the control group in those conditions that do not require the engagement of the caudate nucleus, hence those not requiring a set-shift or those where the shift is implicitly given by the task (i.e. retrieval ‘without’ shift and continuous shift) since a mesocortical DA deficit (which should result in unfocused cortical activity) may be greatly responsible (Mattay et al., 2002; Monchi et al; 2004a).
Material and methods
Subjects
Seven right-handed patients at Hoehn and Yahr stages 1 and 2 of Parkinson’s disease (mean age, 62 years; range, 56–70) participated in this study. All patients met the ‘core assessment program for surgical interventional therapy’ criteria for the diagnosis of idiopathic Parkinson’s disease (Langston et al., 1992; Defer et al., 1999), namely two of the three cardinal signs (bradykinesia, tremor and rigidity), response to L-dopa or DA agonists, and lack of evidence of other medical conditions associated with parkinsonism. All of these patients were asked to stop taking any medication prescribed for their condition (DA agonist or L-dopa) 12–18 h before the scanning session. Their mean score on the motor subset of the Unified Parkinson’s Disease Rating Scale prior to scanning (OFF medication) was 25.1 out of a maximum of 108. Patients were screened for dementia and depression using the Mini-Mental State Examination (MMSE) and the Beck Depression Inventory (BDI), respectively.
Seven right-handed control subjects (mean age, 51.1 years; range, 47–60) with no history of neurological or psychiatric disorder also participated in this study. The control subjects were also screened for dementia and depression using MMSE and BDI. For both the patients and the matched-control subjects, the exclusion criteria were a score of ≤27 on the MMSE and a score of ≥15 on the BDI. Handedness was assessed using the Edinburgh handedness inventory (Oldfield, 1971). All participants gave informed consent after reading the protocol, which was reviewed and approved by the Research Ethics Committee of the Montreal Neurological Institute.
Cognitive task
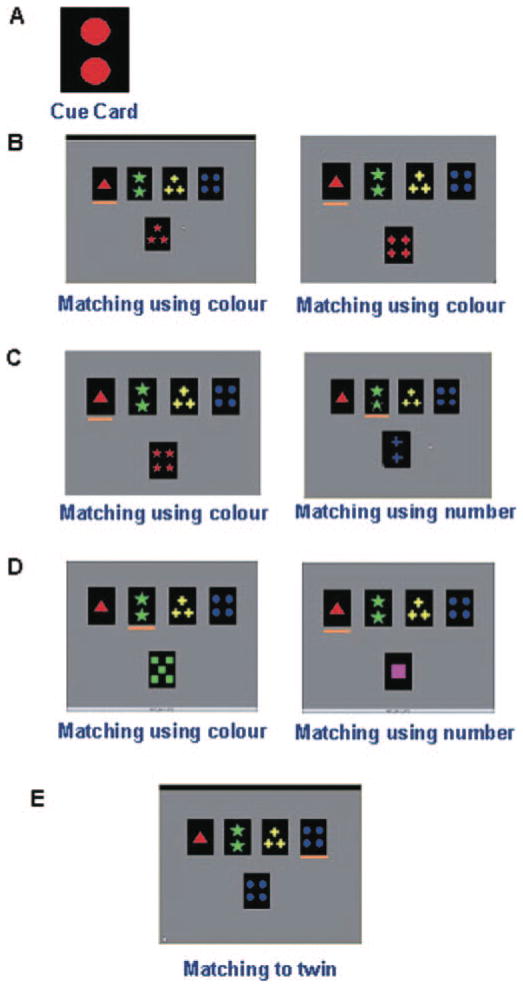
In the MCST used here (Monchi et al., 2004b, 2006), four reference cards are permanently on display in a row in the top half of a computer screen, displaying one red triangle, two green stars, three yellow crosses and four blue circles, respectively (Fig. 1). On each classification trial, a new test card is presented in the middle of the screen below the reference cards and the subject has to match the test card to one of the four reference cards. The matching response is indicated by pressing one of four buttons with the right hand, using one of four fingers, each corresponding to one of the reference cards. The match of each test card to one of the reference cards is determined by a classification rule that differs across experimental conditions. On each trial, as soon as the response is made, a period of 2.3 s occurs when the screen becomes bright if the response was correct and dark if the response was incorrect. It should be noted that in the present task, unlike in the Wisconsin Card-Sorting Task, the feedback provided does not help with the response on the following trial. The duration of each trial varies randomly according to the subject’s reaction time during the matching, providing the asynchrony between stimulus presentation and frame acquisition required for event-related acquisition. There were four different conditions in this experiment: (i) retrieval without shift; (ii) retrieval with shift; (iii) continuous shift; and (iv) control. Trials of the continuous shift and the control conditions occur in different blocks presented in a random order. The retrieval without shift and retrieval with shift are interleaved within the same blocks.
Fig. 1.
The different conditions of the MCST. (A) An example of the cue card that appears for 3.5 s at the beginning of a block of retrieval trials. In this example, the cue card contains two red circles. (B) An example of two consecutive retrieval trials without shift. Since the colour red is the only attribute shared by the test card and the cue on both trials, matching must be based on colour. In this and the other examples (C–E), the orange line below one of the reference cards indicates the selected response. (C) An example of two consecutive retrieval trials with shift. Left: the test card contains four red stars and hence shares the colour attribute with the cue card (containing two red circles, shown in A). Right: on the subsequent trial, the test card now shares a different attribute with the cue card (in this example ‘number’). Thus, a shift in classification category occurs requiring a novel response. (D) An example of two consecutive trials in the continuous shift condition. Left: the only reference card that shares an attribute with the test card is the second one and, therefore, the second reference card has to be selected according to colour. Right: in the subsequent trial, a test card containing one pink square is shown. The first reference card must now be selected because it is the only one that shares an attribute with the test card (number) and, therefore, a shift in classification occurs. Thus, in this condition, a continuous shift occurs guided implicitly by the only shared attribute between the test card and one of the reference cards. (E) An example of a control trial. The test card always matches exactly one of the reference cards and the subject simply must select the twin reference card.
