Conspectus
The determination of molecular structures using solid-state NMR spectroscopy requires distance measurement through nuclear-spin dipole-dipole couplings. However, most dipole-coupling techniques compete with the transverse (T2) relaxation of the nuclear spins, whose time constants are at most several tens of milliseconds, which limits the ability to measure weak dipolar couplings or long distances.
In the last ten years, we have developed a number of magic-angle-spinning (MAS) solidstate NMR techniques to measure distances of 15-20 Å. These methods take advantage of the high gyromagnetic ratios of 1H and 19F spins, multi-spin effects that speed up dipolar dephasing, and 1H and 19F spin diffusion that probes distances in the nanometer range. Third-spin heteronuclear detection provides a method for determining 1H dipolar couplings to heteronuclear spins. We have used this technique to measure hydrogen-bond lengths, torsion angles, the distribution of protein conformations, and the oligomeric assembly of proteins. We developed a new pulse sequence, HARDSHIP, to determine weak long-range 1H-heteronuclear dipolar couplings in the presence of strong short-range couplings. This experiment allows us to determine crystallite thicknesses in biological nanocomposites such as bone. The rotational-echo double-resonance (REDOR) technique allows us to detect multi-spin 13C-31P and 13C-2H dipolar couplings. Quantitative analysis of these couplings provides information about the structure of peptides bound to phospholipid bilayers and the geometry of ligand-binding sites in proteins.
Finally, we also use relayed magnetization transfer, or spin diffusion, to measure long distances. Z-magnetization can diffuse over several nanometers because its long T1 relaxation times allow it to survive for hundreds of milliseconds. We developed 1H spin diffusion to probe the depths of protein insertion into the lipid bilayer and protein-water interactions. On the other hand, 19F spin diffusion of site-specifically fluorinated molecules allowed us to elucidate the oligomeric structures of membrane peptides.
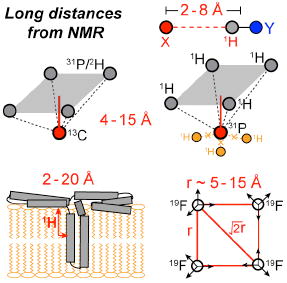
Introduction
An essential element in solid-state NMR based structure determination is distance measurement through nuclear-spin dipole-dipole couplings. The internuclear dipolar coupling ωd scales with the gyromagnetic ratios (γ) of the two spins I and S and the inverse third power of the distance rIS, . For common spin-1/2 nuclei in organic compounds, the dipolar couplings for a 5-Å distance are, respectively, 61 Hz for 13C-13C, 24 Hz for 13C-15N, and 99 Hz for 13C-31P spin pairs. Since most dipolar-coupling techniques such as rotational-echo doubleresonance (REDOR) 1 compete with the transverse (T2) relaxation of the nuclear spins, whose time constants are at most several tens of milliseconds, the ability to measure weak dipolar couplings is intrinsically limited by the coherence lifetime of the nuclear spins.
We have developed several strategies to increase the distance reach of magic-anglespinning (MAS) solid-state NMR. The first approach is to choose high-γ nuclear spins such as 1H and 19F. The main challenge in measuring distances to proton spins is that the multi-spin 1H-1H dipolar couplings in organic compounds cause very short 1H T2 relaxation times, which can be increased only by special decoupling sequences or very fast MAS. We review a REDOR technique designed to measure 1H-X heteronuclear dipolar couplings up to 8 Å while suppressing the undesired 1H-1H couplings 2.
The second strategy for extending the distance reach of solid-state NMR is to take advantage of multi-spin effects. For dipolar couplings between one X spin and multiple Y spins, the dipolar Hamiltonians for the various X-Y spin pairs commute, and combine to speed up dipolar evolution, thus facilitating distance measurements. We review the principle of a multispin 1H-X distance-measuring technique, HARDSHIP 3, and show its application to biological nanocomposites. We also describe a 13C-2H REDOR technique for perdeuterated molecules, which can measure distances to about 7 Å.
The third strategy to measure long-range distances is to use relayed magnetization transfer, i.e. spin diffusion 4. Although semi-quantitative, z-magnetization can diffuse over several nanometers because it can survive for hundreds of milliseconds due to the long T1 relaxation times. We review 1H and 19F spin diffusion techniques that have been used successfully to determine the topology of membrane-bound peptides and proteins in lipid bilayers 5,6.
1. 1H-X distances by REDOR
In the 1H-X REDOR distance experiment (Fig. 1a), 1H magnetization evolves in the recoupled 1H-X dipolar field. A single 1H 180° pulse is applied in the center of the X-H dipolar dephasing period to refocus the 1H chemical shift while two 180° pulses per rotor period are applied to the X-spin. A homonuclear decoupling sequence is used to suppress the 1H-1H dipolar couplings. Therefore, the magnetization of each proton is modulated independently by its coupling to the X-spin. To detect the dipolar modulation of a specific proton, the magnetization of that proton is selectively transferred to its directly bonded spin Y, which differs from X 2. This 1H-Y transfer step can be made site-specific by using sequences that suppress 1H spin diffusion. This Y-detected 1H-X REDOR technique is related to the medium- and long-distance (MELODI) heteronuclear correlation experiment 7, where the X and Y spins are the same. The use of two unlike spins for dipolar dephasing (X) and detection (Y) affords the flexibility necessary to measure long distances.
Fig. 1.
