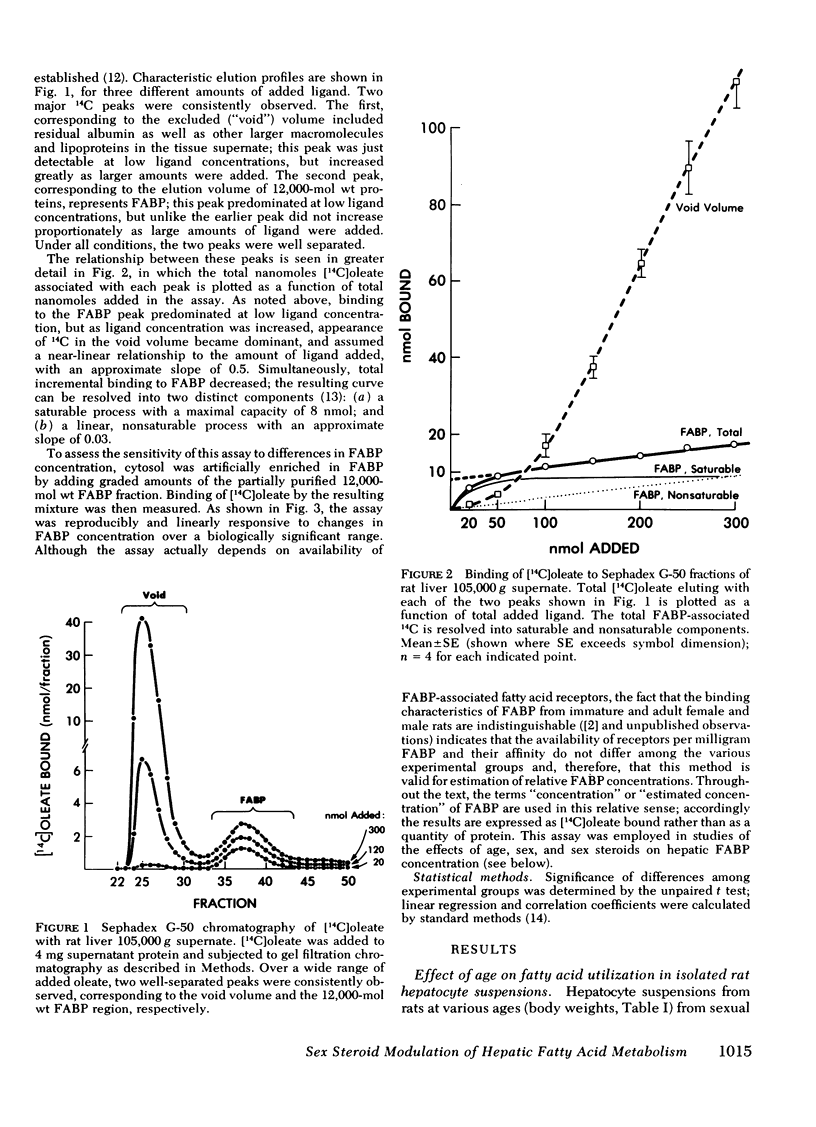
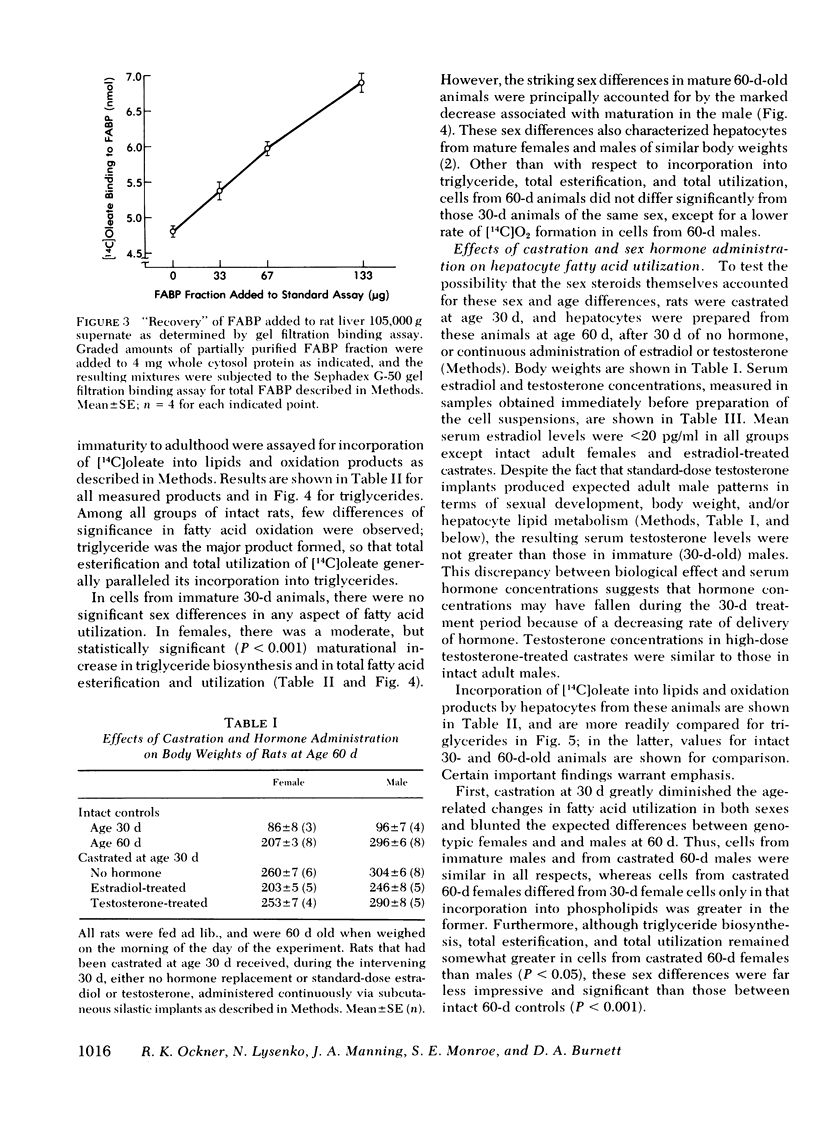
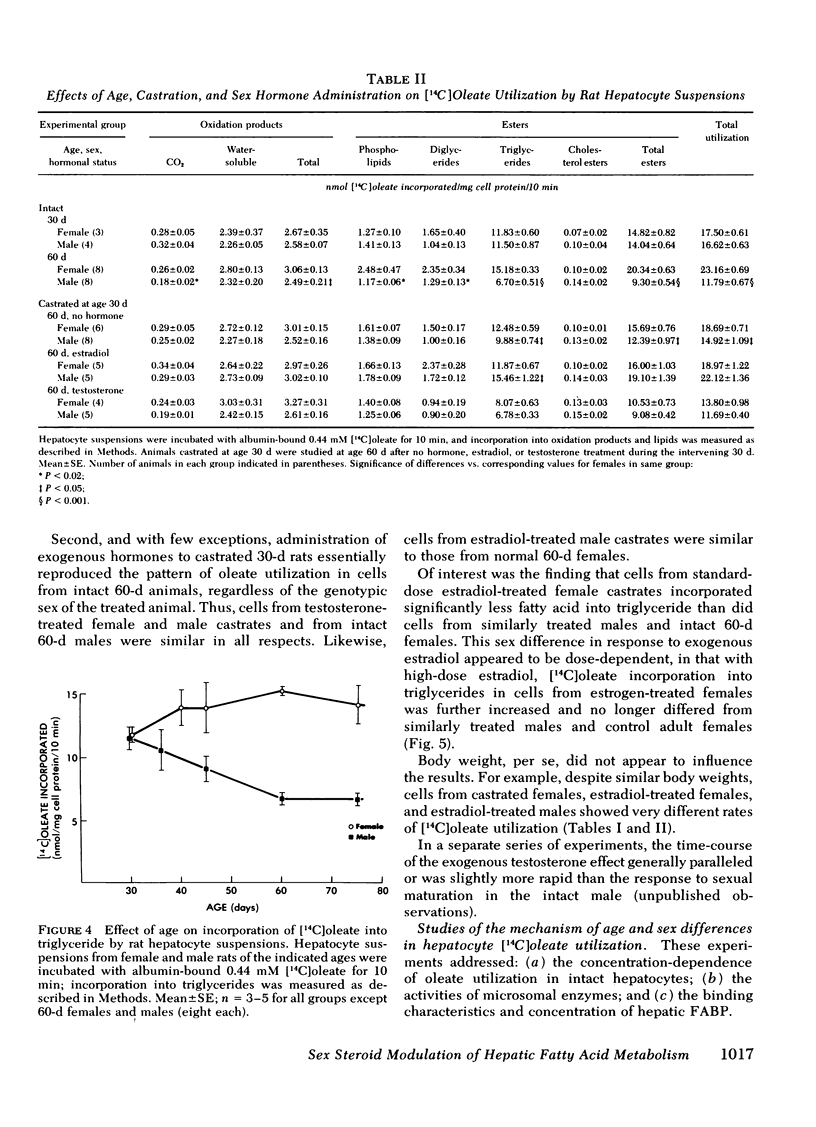
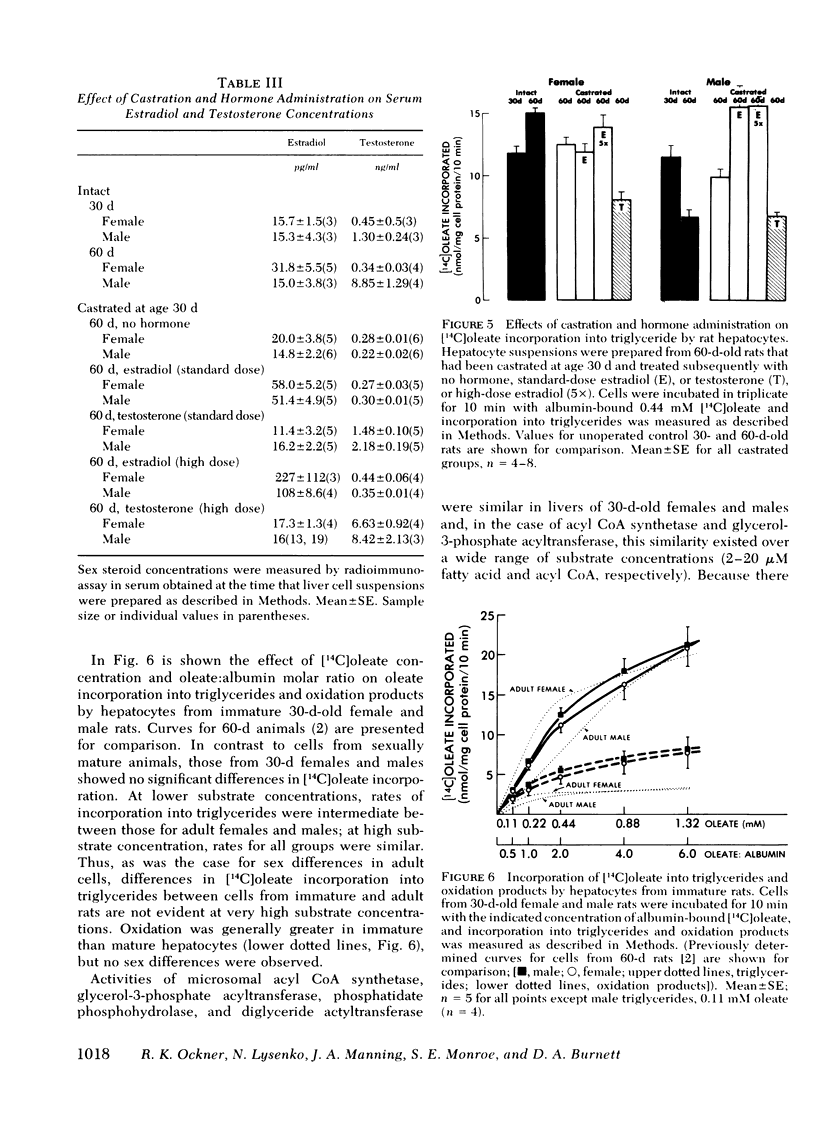
Abstract
The mechanism by which sex steroids influence very low density hepatic lipoprotein triglyceride production has not been fully elucidated. In previous studies we showed that [14C]oleate utilization and incorporation into triglycerides were greater in hepatocyte suspensions from adult female rats than from males. The sex differences were not related to activities of the enzymes of triglyceride biosynthesis, whereas fatty acid binding protein (FABP) concentration in liver cytosol was greater in females. These findings suggested that sex differences in lipoprotein could reflect a sex steroid influence on the availability of fatty acids for hepatocellular triglyceride biosynthesis. In the present studies, sex steroid effects on hepatocyte [14C]oleate utilization and FABP concentration were investigated directly.