In the retrieval blocks, a series of classification trials are preceded by the brief presentation (3.5 s) of a single cue card containing one to four stimuli (Fig. 1A), each one being one of four shapes in one of four possible colours, followed by a blank period also of 3.5 s. The cue card does not reappear and has to be remembered throughout the series of classification trials. On every trial, a new test card that shares a single attribute (colour, shape or number) with the cue card held in memory is presented underneath the reference cards. The subject has to select one of the four reference cards based on this attribute. For instance, if the test card and the cue card share the colour red (as in the example shown in Fig. 1B), a match to the reference card that has red in it is required. There are two types of classification trials in the retrieval condition: trials without shift and trials with shift. Trials without shift (Fig. 1B) occur when the test cards on two or more consecutive trials share the same attribute with the cue card. Trials with shift (Fig. 1C) occur when the test cards on two consecutive trials do not share the same attribute with the cue card and, therefore, a ‘shift’ is required from the previous shared attribute to the current shared attribute. Five shift trials occur within each retrieval block of trials. Shifts in classification occur randomly after 2, 3, 4 or 5 consecutive classifications in a row using the same criterion, the total number of trials per block being variable. In the retrieval with shift condition, an active comparison needs to be performed between the cue and the test cards in order to execute the shift.
In the continuous shift condition (Fig. 1D), no cue card is presented. Each test card contains only one attribute shared with a single reference card and, thus, there is only one possible response based on this attribute. In this condition, test cards are presented so that shifts in classification occur on each trial in a random order. For instance, a trial in which the test card only shares the colour green with the second reference card (such as in Fig. 1D) will be followed by a trial in which the test card only shares the number (one object) with the first reference card. Twelve trials occur within each block of the continuous shift condition. Unlike the retrieval with shift condition, no previously presented stimuli needs to be held in memory and no active comparison is needed to execute the shift since the new rule for classification is implicitly given by the task.
In the control condition, the test card on each trial is a replica of one of the four reference cards (Fig. 1E) and the subject is only required to match the test card to its twin within the four reference cards. Twelve trials occur within each block of the control condition.
Subjects performed four retrieval blocks that included both shift and non-shift trials and two blocks of the continuous shift and control conditions per run. Before scanning, subjects trained on the task on a personal computer until their performance reached a plateau, i.e. no further significant improvement on the task occurred within a period of 45 min.
fMRI scanning
Subjects were scanned using a 1.5 T Siemens Sonata MRI scanner at the McConnell Brain Imaging Centre, Montreal Neurological Institute (MNI). Each scanning session began with a T1-weighted 3D volume acquisition for anatomical localization, followed by acquisitions of echo planar T2*-weighted images with BOLD contrast. Functional images were acquired in six runs containing 200 volumes each acquired every 2.5 s. Volumes contained 24 slices, voxel size 4.7 × 4.7 × 4.7 mm3.
Data analysis
The methods for data analysis were the same as in our previous studies (Monchi et al., 2001, 2004a, 2006) and made use of the fMRIstat software developed by Worsley et al. (2002). The first three frames in each run were discarded. Images from each run were first realigned to the fourth frame for motion correction and smoothed using a 6 mm full width half-maximum (FWHM) isotropic Gaussian kernel. The statistical analysis of the fMRI data was based on a linear model with correlated errors. The design matrix of the linear model was first convolved with a difference of two gamma haemodynamic response functions timed to coincide with the acquisition of each slice. The correlation structure was modelled as an autoregressive process. At each voxel, the autocorrelation parameter was estimated from the least squares residuals, after a bias correction for correlation induced by the linear model. The autocorrelation parameter was first regularized by spatial smoothing and was then used to ‘whiten’ the data and the design matrix. The linear model was re-estimated using least squares on the whitened data to produce estimates of effects and their standard errors. The resulting effects and standard effect files were then spatially normalized by non-linear transformation into the MNI standard proportional stereotaxic space, which is based on that of Talairach and Tournoux (1988), using the algorithm of Collins et al. (1994). Anatomical images were also normalized to the MNI space using the same transformation. In a second step, runs and subjects were combined using a mixed effects linear model for the data taken from the previous analysis. A random effects analysis was performed by first estimating the ratio of the random effects variance to the fixed effects variance, then regularizing this ratio by spatial smoothing with a Gaussian filter. Inter-group analyses were performed by direct comparisons using the effects and standard deviations files of all individuals from both groups. The amount of smoothing was chosen to achieve 100 effective degrees of freedom (Worsley et al., 2002, 2005). Statistical maps were thresholded at P < 0.05 correcting for multiple comparisons using the minimum between a Bonferroni correction and random field theory in the single group analysis. This yields a threshold of t > 4.40 for a single voxel or a cluster size >540 mm3 for a significance assessed on the special extent of contiguous voxel (Friston et al., 1995). For the inter-group analysis, statistical maps were thresholded at P < 0.0001 uncorrected for multiple comparisons (corresponding to t > 3.90 for a single voxel, or to a cluster size of >96 mm3). Peaks within the basal ganglia, thalamus, and PFC that were observed in our previous study using the same protocol in young healthy adults (Monchi et al., 2004b, 2006) were considered predicted and are reported at a significance of P < 0.005 uncorrected [indicated by an asterisk (*) in the tables].
For every single condition, all error trials were removed from the fMRI analysis. The length during the matching period (from the moment a new test card is presented to the moment a response is made) on each trial of the four conditions (retrieval without shift, retrieval with shift, continuous shift, and control) was explicitly included in the design matrix. Thus, under the assumption of linearity, a change of BOLD response could not be attributed to a difference in reaction times across the two groups. Anyhow it should be noted that the matching periods were comparable in length across the two groups (see Behaviour in the Results section). Activity during the matching period of each condition was combined to generate four contrasts for statistical analysis. Two contrasts that do not significantly require caudate nucleus in young healthy adults (Monchi et al., 2006) are (i) retrieval without shift minus control and (ii) continuous shift minus control, and two contrasts that do significantly require caudate nucleus in young healthy adults are (iii) retrieval with shift minus retrieval without shift and (iv) retrieval with shift minus continuous shift.
Results
Behaviour
During the scanning session, the percentage of errors in the retrieval without shift condition was 8.13% (SD 6.19) for the control group and 21.56% (SD 14.24) for the Parkinson’s disease group. During the retrieval with shift condition the control group made on average 20.17% (SD 17.59) errors and the patients 39.86% (SD 15.90) errors. During the continuous shift condition, the mean percentage of errors was 2.38% (SD 2.38) for the healthy subjects and 16.64% (SD 14.64) for the patients. For the control condition, it was 0.47% (SD 0.94) for the control group and 4.40% (SD 3.59) for the patients. An ANOVA on the mean percentage of errors comparing the two groups: patients versus controls by the four task conditions revealed significantly more errors in the patient group than in the control group for all the conditions. More specifically, for the retrieval without shift condition the results are F(1,12) = 5.24, P = 0.041; for the retrieval with shift condition F(1,12) = 4.88, P = 0.047; for the continuous shift F(1,12) = 6.46, P = 0.027, and for the control condition F(1,12) = 7.83, P = 0.016. However, the last result should be tempered by the fact that both groups made less than 5% errors in the control condition.