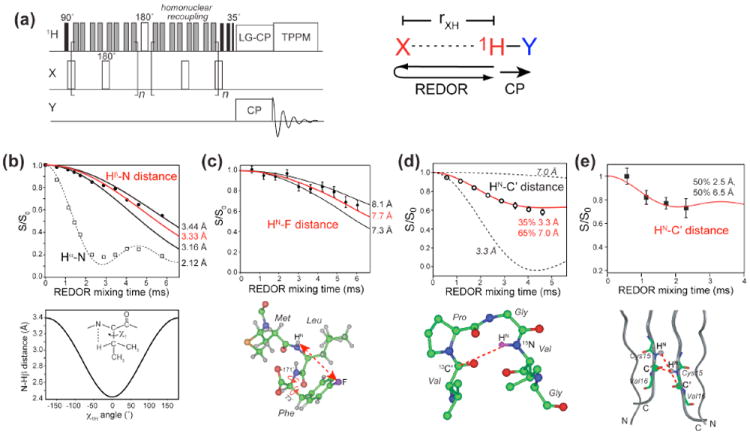
(a) Pulse sequence of the Y-detected 1H-X REDOR experiment. Several applications of this method are shown in (b-e). (b) 13C-detected intra-residue Hβ – N and Hα-N REDOR dephasing in the model compound N-acetyl-valine 8. The Hβ – N distance depends on the χ1 angle as shown below the REDOR panel, where χ1H ≡ N − Cα − Cβ − Hβ = χ1 − 120°. (c) 15N-detected inter-residue HN-19F REDOR dephasing of the tripeptide formyl-Met-Leu-Phe 9. (d) 15N-detected inter-residue HN-13C’ REDOR dephasing between Val6 C’ and Val9 HN in the elastin-mimetic peptide (VPGVG)3 10. (e) Intermolecular HN-C’ distances in Cys15 of the antimicrobial peptide, PG-1 11. The REDOR data restrain the peptide to be parallel packed in an NCCN fashion.
As for any REDOR experiment, the distance upper limit of this Y-detected 1H-X REDOR technique is dictated by the T2 relaxation time of the evolving 1H spin. Under slow MAS, the MREV-8 homonuclear-decoupled 1H T2 values are usually about 5 ms. Longer T2 values can be achieved by using more advanced homonuclear decoupling sequences or by reducing the 1H spin density by partial deuteration.
Fig. 1b-e displays some of the applications of this 1H-X REDOR technique. An 15N-detected HN-CO REDOR experiment allows the measurement of hydrogen-bond lengths in suitably labeled proteins, and HN-CO distances up to 6 Å have been measured 2. An HN-CO distance between Val6 and Val9 of the elastin-mimetic peptide, (VPGVG)3, showed a bimodal distribution of two distances centered at 3.3 Å and 7.0 Å 10, indicating that elastin adopts a mixture of a tight β-turn (35%) and an extended structure (65%) to facilitate its reversible conformational transitions. The HN-CO distance within a residue constrains the backbone ϕ angle of that residue. This is useful for determining the conformation of Gly, where the more conventional method correlating Cα-H dipolar coupling with the N-HN coupling is less advantageous 8. Similarly, sidechain Hβ to backbone 15Nα distances can be measured with 13C detection to constrain the χ1 torsion angle 8.
The longest distances that can be measured with this 1H-X REDOR technique are 1H-19F distances because of the high γ of 19F spins. 19F spins are usually incorporated into proteins site-specifically and are ideal distance probes. We demonstrated this technique on a para-19F-labeled Phe in the tripeptide, formyl-MLF 9. A distance of 7.7 Å was measured between 4-19F –Phe and Leu HN, which constrained the intervening Phe ϕ and χ1 torsion angles. A unique challenge of 1H-19F REDOR is the large chemical shift anisotropy of 19F: composite pulses such as 90°225°315° are important for achieving broadband inversion of the 19F polarization.
2. Multi-spin distance experiments
2.1 HARDSHIP for biological nanocomposites
Many hard and stiff biological materials are nanocomposites of inorganic particles embedded in an organic matrix. Examples include the load-bearing material of bone, which consists of 3-nm thick bioapatite nanocrystals in a collagen matrix (Fig. 2a); dentin in teeth, with a similar overall composition; and nacre, with ~300-nm thick “bricks” of crystalline CaCO3 held together by thin layers of organic “cement”. Electron microscopy and scattering methods can provide information on the particle spacing and some of their dimensions, but for probing spatial variations in nanoparticle composition, interfacial structure, and other features on the 1-nm scale, solid-state NMR holds the most promise. NMR is particularly suited for studies of bone and dentin, because the nanocrystals in these materials are particularly thin (only ~3 nm) and thus less accessible to microscopy methods. At the same time, the nano-size gives a large number of spins near the organic-inorganic interfaces, which enable NMR studies of the composition and structure of organic-inorganic interfaces.
Fig. 2.
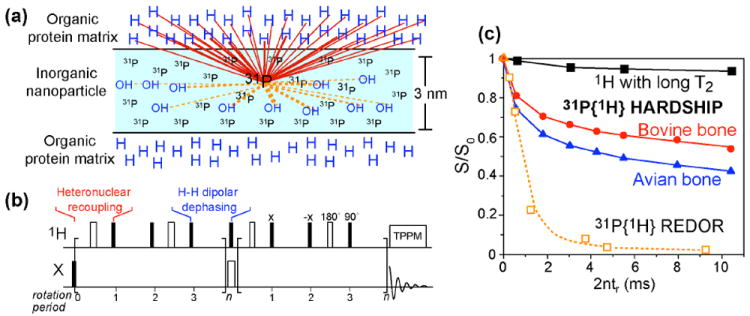
Principle and application of the HARDSHIP technique for measuring long-range distances in nanocomposites. (a) Schematic of a 1H-poor inorganic nanoparticle embedded in a 1H-rich organic matrix. HARDSHIP selectively probes 1H-X dipolar couplings across the interface. (b) HARDSHIP pulse sequence, where 1H-X heteronuclear recoupling alternates with 1H-1H dipolar dephasing to remove the undesired strong 1H-31P dipolar couplings within the inorganic phase while retaining the long-range 1H-31P couplings between the organic and inorganic phases. (c) Application of the HARDSHIP experiment to two bone samples with different nanocrystal thicknesses (triangles and circles) and to hydroxyapatite without an organic matrix (squares).