Hepatocytes from immature (30-d-old) rats exhibited no sex differences in [14C]oleate utilization. With maturation, total [14C]oleate utilization and triglyceride biosynthesis increased moderately in female cells and decreased markedly in male cells; the profound sex differences in adults were maximal by age 60 d. Fatty acid oxidation was little affected.
Rats were castrated at age 30 d, and received estradiol, testosterone, or no hormone until age 60 d, when hepatocyte [14C]oleate utilization was studied. Castration virtually eliminated maturational changes and blunted the sex differences in adults. Estradiol or testosterone largely reproduced the appropriate adult pattern of [14C]oleate utilization regardless of the genotypic sex of the treated animal.
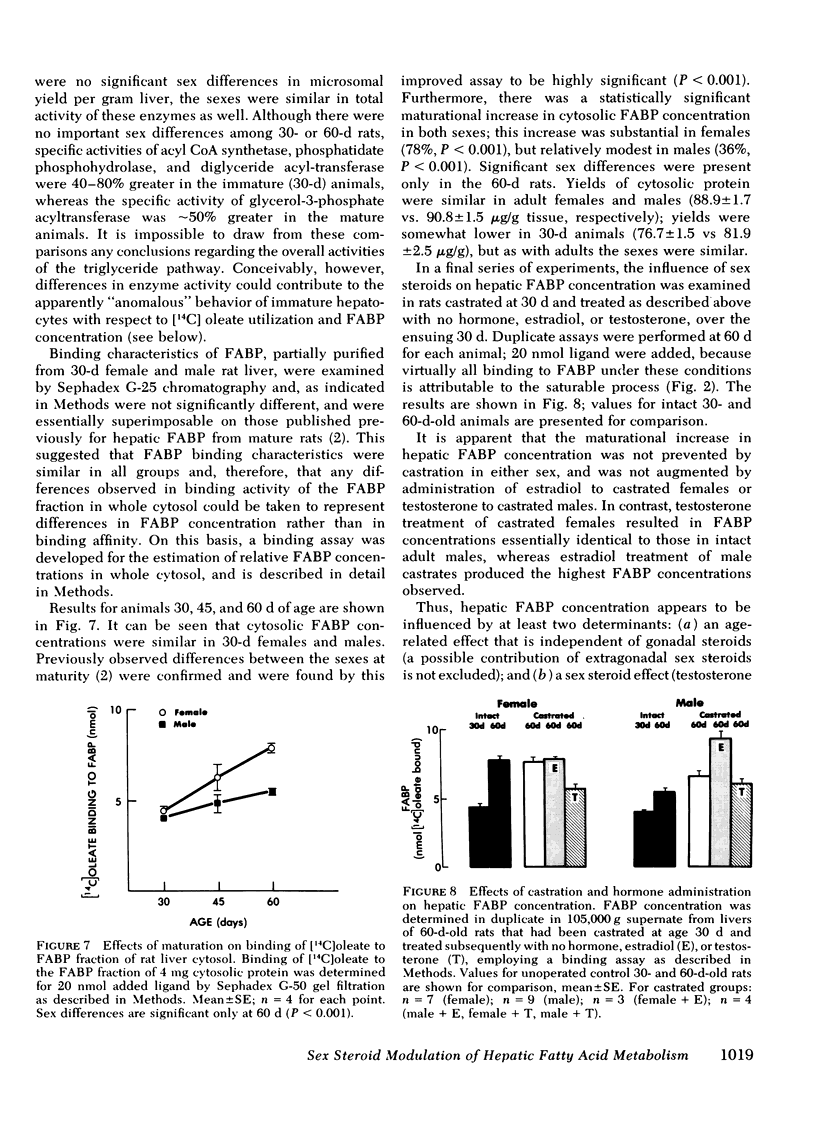
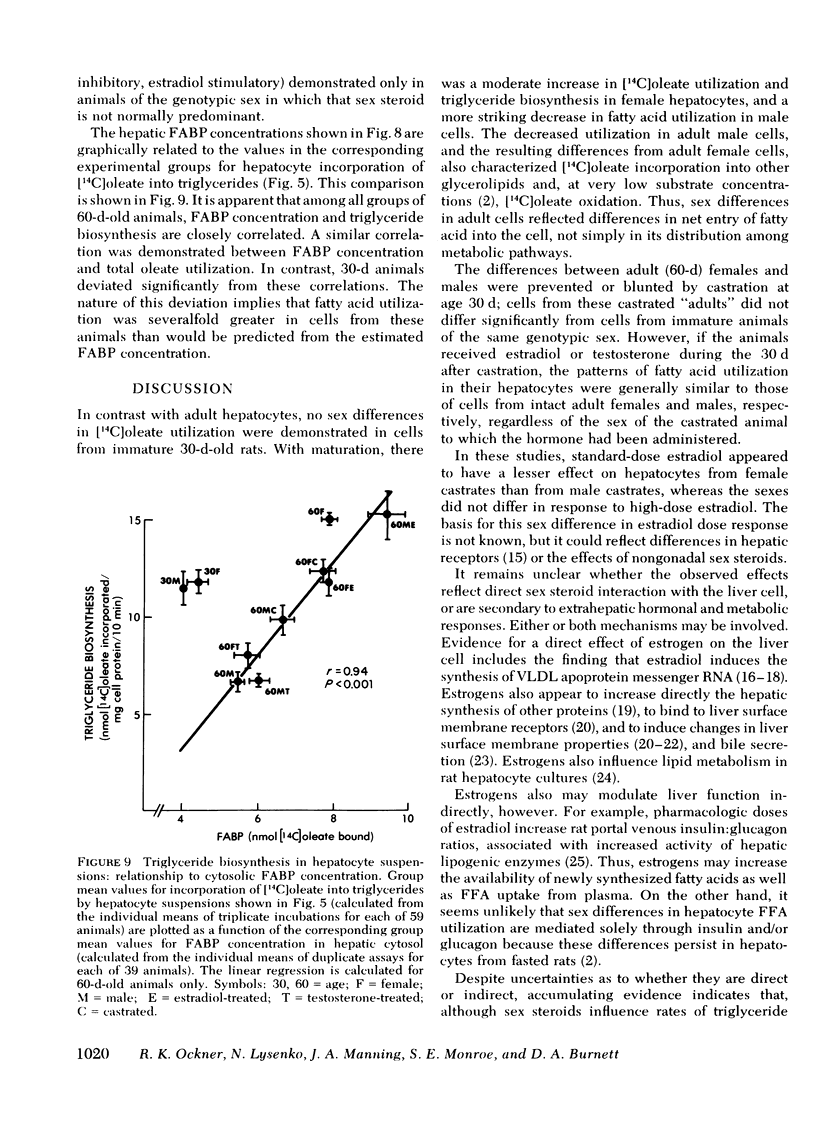
In immature females and males, total cytosolic FABP concentrations were similar. In 60-d-old animals, there was a striking correlation among all groups (females, males, castrates, and hormone-treated) between mean cytosolic FABP concentration on the one hand, and mean total [14C]oleate utilization (r = 0.91) and incorporation into triglycerides (r = 0.94) on the other. In 30-d-old animals rates of [14C]oleate utilization were greater, relative to FABP concentrations, than in 60-d-old animals.