The average reaction time over all responses during the retrieval without shift condition was 1493.5 ms (SD 524.1) for the control group and 1600.3 ms (SD 438.7) for the patient group. For the retrieval with shift, it was 1820.9 ms (SD 614.9) for the controls and 2157.7 ms (SD 667.1) for the patients. For the continuous shift condition the mean reaction time was 1841.0 ms (SD 604.7) for the controls and 2395.0 ms (SD 869.2) for the patients. Finally the mean reaction time for the control condition was 952.5 ms (SD 287.5) for the controls and 1187.6 ms (SD 640.7) for the patients. An ANOVA on the mean reaction time comparing the two group patients versus controls by the four task conditions revealed ‘no’ significantly longer reaction times in the patient group than in the control group across all the conditions. More specifically, for the retrieval without shift condition the results are F(1,12) = 0.17, P = 0.69; for the retrieval with shift condition F(1,12) = 0.964, P = 0.35; for the continuous shift F(1,12) = 1.92, P = 0.19, and for the control condition F(1,12) = 0.90, P = 0.36.
fMRI
As predicted, significantly greater cortical activity was observed in the patient group compared with the healthy group in terms of t-statistic, size of clusters and number of brain regions in the two comparisons that did not show increased activity in the caudate nucleus in controls: retrieval without shift versus control (Table 1, Fig. 2) and continuous shift versus control (Table 2, Fig. 3). On the other hand, less activity was observed in the patient group compared with the healthy group in the contrasts that did result in increased activity in the caudate nucleus in healthy subjects: retrieval with shift versus retrieval without shift (Table 3, Fig. 4) and retrieval with shift versus continuous shift (Table 4, Fig. 5). In the following paragraphs, a summary of the results is provided and a full description of the observed peaks is given in Tables 1–4.
Table 1.
Retrieval without shift versus control
| Anatomical area | Control subjects
|
PD patients
|
|||||
|---|---|---|---|---|---|---|---|
| x, y, z | t-stat | Cluster | x, y, z | t-stat | Cluster | ||
| Frontopolar cortex | (10) L | −32, 56, 2 | 4.57 | >3000 | −10, 56, −16 | 4.05 | 856 |
| R | 30, 48, 0 | 4.41 | 864 | ||||
| DLPFC | (8) L | −48, 12, 44 | 4.22 | >5000 | |||
| (9) L | −50, 18, 26 | 5.0 | >3000 | −46, 20, 30 | 4.76 | sc | |
| R | 52, 22, 34 | 4.63 | 2880 | 44, 14, 32 | 5.56 | >5000 | |
| (8) R | 50, 26, 40 | 4.66 | sc | ||||
| VLPFC | (47/12) L | −28, 24, 4 | 4.55 | 752 | −36, 20, −6 | 4.21 | 1888 |
| L | −42, 42, −6 | 4.22 | >3000 | −42, 34, 16 | 4.22 | >5000 | |
| L | 36, 20, −8 | 4.07 | 776 | 46, 20, 2 | 3.93 | 1184 | |
| R | 46, 46, −8 | 4.42 | 1048 | 30, 48, 0 | 4.41 | 864 | |
| (45, 46) L | −50, 18, 26 | 5.07 | >3000 | −50, 22, 22 | 4.11 | >5000 | |
| PMC | (6) L | −38, 8, 28 | 4.55 | >3000 | −34, 6, 34 | 5.71 | >5000 |
| R | 32, 14, 60 | 4.28 | 2304 | 38, 8, 28 | 4.81 | >5000 | |
| 26, 6, 48 | 3.80 | 744 | |||||
| SMA | (6) L | −2, 12, 56 | 5.13 | 4048 | −8, 32, 38 | 4.44 | 3872 |
| R | 2, 12, 56 | 5.13 | sc | 2, 22, 52 | 4.85 | sc | |
| Post CC | (24) L | −2, −22, 34 | 3.73 | 1928 | |||
| R | 2, −36, 34 | 4.66 | sc | ||||
| TC | (21) L | −54, −46, 6 | 3.91 | 1076 | −52, −44, −8 | 4.27 | 3888 |
| (39) L | −28, −56, −16 | 4.49 | 2432 | ||||
| PPC | (7) R | 38, −62, 44 | 4.48 | >10 000 | |||
| Prestriate cortex | (18) L | −10, −66, −2 | 5.22 | >10 000 | |||
| R | 10, −68, 0 | 4.59 | >10 000 | ||||
| (19) L | −10, −74, 32 | 6.03 | >10 000 | ||||
| R | 38, −68, 30 | 4.25 | 616 | 28, −76, 30 | 6.32 | >10 000 | |
| Caudate | R | 12, 4, 6 | 4.01 | 904 | |||
| Controls greater than patients | Patients greater than controls | ||||||
| MC | (4, 6) R | 54, −4, 8 | 3.56 | 120 | |||
| Post CC | (24) L | −2, −24, 36 | 4.50 | 656 | |||
| PPC | (7, 40) R | 40, −46, 54 | 3.63 | 96 | |||
| Prestriate cortex | (18, 19) L | −8, −72, 32 | 4.27 | 808 | |||
| (19) L | −38, −78, 18 | 4.05 | 384 | ||||
L = left, R = right, DL = dorsolateral, VL = ventrolateral, PFC = prefrontal cortex, CC = cingulate cortex, TC = temporal cortex, PMC = premotor cortex, MC = motor cortex, SMA = supplementary motor area, PPC = posterior parietal cortex, Ant = anterior, Post = posterior. Cluster sizes are reported in mm3. sc indicates that the peak is part of the same cluster as the peak listed immediately above in the table and that its size therefore is included in the preceding reported volume. Numbers in parentheses refer to architectonic areas. The same abbreviations are used in all tables (1–4).
P < 0.005 uncorrected. When no star is indicated P < 0.05 corrected for multiple comparision in single group analysis and P < 0.0001 uncorrected for inter-group analysis.