Using heteronuclear dipolar couplings, NMR can measure distances from the organicinorganic interface to molecular segments in both the organic matrix and the inorganic nanoparticles. With a high density of dephasing spins, for example protons in the organic matrix, the dipolar fields are significantly stronger than a single spin pair, and dephasing at 1 nm from the interface can be significant. In fact, the dependence of the dipolar fields on the distance r from the interface is reduced to approximately ~ r3/2, since the number of spins with significant dipolar coupling increases with r3.
The composition of the organic matrix near the interface is probed most easily in phosphates, where 13C-31P REDOR yields difference spectra that are dominated by the 13C spins at the interface. Using this approach and spectral editing, we have shown that citrate is strongly bound to the calcium phosphate nanocrystals in bone of a wide range of vertebrate species 12. The bound citrate provides more carboxyl groups than all non-collageneous proteins taken together. Its carboxyl-carboxyl distance favorably matches the Ca2+ spacing along the c-axis of bone apatite. This bound citrate prevents thickening of the nanocrystals by covering about 1/6 of the nanocrystal surfaces and by being too large to be incorporated into the growing crystals.
The inorganic side of the interface can be probed using long-range, multi-spin couplings between the abundant protons in the organic phase and X-nuclei such as 31P, 29Si, 13C, 27Al, 23Na in the inorganic phase. Quantitative analysis of the dipolar dephasing can reveal the nanoparticle thickness from the dephasing of the dominant ions such as PO43-, and the preferential depth of minor components from the interface.
To avoid dephasing by the strong local fields of dispersed OH or water protons in the inorganic phase, we engineered a selective pulse sequence, HARDSHIP, that achieves heteronuclear dephasing only by the organic-matrix or surface protons but not by the proximal protons in the inorganic phase. These two types of protons can be distinguished based on the strength of the 1H-1H homonuclear couplings, or equivalently the transverse relaxation time T2,H. In this HARDSHIP experiment, heteronuclear recoupling for ~0.15 ms alternates with periods of 1H homonuclear dipolar dephasing that are flanked by canceling 90° pulses 3. The heteronuclear dipolar evolution of the long-T2, H protons is refocused within two recoupling periods (~0.6 ms). For the short-T2,H protons of the organic matrix, the homonuclear dipolar dephasing of transverse 1H coherence during the period between the two 90° pulse prevents refocusing of the heteronuclear 1H-X dipolar interaction. This results in exponential dipolar dephasing of the X-spin coherence by the organic matrix protons only. During the heteronuclear recoupling period, the strong homonuclear couplings do not affect heteronuclear dephasing, because long-range 1H-X dipolar couplings approximately commute with short-range 1H-1H couplings. The exponential dephasing curves, reflecting 1H-X dephasing only by the short-T2,H protons of the organic matrix, can be simulated accurately to take into account multi-spin effects, because the Hamiltonians for all heteronuclear spin pairs commute. This method was demonstrated on the bioapatite-collagen nanocomposite in bone and on pure hydroxyapatite 3, and can be used to analyze layered structures of up to 3-nm thickness and spherical particles of up to 10-nm diameter.
2.2 13C-31P REDOR for membrane-bound peptides and proteins
REDOR can also be used to measure distances between a single 13C and multiple 31P spins in phospholipid-bilayer-bound peptides and proteins. Analogous to HARDSHIP, the effect of multiple 31P spins can be taken into account by geometric considerations and by numerical simulations. The average 31P-31P distance between phospholipids is about 9 Å, based on the typical phospholipid headgroup area. This separation translates to a weak 31P-31P dipolar coupling of 20 Hz, which is completely removed by MAS. Qualitatively, this means that a 13C spin that is 9 Å from the 31P plane will experience significant dipolar couplings to multiple 31P spins, while a 13C spin that is much closer to the 31P plane will predominantly interact with only one 31P spin. When multiple 13C-31P couplings need to be considered, the fact that they commute with each other means that the REDOR intensity, S/S0, is the powder average of the product of the spin-pair REDOR dephasing, , where ω̄D,m is the time-averaged dipolar coupling of one spin pair, tr is the MAS rotation period, and N is the number of rotation periods in the REDOR pulse train.
Multi-spin REDOR simulations quantitatively confirm the insight that multi-spin consideration is relevant only when the observed 13C-31P dipolar coupling is weak 13. For example, for a rapid REDOR dephasing equivalent to a two-spin distance of 4.0 Å and 5.1 Å, which was measured for Arg11 of the antimicrobial peptide (AMP) PG-1 in POPE/POPG membranes, inclusion of a second 31P spin to create a three-spin system only affected the individual 13C-31P distance modestly, to 5.2 Å each (Fig. 3a, b). No additional 31P spins can be included without violating the physical constraint of the 31P-31P separations in phospholipid membranes. In general, for REDOR dephasing equivalent to two-spin distances of 5 Å or longer, the inclusion of multiple 31P spins increases the length of each 13C-31P vector, but the vertical distance between 13C and the 31P plane increasingly approaches the nominal two-spin distance (Fig. 3d-f). For example, REDOR dephasing for a nominal 8-Å spin-pair distance is equivalent to a vertical distance of 7 Å in a five-spin system.
Fig. 3.