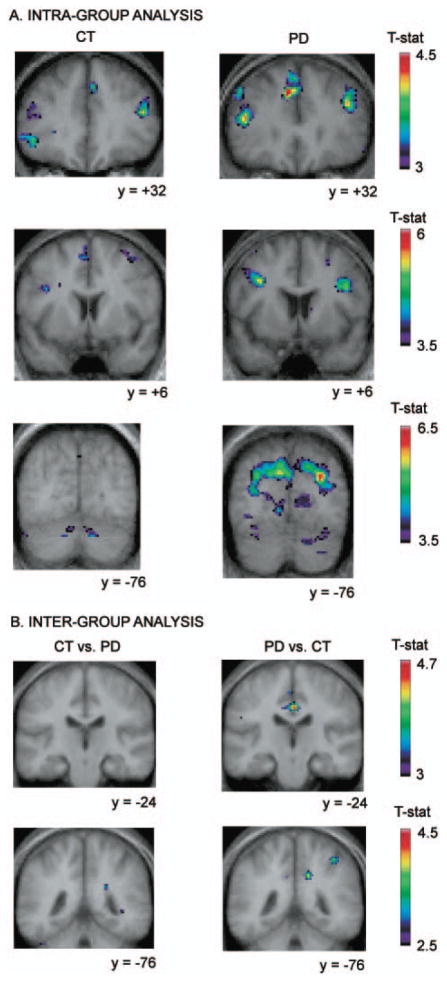
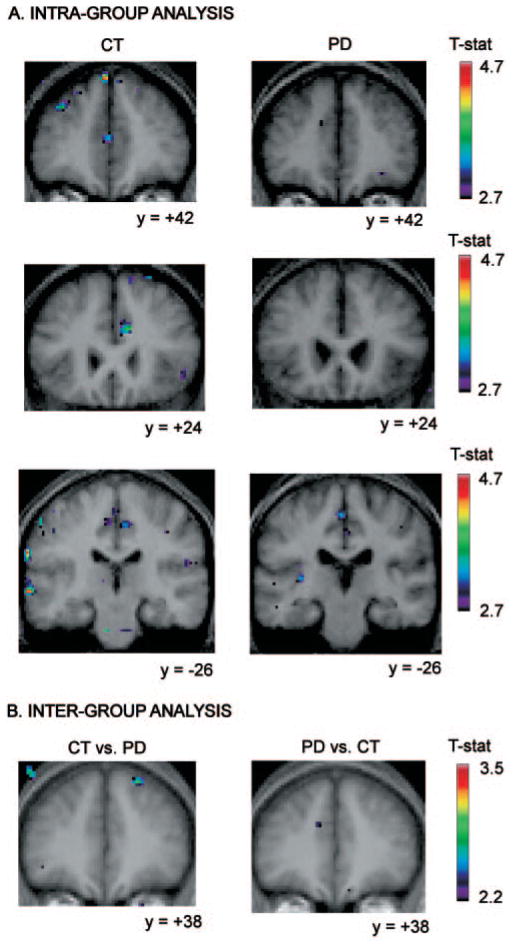
Fig. 2.
Location of peaks within the retrieval without shift versus the control condition. Coronal sections are shown. The anatomical MRI images shown are the average of T1 acquisitions transformed into stereotaxic space for each group in the intra-group analysis and for both groups in the inter-group analysis. (A) Intra-group analysis. The images display larger activations in the Parkinson’s disease group than in the control group, bilaterally, in the supplementary motor area, in the dorsolateral PFC, in the premotor cortex, and in the prestriate cortex. (B) Inter-group analysis. Images display significantly greater activity in the patient group versus the control group in the posterior cingulate cortex and the right posterior parietal cortex, while no significantly increased activity is observed in the control versus patient group analysis.
Table 2.
Continuous shift versus control
| Anatomical area | Control subjects
|
PD patients
|
|||||
|---|---|---|---|---|---|---|---|
| x, y, z | t-stat | Cluster | x, y, z | t-stat | Cluster | ||
| DLPFC | (46, 9/46) L | −46, 28, 28 | 4.52 | >10 000 | |||
| (9) L | −46, 16, 34 | 5.75 | sc | ||||
| (46, 9/46) R | 46, 32, 20 | 4.51 | 1448 | 48, 22, 30 | 4.47 | >5000 | |
| VLPFC | (47/12) L | −28, 22, 4 | 5.14 | 896 | −30, 18, 0 | 4.62 | 2056 |
| R | 32, 22, 2 | 3.92 | 690 | 36, 20, 2 | 4.31 | 1864 | |
| Posterior PFC | (6, 8, 44) L | −52, 18, 26 | 5.9 | 7368 | −44, 10, 40 | 5.29 | >10 000 |
| R | 48, 14, 32 | 6.60 | >5000 | ||||
| PMC | (6) L | −36, −4, 66 | 4.65 | 2232 | −36, 6, 34 | 6.34 | >10 000 |
| R | 36, 8, 58 | 4.81 | 3568 | 50, −8, 24 | 5.33 | >5000 | |
| SMA | (6) L | −2, 6, 66 | 5.33 | 3904 | −2, 22, 52 | 5.27 | 6016 |
| Post CC | (31) L | 0, −36, 34 | 4.03 | 1032 | |||
| TC | (21) L | −52, −44, 0 | 3.89 | 632 | |||
| PPC | (7) R | 32, −70, 46 | 4.31 | >5000 | 34, −56, 54 | 5.00 | >10 000 |
| Precuneus | (7) R | 2, −74, 46 | 4.51 | Sc | 6, −40, 62 | 5.24 | >10 000 |
| Prestriate cortex | (18) L | −32, −92, 2 | 4.28 | 1792 | −10, −68, −2 | 5.58 | >20 000 |
| R | 34, −54, −12 | 5.25 | >10 000 | ||||
| (19) L | −48, −66, −10 | 3.85 | tbc | −6, −78, 42 | 6.83 | >20 000 | |
| L | −26, −76, 34 | 4.14 | 3848 | −28, −76, 34 | 6.11 | sc | |
| R | 36, −84, 18 | 3.85 | 600 | 32, −76, 28 | 5.99 | >20 000 | |
| Thalamus | R | 16, −20, 0 | 4.15 | 1532 | |||
| Subthalamic nucleus | R | 10, −16, −10 | 3.99 | sc | |||
| Controls greater than patients | Patients greater than controls | ||||||
| DLPFC | (9) R | −46, 36, 36 | 2.98* | 152 | |||
| MC | (4, 6) R | 50, −8, 24 | 3.92 | 64 | |||
| Post CC | (24) L | −2, −24, 36 | 3.92 | 150 | |||
| Prestriate cortex | (18, 19) L | −14, −72, 32 | 3.99 | 904 | |||
| (19) L | −38, −78, 18 | 3.69 | 176 | ||||
Abbreviations same as in Table 1.
P < 0.005 uncorrected. When no star is indicated P < 0.05 corrected for multiple comparision in single group analysis and P < 0.0001 uncorrected for inter-group analysis.
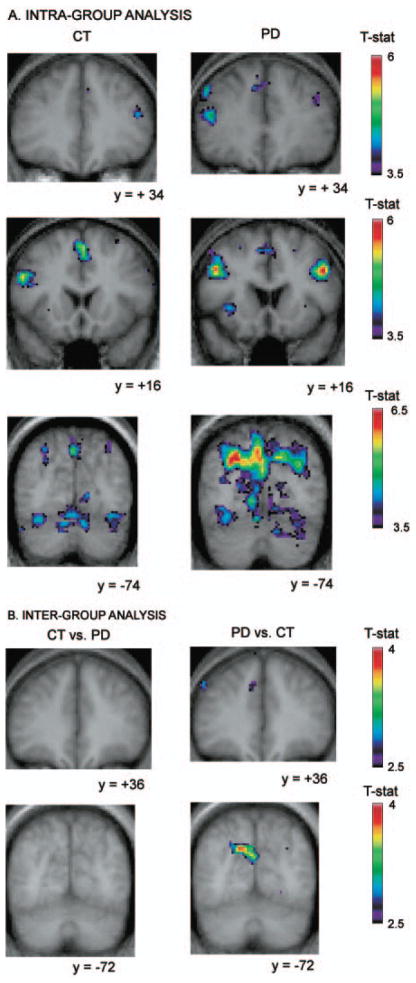
Fig. 3.
Location of peaks within the continuous shift versus the control condition. Coronal sections are shown. The anatomical MRI images shown are the average of T1 acquisitions transformed into stereotaxic space for each group in the intra-group analysis and for both groups in the inter-group analysis. (A) Intra-group analysis. The images display significant activation in the left dorsolateral PFC and the right posterior PFC in the Parkinson’s disease group, while none is observed in the control group. They also show larger activations in the patient group than in the control group bilaterally in the prestriate cortex. (B) Inter-group analysis. Images display significantly greater activity in the patient group versus the control group in the left dorsolateral PFC and the left prestriate cortex, while no significantly increased activity is observed in the control versus patient group subtraction.
Table 3.
Retrieval with shift versus retrieval without shift
| Anatomical area | Control subjects
|
PD patients
|
|||||
|---|---|---|---|---|---|---|---|
| x, y, z | t-stat | Cluster | x, y, z | t-stat | Cluster | ||
| DLPFC | (8) L | −30, 34, 36 | 4.10 | 216 | −38, 40, 38 | 4.50 | 584 |
| VLPFC | (47/12) L | −54, 20, −6 | 5.72 | 7408 | |||
| −28, 20, −2 | 4.69 | sc | |||||
| R | 30, 18, −2 | 3.78 | 208 | 38, 18, −2 | 4.94 | 1088 | |
| MC | (4, 6) L | −26, −6, 56 | 5.80 | 2952 | |||
| R | 28, −14, 60 | 4.92 | 3720 | ||||
| SMA | (6) L | −2, 10, 50 | 5.64 | >10 000 | 0, 2, 50 | 5.63 | 4136 |
| −2, 10, 60 | 4.15 | sc | −10, 8, 70 | 4.51 | 528 | ||
| R | 2, −2, 58 | 4.98 | > 5000 | ||||
| PMC | (6) L | −50, 4, 38 | 5.03 | 2616 | −54, 4, 42 | 4.33 | 1560 |
| R | 52, −2, 12 | 4.69 | 6.24 | ||||
| Ant CC | (32) L | −4, 16, 42 | 4.72 | >5000 | |||
| Post CC | (31) L | −2, −42, 32 | 4.60 | 2176 | |||
| R | 2, −46, 28 | 5.20 | sc | ||||
| PPC | (40) L | −48, −42, 52 | 4.70 | >5000 | −32, −52, 42 | 3.90 | 864 |
| R | 50, −36, 48 | 4.95 | 3376 | ||||
| Precuneus | (7) L | −4, −78, 46 | 5.34 | >10 000 | −12, −76, 54 | 4.80 | >5000 |
| R | 18, −76, 46 | 4.87 | >10 000 | 6, −78, 46 | 4.78 | >5000 | |
| TC | (20) L | −32, −38, −16 | 4.97 | 1264 | |||
| (21) L | −60, 4, −24 | 4.22 | 64 | ||||
| (37) L | −46, −56, −12 | 4.68 | 2248 | ||||
| Prestriate cortex | (17, 18) L | −16, −92, −14 | 4.17 | 2664 | −12, −96, −2 | 4.56 | 984 |
| (18) R | 14, −86, 0 | 4.29 | 752 | ||||
| (19) L | −12, −78, 26 | 5.77 | >10 000 | ||||
| R | 26, −70, 38 | 4.70 | >10 000 | 28, −78, 46 | 4.70 | 1752 | |
| R | 30, −90, 6 | 4.27 | 376 | 32, −84, 22 | 4.35 | 1104 | |
| Caudate | L | −8, −8, 4 | 5.45 | 1264 | −14, 6, 8 | 4.31* | 480 |
| R | 14, −2, −2 | 4.29 | 1520 | ||||
| Putamen | L | −4, −14, −6 | 3.22* | 24 | |||
| R | 30, 4, 0 | 3.35* | 32 | ||||
| Thalamus | L | −10, −18, 8 | 4.69 | 5128 | |||
| Subthalamic nucleus | L | −8, −16, −10 | 4.14 | sc | |||
| Controls greater than patients | Patients greater than controls | ||||||
| Orbitofrontal | (11) L | −34, 32, −20 | 4.06 | 592 | |||
| L | −32, 56, −16 | 3.95 | 128 | ||||
| DLPFC | (8) L | −34, 24, 32 | 3.29* | 16 | |||
| VLPFC | (47/12) L | −38, 18, −16 | 3.95 | 88 | |||
| (45) R | 36, 36, 10 | 3.7* | 16 | ||||
| PMC | (6) R | 30, 2, 54 | 4.03 | 32 | |||
| TC | (21) L | −58, 2, −28 | 3.92 | 32 | |||
| PPC | (40) R | 50, −40, 54 | 4.22 | 176 | |||
| Post CC | (31) R | 2, −46, 28 | 4.09 | 56 | |||
| Caudate nucleus | L | −8, −8, 8 | 3.64* | 24 | |||
| R | 18, 16, 14 | 3.00* | 16 | ||||
| Putamen | R | 26, 8, 2 | 2.87* | 32 | |||
Abbreviations same as in Table 1.