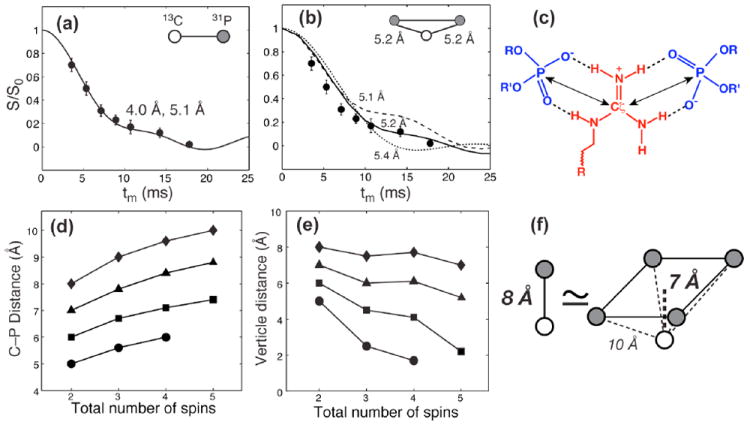
13C-31P REDOR for determining protein distances to lipid 31P groups. (a) REDOR dephasing of Arg11 Cζ in membrane-bound PG-1 13. Assuming a two-spin system, the data is best fit with a 1:1 mixture of a 4.0 Å and a 5.1 Å distance. (b) Assuming two 31P spins interact with Cζ at equal distances, each Cζ-P distance is 5.2 Å. (c) Guanidinium-phosphate bidentate complex, stabilized by N-H…O-P hydrogen bonds and electrostatic attraction. (d) Dependence of individual 13C-31P distances on the number of spins in the REDOR simulation. (e) Dependence of the vertical distance from 13C to the 31P plane on the number of spins in the simulation. (f) A two-spin distance of 8.0 Å is equivalent to a vertical distance of 7.0 Å in a five-spin cluster (4 31P spins and 1 13C) in the REDOR data.
This 13C-31P REDOR approach has been extensively used to study peptide-lipid interactions of arginine-rich membrane peptides 14. These cationic peptides exhibit antimicrobial or membrane-translocation abilities. Arg-rich domains are also found in voltage-gated potassium channels where they act as the voltage sensor 15. 13C-31P distances between the Arg’s and the lipid phosphates have provided useful mechanistic insights into how these cationic peptides bind and insert into the lipid membranes and how their orientation and depth in the membrane explain their biological function. For beta-hairpin disulfide-linked AMPs such as PG-1, 13C-31P distances as short as 4.0 Å have been found for guanidinium Cζ (Fig. 3a). The data suggests the possibility that each guanidinium can form a bidentate complex with two lipid headgroups (Fig. 3b, c). Such a complex would be stabilized by N-H…O-P hydrogen bonds as well as by electrostatic attraction. Such guanidinium-phosphate complexation can reduce the free energy of insertion of these cationic peptides into the lipid membrane. Moreover, short 13C-31P distances were also observed for an Arg embedded in the hydrophobic middle of the beta-hairpin. Since PG-1 is known to be inserted into the hydrophobic part of the membrane based on 1H spin diffusion data 16, the short 13C-31P distances dictate that some lipid molecules have rotated to embed their polar headgroups into the hydrophobic region of the bilayer, which is the key structural feature of toroidal pores 17,18.
The 13C-31P distances of these cationic peptides indicate that short 13C-31P distances are a specific feature of cationic Arg and Lys residues but not hydrophobic residues 19. However, at physiological temperature, Arg and Lys differ in their mobilities: the guanidinium moiety is significantly less mobile than the Lys ammonium moiety, as seen in the measured order parameters. Therefore, the guanidinium ions form stable hydrogen bonds with the lipid phosphate whereas the Lys ammonium only has transient interactions. This difference likely accounts for the more essential functional role of Arg than Lys in these cationic peptides.
2.3 13C-2H REDOR for perdeuterated ligands
The 13C-2H dipolar coupling is typically too weak to be useful for measuring long distances 20. However, the ease of perdeuteration of small molecules makes multi-spin 13C-2H REDOR a viable approach to measure much longer distances than possible by single-pair 13C-2H dipolar coupling. An example is given by the drug-complexed structure of the influenza virus M2 transmembrane peptide (M2TM), where the drug, amantadine, was perdeuterated 21. In the three-fold symmetric drug, twelve C-D bonds are oriented at the tetrahedral angle of 71° from the axis of the adamantane cage, while three C-D bonds are parallel to the molecular axis. Each 13C spin of M2TM experiences dipolar couplings to all fifteen deuterons. Since the three axial deuterons are further from the surrounding peptides, a first approximation is to consider only the dipolar field of the twelve equatorial deuterons. The second moment of these twelve 13C-2H couplings is about 12-fold stronger than a single 13C-2H pair, which significantly speeds up dipolar dephasing. In the REDOR experiment, a single 13C-2H pair exhibits dipolar dephasing as , where the constant value of 1/3 results from the fact that the spin-1 deuteron causes three lines of equal intensity at 0 and ± ω̄CD in its dipolar spectrum. For 12 deuterons, the REDOR dephasing is simply the product of the spin-pair dephasing, . Quantitative distance extraction for amantadine-complexed M2TM was simplified by the fast uniaxial diffusion of the drug, which allows the distances to be parameterized in terms of the pore radius and height of each 13C spin from the center of the drug (Fig. 4c-d). For general situations where no symmetry is present, distances can be quantified using additional parameters 22.
Fig. 4.
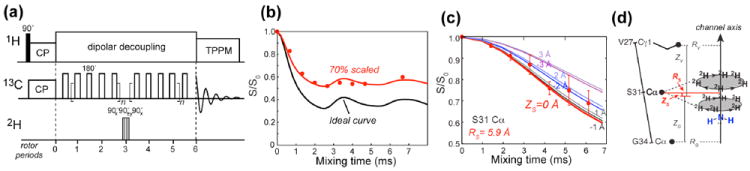
Multi-spin 13C-2H REDOR for measuring long distances 21. (a) Pulse sequence of the 13C-2H REDOR experiment. (b) 13C-2H REDOR dephasing of 13Cα, 2Hβ-labeled alanine. The REDOR minimum is 30% higher than the theoretical minimum due to incomplete inversion of the 2H polarization. (c, d) 13C-2H REDOR dephasing of Ser31 Cα by perdeuterated amantadine in membrane-bound M2TM. The measured dephasing was simulated in terms of the pore radius R and height Z of the carbon from the center of the drug. The geometry is displayed in (d).
To obtain quantitative 13C-2H REDOR dephasing, it is preferable to apply multiple 180° pulses on the 13C spins and a single inversion pulse on the 2H spin, instead of the other way around (Fig. 4a), because the large 2H spectral width makes it difficult to achieve complete inversion of the 2H polarization by routine 180° pulses with field strengths of about 50 kHz, and cumulative incomplete inversion by multiple 2H pulses will severely slow down REDOR dephasing 21. Composite pulses should be used to increase the inversion bandwidth. The degree of inversion efficiency should be calibrated using model compounds (Fig. 4b).
3. Long-range distances from 1H and 19F spin diffusion
Direct spin diffusion among abundant and high-γ spins such as 1H and 19F is a powerful approach for measuring distances in the 10-20 Å range. 1H spin diffusion is particularly useful for determining the immersion depth of membrane proteins in lipid bilayers 5,6. 19F spin diffusion is an elegant and background-free method for measuring site-specific distances in oligomeric protein complexes.
3.1 1H spin diffusion in lipid-bilayer bound membrane peptides and proteins
The pulse sequence for the heteronuclear-detected 1H spin diffusion experiment is shown in Fig. 5a. The 1H magnetization of mobile lipids and water is first selected using a T2 filter, then is allowed to transfer to the 1H-suppressed rigid protein during a mixing time. The result of spin diffusion is detected through the protein 13C or 15N signals 5,6. Only when the protein is in close proximity to the lipid chains or water will there be a lipid-protein or water-protein 1H-13C cross peak. The T2 filter requires that the experiment be conducted in the liquid-crystalline phase of the membrane where the lipid mobility is higher than the protein mobility and spin diffusion within the lipid matrix is rate-limiting, occurring on the timescale of tens to hundreds of milliseconds. Once the 1H magnetization diffuses to a protein site, it will be rapidly equilibrated in the protein due to the protein’s rigidity. Applied in this way, the immersion depth is not site-specific, but is the minimum distance between the protein and the hydrophobic center of the membrane. The lipid-chain-end methyl protons (0.9 ppm) are more desirable depth probes than the methylene protons due to the former’s better defined locations.
Fig. 5.
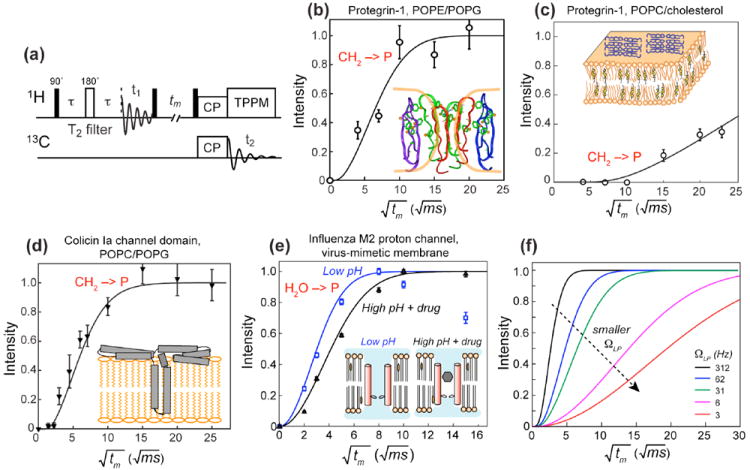
1H spin diffusion for to determine depth of insertion and water interaction of membrane peptides and proteins. (a) Pulse sequence of the 13C-detected 1H spin diffusion experiment. (b) Lipid-chain to PG-1 spin diffusion in bacteria-mimetic POPE/POPG membranes. The best-fit distance of 2 Å indicates a TM topology. (c) Lipid-chain to PG-1 spin diffusion in eukaryote-mimetic POPC/cholesterol membranes. The slow buildup rate is best fit to a 20-Å distance, indicating that PG-1 lies on the surface of this membrane. (d) Lipid-chain to colicin Ia channel domain spin diffusion in POPC/POPG membranes. The fast buildup and the 2-Å distance indicates that the protein has a TM domain, thus supporting the umbrella model shown here. (e) Water spin diffusion to M2TM in two different states. The buildup is faster for the open channel at low pH than for the drug-bound closed channel at high pH (filled triangles). (f) Simulated lipid-protein spin diffusion buildup curves for various interfacial transfer rate ΩLP. In the simulations, DP = 0.3 nm2/ms, DL = 0.012 nm2/ms, and the peptide-lipid separation is fixed at 2 Å.