P < 0.005 uncorrected. When no star is indicated P < 0.05 corrected for multiple comparision in single group analysis and P < 0.0001 uncorrected for inter-group analysis.
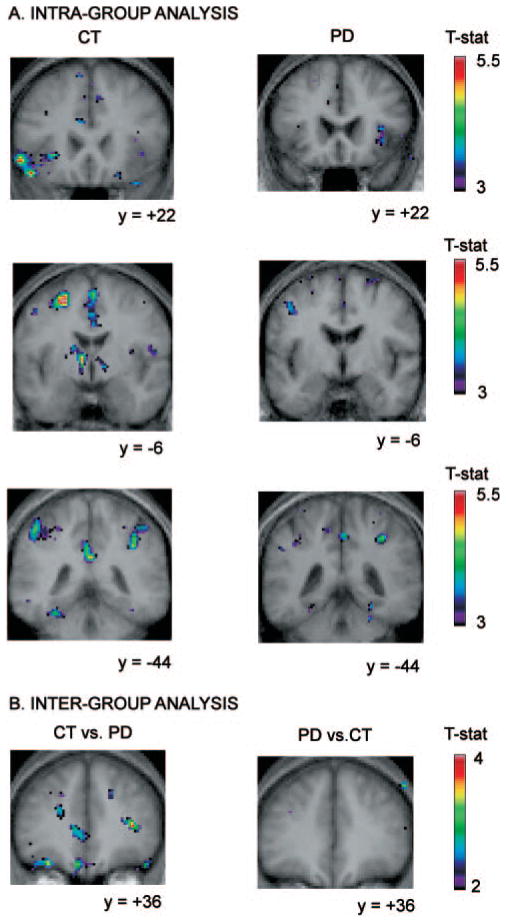
Fig. 4.
Location of peaks within the retrieval with shift versus the retrieval without shift condition. Coronal sections are shown. The anatomical MRI images shown are the average of T1 acquisitions transformed into stereotaxic space for each group in the intragroup analysis and for both groups in the inter-group analysis. (A) Intra-group analysis. The images display significant activation in the left ventrolateral PFC and caudate nucleus in the control group while none is observed in the Parkinson’s disease group. They also show larger activations in the control group than in the patient group in the posterior cingulate cortex and the posterior parietal cortex bilaterally. (B) Inter-group analysis. Images display significantly greater activity in the control group versus the patient group in the left dorsolateral PFC and orbitofrontal cortex, as well as the right ventrolateral PFC, while no significantly increased activity is observed in the patient versus control group subtraction.
Table 4.
Retrieval with shift versus continuous shift
| Anatomical area | Control subjects
|
PD patients
|
|||||
|---|---|---|---|---|---|---|---|
| x, y, z | t-stat | Cluster | x, y, z | t-stat | Cluster | ||
| DLPFC | (8) L | −4, 42, 54 | 4.47 | 144 | |||
| R | 22, 38, 52 | 3.81* | 200 | ||||
| VLPFC | (45) L | −54, 14, 10 | 3.42* | 48 | −32, 18, 8 | 3.6* | 80 |
| PMC | (6) L | −16, 14, 66 | 4.38 | 176 | |||
| Ant CC | (32) R | 10, 24, 28 | 3.9* | 152 | |||
| TC | (21) L | −66, −24, −4 | 4.74 | 576 | |||
| Caudate | L | −14, 8, 14 | 3.00* | 16 | |||
| R | 14, 12, 14 | 2.92* | 16 | ||||
| Thalamus | L | −16, −10, 12 | 3.83* | 224 | |||
| R | 10, −12, 4 | 3.66* | 136 | ||||
| Controls greater than patients | Patients greater than controls | ||||||
| DLPFC | (8) L | −4, 42, 54 | 3.27* | 64 | |||
| R | 22, 38, 52 | 2.88* | 32 | ||||
| VLPFC | (45) L | −54, 14, 10 | 2.73* | 16 | |||
| TC | (20, 37) L | −42, −40, −18 | 4.03 | 496 | |||
| (21) L | −68, −28, −4 | 4.46 | 192 | ||||
Abbreviations same as in Table 1.
P < 0.005 uncorrected. When no star is indicated P < 0.05 corrected for multiple comparision in single group analysis and P < 0.0001 uncorrected for inter-group analysis.
Fig. 5.
Location of peaks within the retrieval with shift versus the continuous shift condition. Coronal sections are shown. The anatomical MRI images shown are the average of T1 acquisitions transformed into stereotaxic space for each group in the intra-group analysis and for both groups in the inter-group analysis. (A) Intra-group analysis. The images display significant activation in the left dosolateral PFC and the right anterior cingulate cortex, and the left temporal cortex, while none is observed in the Parkinson’s disease group. (B) Inter-group analysis. Images display significantly greater activity in the control group versus the patient group in the right dorsolateral PFC while no significantly increased activity is observed in the patient versus control group subtraction.
Retrieval without shift versus control
Control subjects
Significant increases in BOLD signal were observed when comparing the retrieval with shift to the control condition in various regions of the PFC including left frontopolar, bilateral dorsolateral, and bilateral ventrolateral PFC. Other more posterior sites of significant increased activity were also observed and are given in detail in Table 1.
Parkinson’s disease patients
In the patient group, significant peaks included bilateral frontopolar cortex, bilateral dorsolateral PFC, bilateral ventrolateral PFC, as well as, in the premotor cortex and the supplementary motor area, bilaterally. More posteriorly, significant activity was observed in the right posterior cingulate cortex and the right posterior parietal cortex, while it was not observed in the control group. As can be seen in Table 1, regions that exhibited significantly increased activity in both the patients and the control group had larger cluster size in the patients than in the control subjects.
Inter-group comparison
The inter-group comparison revealed significantly greater activity in the patient group compared with the control group in the right motor cortex, in the left posterior cingulate cortex, the right posterior parietal cortex and in the left occipital cortex. Importantly, no significant greater activation was found in the control subjects compared with the patients for this contrast (Fig. 2).
Continuous shift versus control
Control subjects
When comparing the continuous shift with the control condition, regions of significantly increased activation included bilateral dorsolateral PFC, left ventrolateral PFC, left posterior PFC, as well as, right posterior parietal cortex and bilateral occipital cortex.
Parkinson’s disease patients
In the patient group, significant peaks included bilateral dorsolateral PFC and posterior PFC as well as right posterior parietal cortex and bilateral occipital cortex. Furthermore, significant increases in the BOLD signal were found in other brain regions that were not activated significantly in the control subjects for this subtraction. These included the left dorsolateral PFC, the right posterior cingulate cortex, the right temporal cortex, the right thalamus and a region comprising the subthalamic nucleus. Also in this comparison (Table 2), regions that were significantly activated in both the patients and controls had more significant peaks and larger cluster size in the patients than in the control subjects.
Inter-group comparison
The comparison between the two groups showed significantly greater activity in the Parkinson’s disease group compared with the control group in the left dorsolateral PFC, the right motor cortex, in the left posterior cingulate cortex, and in the left occipital cortex. Importantly, no significant greater activation was found in the control subjects compared with the patients for this subtraction (Fig. 3).
Retrieval with shift versus retrieval without shift
Control subjects
Significant increases in activity were observed, bilaterally, in the ventrolateral PFC, the posterior parietal cortex, the occipital cortex and in the premotor cortex. Significantly increased activity was also found in subcortical regions, including the caudate nucleus bilaterally, the putamen bilaterally, as well as the thalamus and a region comprising the subthalamic nucleus on the left (Table 3, Fig. 4).
Parkinson’s disease patients
In the patient group, regions where significant BOLD signal increases where found included, in the right hemisphere, the ventrolateral PFC, and the posterior parietal cortex. Significant activation was also found in the left caudate nucleus. Unlike the control group, no significant activation was found in the left ventrolateral PFC, the left premotor cortex, or anywhere in the temporal cortex, the anterior or posterior cingulate cortex, the putamen, the thalamus or the subthalamic nucleus.
Inter-group comparison
The inter-group comparison (controls versus patients) revealed significantly greater activity, in the control compared with the patient group, bilaterally in the ventrolateral PFC, the caudate nucleus, and in the right putamen. In the left hemisphere, significantly greater increased activity was found in the frontopolar cortex, the dorsolateral PFC, the anterior cingulate cortex, the prestriate cortex and in the right hemisphere, in the premotor cortex, the posterior cingulate cortical region (area 31), and the posterior parietal cortex. Importantly, no significant activation was found when the patients were compared to the control group.
Retrieval with shift versus continuous shift
Control subjects
Significant increases in activity were observed bilaterally in the dorsolateral PFC, and, in the left hemisphere, in the ventrolateral PFC, the anterior cingulate cortex, the posterior parietal cortex, and the caudate nucleus, as well as the thalamus on the right (Table 4, Fig. 5).
Parkinson’s disease patients
In the patient group, significant activation was located in the left ventrolateral PFC and left thalamus.
Inter-group comparison
The control versus patient group comparison presented significantly greater activity in the dorsolateral PFC bilaterally, the left ventrolateral PFC and left medial temporal gyrus (area 21). In the reverse comparison, i.e. patients versus control subjects, the only significant activation was found in the fusiform gyrus (intersection of areas 20 and 37).
Discussion
As predicted, a pattern of reduced cortical activation in Parkinson’s disease was observed in the two comparisons that demonstrated significant involvement of the caudate nucleus, i.e. the retrieval with shift versus retrieval without shift (Table 3, Fig. 4) and the retrieval with shift versus continuous shift condition (Table 4, Fig. 5). In particular, this reduction was observed in the dorsolateral and the ventrolateral PFC, as revealed both by the intra- and the inter-group analysis. For these conditions this pattern of activation is in agreement with the traditional model of the pathophysiology of the disease, which proposes that the nigrostriatal DA depletion results in decreased cortical activity via increased inhibitory output of the basal ganglia to the thalamus (Albin et al., 1989). This pattern of reduced cortical activity observed in the retrieval with shift condition versus the continuous shift appears to support the findings of Lewis et al. (2005) showing that L-dopa replacement therapy ameliorated manipulation within working memory but not ‘attentional’ set-shifting per se. In the present study, manipulation constitutes an important component of the retrieval with shift condition, while it is not the case in the continuous shift condition.
In contrast to the comparisons described in the previous paragraph, greater overall cortical activity was found in the Parkinson’s disease group in comparisons that do not normally require the caudate nucleus, i.e. the retrieval without shift versus control condition (Table 1, Fig. 2) and the continuous shift versus the control condition (Table 2, Fig. 3). This was observed both in the single group analysis and in the inter-group comparisons. In particular, there were two main findings. First, patients recruited more and larger regions of the PFC than the healthy subjects such as the dosolateral and ventrolateral PFC, the premotor cortex, the posterior PFC and the frontopolar cortex (Tables 1 and 2). Second, the patient group also recruited more regions than the healthy subjects in the more posterior cortical areas, such as the posterior parietal cortex, the posterior cingulate cortex and the prestriate cortex (Tables 1 and 2, Figs 2 and 3). This pattern of activation is consistent with previous functional neuroimaging studies that showed increased activity in various cortical areas including posterior areas such as the posterior parietal cortex and the prestriate cortex (Samuel et al., 1997; Owen et al., 1998; Dagher et al., 2001; Cools et al., 2002). It has been proposed that these patterns of increased activation may be at the origin of compensatory mechanisms (Dagher et al., 2001; Samuel et al., 1997). Alternatively, the occurrence of a secondary DA deficit may be responsible for the loss of focusing of neural activity in the cortex (Javoy-Agid and Agid, 1980, Swagushi et al., 2001), resulting in increased activity in a variety of neural structures (Cools et al., 2002; Mattay et al., 2002; Monchi et al., 2004a). In our previous fMRI studies in Parkinson’s disease (Monchi et al., 2004a), we did also observe an over-activation in the PFC during those WCST periods that did not specifically require the striatum, such as when receiving positive feedback and matching following positive feedback and, to a lesser extent, in more posterior regions of the cortex.
We should also mention that while the error rate during executive performance in patients was higher than normal controls in all conditions, it was decisively worse during the retrieval with shift condition (i.e. the condition requiring significant caudate nucleus). Therefore, the observed reduced activity in the Parkinson’s disease group while performing this condition could also be explained by the high rate of errors. However, while it is true that this condition was the most demanding for the patients, the error trials were removed from the reported brain imaging analysis.