Fig. 5b-d shows examples of the lipid-protein 1H spin diffusion experiment to determine the immersion depths of membrane proteins. Small membrane peptides such as PG-1 can adopt different insertion motifs depending on the membrane composition 16: it inserts into the bacteriamimetic anionic membrane but lies on the surface of the eukaryote-mimetic membrane, which explains the selective activity of this AMP. Larger membrane proteins such as the bacterial toxin colicin Ia channel domain can possess both surface-bound and TM domains. The presence or absence of a TM domain can be unambiguously determined from the lipid-protein spin diffusion buildup curve (Fig. 5d). For this toxin, the fast spin diffusion from the lipid chains supports an umbrella model while disproving a penknife model 6.
Distance quantification of the cross peak buildup curves requires knowledge of the spin diffusion coefficients. For lipid-protein spin diffusion, the diffusion coefficients within the lipid matrix (DL) and within the protein (DP) are necessary 6. For water-to-protein spin diffusion, an analogous DW is involved. These diffusion coefficients can be estimated from the dipolar couplings of lipids, protein and water using the relation D = Ωa2, where Ω is the dipolar coupling and a is the lattice spacing between spins. For rigid proteins, a DP of 0.8 nm2/ms was estimated, corresponding to a 1H-1H dipolar coupling of 20 kHz for a 2 Å separation. The dipolar couplings between vicinal protons of liquid-crystalline lipids correspond to a DL of 0.01 - 0.03 nm2/ms. The exact value depends on the membrane dynamics: cholesterol-rich membranes have higher rigidity and hence larger DL, whereas low-melting phospholipid membranes such as POPC and POPG have smaller DL’s. The interfacial transfer rate ΩLP is an important adjustable parameter in spin diffusion simulations. ΩLP is small due to the low probability of intermolecular contact. It can be estimated from the shape of the observed buildup curve (Fig. 5f): lower ΩLP decreases the slope of the buildup curve 16. For water-protein spin diffusion, DW is typically 3 nm2/ms and ΩWP is about 200 Hz. Most membrane proteins exhibit rapid water-protein magnetization transfer that is equilibrated by ~100 ms. This reflects the fact that both surface-bound and TM membrane peptides have some residues exposed to water on the membrane surface.
When 1H spin diffusion is used to determine the depth of membrane proteins, a 1D lattice model for magnetization transfer along the bilayer normal is the most appropriate framework. For water-protein spin diffusion, an alternative 3D lattice model can be more informative. If we assume an average diffusion coefficient Deff for the entire protein-lipid-water system, then the spin diffusion buildup is proportional to the water-exposed surface area (SWP) of the protein 23,24. This approach has been used to investigate the conformational changes of ion channels and other membrane proteins. For example, the low-pH state of the influenza M2 proton channel has a faster buildup rate than the drug-bound state of the channel; the difference suggests a two-fold larger SWP in the open state of the channel (Fig. 5e)24.
A specific question about water-protein interactions is how water interacts with cationic residues in membrane-bound AMPs and cell-penetrating peptides. The 13C-detected 1H spin diffusion experiments revealed that the Arg guanidinium ions are well solvated by water molecules and that water-peptide spin diffusion can be observed in as short as 1 ms 25,26. This observation, together with the short Arg-phosphate distances, supports the notion that Arg’s are stabilized in lipid membranes by both salt bridges and water dimples.
31P-detected 1H spin diffusion is also useful for understanding membrane phenomenon. Lipid-chain to headgroup spin diffusion usually requires over hundreds of milliseconds to equilibrate, but the presence of TM protein domains speed up this spin diffusion by providing a rigid conduit along which magnetization transfer can occur. Therefore, even without 13C labeled proteins, one can use the 31P-detected 1H spin diffusion experiment to measure the depth of insertion of membrane proteins indirectly 6. Since the membrane surface is almost always hydrated, water-31P cross peaks are usually readily detectable. However, these cross peaks require exchangeable protons in the membrane, either from the lipid headgroups such as the NH2 group in phosphatidylethanolamine or from the protein 27. In protein-free phosphatidylcholine membranes, no water-31P cross peaks can be observed, even though the membrane is hydrated.
3.2 19F -19F distances from CODEX spin diffusion
19F spin diffusion provides a novel method to measure distances to ~15 Å as well as to determine the oligomeric number of protein assemblies. This approach is based on the CODEX technique initially developed to measure slow reorientational motions 28. The idea is to use rotation-synchronized 180° pulses to recouple the anisotropic 19F chemical shift interaction to create a stimulated echo (Fig. 6a). The echo intensity decreases when the orientation-dependent chemical shift frequency changes due to molecular motion or magnetization transfer to another spin with a different orientation. This magnetization transfer is driven by distance-dependent dipole couplings. When spin diffusion reaches equilibrium among all spins in an n-spin cluster, the fraction of magnetization remaining at the original site is 1/n, which is the equilibrium echo intensity 29. Therefore, the CODEX intensity at equilibrium gives the oligomeric number, while the decay rate to equilibrium reveals internuclear distances. The distances can be quantified using an exchange-matrix formalism: the time-dependent magnetization M(t) follows the first-order differential equation 30 dM(t)/dt = −KM(t), where K is the n-dimensional exchange matrix containing rate constants kij. The rate constants depend on the dipolar coupling according to , where Fij(0) is the overlap integral describing the probability that single-quantum transitions occur at the same frequency for spins i and j. The overlap integral can be measured from model compounds with known 19F-19F distances. A consensus value of F(0) = 37 μs has been found for an MAS frequency of 8 kHz for 19F spins in aromatic compounds 30.
Fig. 6.