The intriguing finding of the present study was that ventrolateral and dorsolateral PFC could exhibit either reduced or increased activity according to whether the caudate nucleus was more or less involved. The reduced activation of the ventrolateral PFC observed in the retrieval with shift (Tables 3 and 4) requiring the contribution of the caudate nucleus cannot be explained simply by a specific hypofunction of this area since in those tasks not involving the caudate nucleus its activation was actually greater in terms of the t-statistic value and the size of the cluster in comparison with healthy subjects. Moreover, the striatal-dependent pattern of cortical activity was not confined to the PFC, but seemed to generalize to other cortical areas such as the motor, cingulate, parietal, temporal and prestriate cortex (Tables 1–4). The involvement of several other cortical areas besides the PFC is not surprising for several reasons. First, many of these regions such as the cingulate cortex and the posterior parietal cortex have also been shown to play an important role in executive processes (e.g. Carter et al., 1998; Asari et al., 2005). Second, the striatum is known to receive input from the entire cortex (Alexander et al., 1986; Parent and Hazrati, 1995), so that a nigrostriatal degeneration could theoretically affect the activity of many of these regions when the striatum is solicited. Finally, mesocortical DA is diffusely present in other regions of the cortex besides the PFC (Bergson et al., 1995), which possibly can explain the increased activity observed in those regions when the striatum is not required.
The present study does not provide direct insight into the relationship between DA receptors and executive deficits in Parkinson’s disease. Work from experimental animals and healthy human volunteers has revealed a high variability regarding dopaminergic drug effects on cognitive processes involving set-shifting and working memory tasks (see review by Cools, 2006). They have proposed that these apparently different effects across different tasks may reflect effects on dissociable neural systems with distinct DA activity. More specifically, while performance on tasks with high demands for cognitive stability (e.g. set-maintenance tasks) may benefit from high DA receptor stimulation in the PFC (predominantly D1 receptor type), tasks with high demands for cognitive flexibility (e.g. set-shifting tasks) may benefit from high DA receptor stimulation in the striatum (predominantly D2 receptor type). Based on these premises and keeping in mind that this disease is characterized primarily by DA depletion in the dorsal striatum, while, at least in the early stages (as for our patients), DA function in the ventral striatum and also the PFC is relatively intact or even upregulated (Owen, 2004; Cools, 2006), it is reasonable to speculate a primary role (but not the only one) of striatal dopaminergic receptor impairment in set-shifting processes.
In conclusion, the pattern of activation described in the present study does not support the traditional model of the pathophysiology of Parkinson’s disease, which proposes that the nigrostriatal DA depletion results simply in decreased cortical activity (Albin et al., 1989). Our findings, instead, support the hypothesis that the impaired performance in executive functions may be the result of deficient interplay between the nigrostrial and mesocortical DA system (Monchi et al., 2004a; Cools, 2006). This may well explain the relatively poor beneficial effect of levodopa on a number of cognitive functions (Feigin et al., 2003; Sohamy et al., 2005). Furthermore, our results show the importance of teasing out the functional role of the caudate nucleus and the PFC in executive processes to properly understand the abnormal mechanisms at the origins of the cognitive deficits observed in Parkinson’s disease.
Acknowledgments
This work is supported by grants from the Parkinson Society of Canada to O.M. and the Fond de la Recherche en Santé du Québec to O.M. and the Canadian Institutes of Health Research to A.P.S. and M.P. The authors would like to thank K. Worsley for advice on the statistical analysis and M. Fraraccio and A. Endo for practical help.
Abbreviations
- DA
dopamine
- fMRI
functional magnetic resonance imaging
- MCST
Montreal Card-Sorting Task
- PFC
prefrontal cortex
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Asari T, Konishi S, Jimura K, Miyashita Y. Multiple components of lateral posterior parietal activation associated with cognitive set shifting. Neuroimage. 2005;26:684–702. doi: 10.1016/j.neuroimage.2004.12.063. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15(12):7821–36. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20 (Suppl 4):415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. ‘Subcortical dementia’: the neuropsychological evidence. Neuroscience. 1988;25:363–87. doi: 10.1016/0306-4522(88)90246-1. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D inter-subject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function—implications for l-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–94. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: a PET activation study in Parkinson’s disease. Brain. 2001;124:1020–32. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease. Mov Disord. 1999;14:572–84. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Feigin A, Ghilardi MF, Carbon M, Edwards C, Fukuda M, Dhawan V, et al. Effects of levodopa on motor sequence learning in Parkinson’s disease. Neurology. 2003;60:1744–9. doi: 10.1212/01.wnl.0000072263.03608.42. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Grossman M, Crino P, Reivich M, Stern MB, Hurtig HI. Attention and sentence processing deficits in Parkinson’s disease: the role of anterior cingulate cortex. Cereb Cortex. 1992;2:513–25. doi: 10.1093/cercor/2.6.513. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson disease? Neurology. 1980;30 (Suppl 12):1326–30. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations. Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not in attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Card-sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related fMRI. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma R, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004a;24:702–10. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J. The caudate nucleus and the preparation of a novel action: an fMRI study. NeuroImage; International Conference on Functional Mapping of the Human Brain; Budapest. 2004b. p. e2041. [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley K, Doyon J. Functional role of the basal ganglia in planning and execution of actions. Ann Neurol. 2006;59:257–64. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW. Planning and spatial working memory in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:757–66. doi: 10.1136/jnnp.51.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield OD. The assessment and analysis of handedness. The Edinburgh Inventory Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, et al. Presynaptic and postsynaptic dopaminergic binding densities in the nigrostriatal and mesocortical systems in early Parkinson’s disease: a double-tracer positron emission tomography study. Ann Neurol. 1999;46 (Suppl 5):723–31. doi: 10.1002/1531-8249(199911)46:5<723::aid-ana7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Quinn NP, Marsden CD, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET: implications for higher cortical functions. Brain. 1998;121:949–65. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992;32:151–61. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Lang AE. Procedural learning and neostriatal dysfunction in man. Brain. 1988;111:941–59. doi: 10.1093/brain/111.4.941. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, et al. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain. 1997;120:963–76. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275 (Suppl 2):321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Sohamy D, Meyers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: evidence from Parkinson’s disease. Behav Brain Res. 2005;156:191–9. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Swagushi T. The role of D1-dopamine related receptors in working memory-guided movements mediated by frontal cortical areas. Parkinsonism Relat Disord. 2001;7:9–19. doi: 10.1016/s1353-8020(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Taylor AE, Saint-Cyr JA. The neuropsychology of Parkinson’s disease. Brain Cogn. 1995;28 (Suppl 3):281–96. doi: 10.1006/brcg.1995.1258. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845–83. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Spatial smoothing of autocorrelations to control the degrees of freedom in fMRI analysis. Neuroimage. 2005;26:635–41. doi: 10.1016/j.neuroimage.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao C, Aston JAD, Petre V, Duncan GH, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]