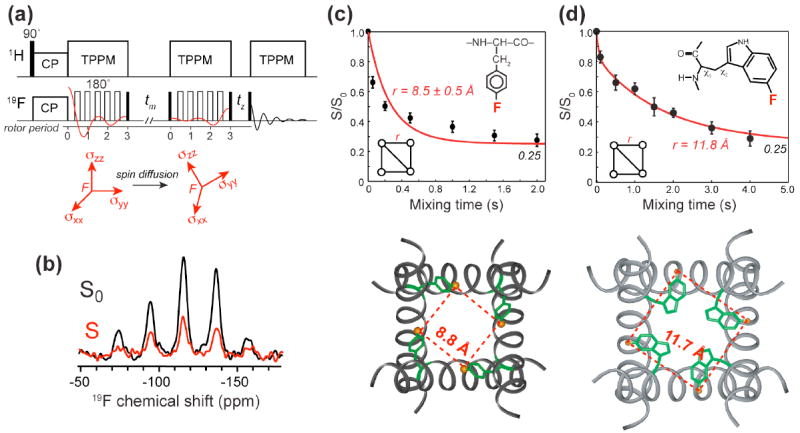
Determination of distances and oligomeric structure using 19F spin diffusion. (a) 19F CODEX pulse sequence 29. Magnetization transfer to 19F with a different chemical shift tensor orientation reduces the intensity of a stimulated echo. (b) Representative 19F CODEX control (S0) and exchange (S) spectra, of 4-19F-Phe30 in M2TM 30. (c) 19F CODEX data of 4-19F-Phe30 in M2TM. The CODEX signals decay to 0.25, proving the tetrameric nature of M2TM in the lipid bilayer. The decay rate yields a nearest-neighbor distance of 8.8 Å. (d) 19F CODEX data of 5-19F-Trp41 in DMPC-bound M2TM. The distance of 11.8 Å constrains both the rotameric structure of Trp41 and interhelical packing 31.
This 19F CODEX technique has been applied to the AMP PG-1 and the influenza M2TM. It was found that PG-1 associates in 2-spin clusters within a distance upper limit of 15 Å in POPE/POPG membranes 16. The association is between like strands: the C-terminal strand with the C-terminal strand and the N-terminal strand with another N-terminal strand. Combined with other site-specific distances 11, this result indicates that PG-1 forms membrane-spanning parallel NCCN beta-barrels in bacteria-mimetic anionic membranes. In contrast, in POPC/cholesterol bilayers, PG-1 forms larger spin clusters of at least 4 for each 19F label, suggesting β-sheets. Moreover, these β-sheets lie on the membrane surface, thus explaining the selective activity of this AMP against microbial membranes.
The 19F CODEX experiment was also used to measure two interhelical distances in the tetrameric influenza M2TM. A para-fluorinated Phe at residue 30 gave a nearest-neighbor distance of 8.8 Å while a 5-19F-labeled Trp41 showed a nearest-neighbor distance of 11.8 Å. These distances constrained the tetramer packing as well as the rotameric conformation of Phe30 and Trp41. The latter gave useful insight into the gating mechanism of this proton channel 31.
Conclusion
The above survey shows an array of techniques that are now available to measure distances up to about 15 Å. As demonstrated for PG-1 and the influenza M2 peptide, just a few sparse long-range distances can already provide powerful constraints to the three-dimensional fold of peptides and proteins. Future directions will be to extend these techniques to measure multiple long-range distances simultaneously in extensively labeled proteins, in order to reduce the need for site-specifically labeled samples.
Acknowledgments
M. H. is grateful to NSF for funding the majority of the techniques described in this review. We thank NIH and DOE for supporting the applications of these methods to many interesting biological and materials problems. We also thank the many coworkers involved in these studies, especially when it was not clear that the experiments would work.
Biography
Mei Hong and Klaus Schmidt-Rohr are Professors of Chemistry at Iowa State University. Mei Hong received her B.A. at Mount Holyoke College and Ph.D. at the University of California Berkeley. Since joining the faculty at Iowa State University in 1999, she has focused on studying membrane protein structure, dynamics and mechanism of action using solid-state NMR. Klaus Schmidt-Rohr received his B.S. and Ph.D. at the University of Mainz, Germany, working at the Max Planck Institute for Polymer Research. After a faculty position at the University of Massachusetts at Amherst, he joined Iowa State University in 2000. Professor Schmidt-Rohr develops and applies solid-state NMR techniques to study the structure and dynamics of complex materials.
References
- 1.Gullion T, Schaefer J. Rotational echo double resonance NMR. J Magn Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Rohr K, Hong M. Measurements of carbon to amide-proton distances by C-H dipolar recoupling with 15N NMR detection. J Am Chem Soc. 2003;125:5648–5649. doi: 10.1021/ja0344415. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Rohr K, Rawal A, Fang XW. A new NMR method for determining the particle thickness in nanocomposites, using T2,H-selective X{1H} recoupling. J Chem Phys. 2007;126:054701. doi: 10.1063/1.2429069. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Rohr K, Spiess HW. Multidimensional Solid-State NMR and Polymers. 1. Academic Press; San Diego: 1994. [Google Scholar]
- 5.Kumashiro KK, Schmidt-Rohr K, Murphy OJ, Ouellette KL, Cramer WA, Thompson LK. A novel tool for probing membrane protein structure: solid-state NMR with proton spin diffusion and X-nucleus detection. J Am Chem Soc. 1998;120:5043–5051. [Google Scholar]
- 6.Huster D, Yao XL, Hong M. Membrane Protein Topology Probed by 1H Spin Diffusion from Lipids Using Solid-State NMR Spectroscopy. J Am Chem Soc. 2002;124:874–883. doi: 10.1021/ja017001r. [DOI] [PubMed] [Google Scholar]
- 7.Yao XL, Schmidt-Rohr K, Hong M. Medium- and Long-Distance 1H-13C Heteronuclear Correlation NMR in Solids. J Magn Reson. 2001;149:139–143. [Google Scholar]
- 8.Sinha N, Hong M. X-1H Rotational-Echo Double-Resonance NMR For Torsion Angle Determination of Peptides. Chem Phys Lett. 2003;380:742–748. [Google Scholar]
- 9.Wi S, Sinha N, Hong M. Long range 1H-19F distance measurement in peptides by Solid-State NMR. J Am Chem Soc. 2004;126:12754–12755. doi: 10.1021/ja0462732. [DOI] [PubMed] [Google Scholar]
- 10.Yao XL, Hong M. Structural Distribution in an Elastin-Mimetic Peptide (VPGVG)3 Investigated by Solid–State NMR. J Am Chem Soc. 2004;126:4199–4210. doi: 10.1021/ja036686n. [DOI] [PubMed] [Google Scholar]
- 11.Mani R, Tang M, Wu X, Buffy JJ, Waring AJ, Sherman MA, Hong M. Membrane-bound dimer structure of a b-hairpin antimicrobial peptide from rotational-echo double-resonance solid-state NMR. Biochemistry. 2006;45:8341–8349. doi: 10.1021/bi060305b. [DOI] [PubMed] [Google Scholar]
- 12.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci U S A. 2010;107:22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang M, Waring AJ, Hong M. Phosphate-Mediated Arginine Insertion Into Lipid Membranes and Pore Formation by a Cationic Membrane Peptide from Solid-State NMR. J Am Chem Soc. 2007;129:11438–11446. doi: 10.1021/ja072511s. [DOI] [PubMed] [Google Scholar]
- 14.Hong M, Su Y. Structure and dynamics of cationic membrane peptides and proteins: Insights from solid-state NMR. Protein Sci. 2011;20:641–655. doi: 10.1002/pro.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty T, Su Y, Hong M. High-Resolution Orientation and Depth of Insertion of the Voltage-Sensing S4 Helix of a Potassium Channel in Lipid Bilayers. J Mol Biol. 2010 doi: 10.1016/j.jmb.2010.06.048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Membrane-dependent oligomeric structure and pore formation of a beta-hairpin antimicrobial peptide in lipid bilayers from solid-state NMR. Proc Natl Acad Sci USA. 2006;103:16242–16247. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 18.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 19.Su Y, Doherty T, Waring AJ, Ruchala P, Hong M. Roles of arginine and lysine residues in the translocation of a cell-penetrating peptide from (13)C, (31)P, and (19)F solid-state NMR. Biochemistry. 2009;48:4587–4595. doi: 10.1021/bi900080d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sack II, Goldbourt A, Vega S, Buntkowsky G. Deuterium REDOR: principles and applications for distance measurements. J Magn Reson. 1999;138:54–65. doi: 10.1006/jmre.1999.1710. [DOI] [PubMed] [Google Scholar]
- 21.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cady SD, Wang J, Wu Y, DeGrado WF, Hong M. Specific binding of adamantane drugs and direction of their polar amines in the pore of the influenza M2 transmembrane domain in lipid bilayers and dodecylphosphocholine micelles determined by NMR spectroscopy. J Am Chem Soc. 2011;133:4274–4284. doi: 10.1021/ja102581n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ader C, Schneider R, Seidel K, Etzkorn M, Becker S, Baldus M. Structural rearrangements of membrane proteins probed by water-edited solid-state NMR spectroscopy. J Am Chem Soc. 2009;131:170–176. doi: 10.1021/ja806306e. [DOI] [PubMed] [Google Scholar]
- 24.Luo W, Hong M. Conformational changes of an ion channel membrane protein detected through water-protein interactions using solid-state NMR spectroscopy. J Am Chem Soc. 2010;132:2378–2384. doi: 10.1021/ja9096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Y, Waring AJ, Ruchala P, Hong M. Membrane-Bound Dynamic Structure of an Arginine-Rich Cell-Penetrating Peptide, the Protein Transduction Domain of HIV TAT, from Solid-State NMR. Biochemistry. 2010;49:6009–6020. doi: 10.1021/bi100642n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Su Y, Luo W, Hong M. Water-protein Interactions of an Arginine-Rich Membrane Peptide in Lipid Bilayers Investigated by Solid-State Nuclear Magnetic Resonance Spectroscopy. J Phys Chem B. 2010;114:4063–4069. doi: 10.1021/jp912283r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty T, Hong M. 2D (1)H-(31)P solid-state NMR studies of the dependence of inter-bilayer water dynamics on lipid headgroup structure and membrane peptides. J Magn Reson. 2009;196:39–47. doi: 10.1016/j.jmr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deAzevedo ER, Bonagamba TJ, Hu W, Schmidt-Rohr K. Centerband-only detection of exchange: efficient analysis of dynamics in solids by NMR. J Am Chem Soc. 1999;121:8411–8412. [Google Scholar]
- 29.Buffy JJ, Waring AJ, Hong M. Determination of Peptide Oligomerization in Lipid Membranes with Magic-Angle Spinning Spin Diffusion NMR. J Am Chem Soc. 2005;127:4477–4483. doi: 10.1021/ja043621r. [DOI] [PubMed] [Google Scholar]
- 30.Luo W, Hong M. Determination of the oligomeric number and intermolecular distances of membrane protein assemblies by anisotropic 1H-driven spin diffusion NMR spectroscopy. J Am Chem Soc. 2006;128:7242–7251. doi: 10.1021/ja0603406. [DOI] [PubMed] [Google Scholar]
- 31.Luo W, Mani R, Hong M. Sidechain conformation and gating of the M2 transmembrane peptide proton channel of influenza A virus from solid-state NMR. J Phys Chem. 2007;111:10825–10832. doi: 10.1021/jp073823k. [DOI] [PubMed] [Google Scholar]