The sex differences that characterize fatty acid utilization in adult rat hepatocytes are not present in cells from immature animals, and reflect in part the influence of sex steroids. It remains to be determined whether the observed relationship of hepatic FABP concentration to [14C]oleate utilization in adult cells is causal or secondary to changes in cellular fatty acid uptake effected through another mechanism. In either case, modulation of triglyceride-rich lipoprotein production by six steroids appears to be mediated to a significant extent by their effects on hepatic fatty acid utilization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Goldberg A. P., Pykälistö O. J., Brunzell J. D., Hazzard W. R. Effect of estrogen on post-heparin lipolytic activity. Selective decline in hepatic triglyceride lipase. J Clin Invest. 1977 Apr;59(4):601–608. doi: 10.1172/JCI108677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N. High-yield preparation of morphologically intact isolated parenchymal cells from rat liver. Methods Enzymol. 1974;32:625–632. doi: 10.1016/0076-6879(74)32064-2. [DOI] [PubMed] [Google Scholar]
- Burnett D. A., Lysenko N., Manning J. A., Ockner R. K. Utilization of long chain fatty acids by rat liver: studies of the role of fatty acid binding protein. Gastroenterology. 1979 Aug;77(2):241–249. [PubMed] [Google Scholar]
- Chait A., Brunzell J. D., Albers J. J., Hazzard W. R. Type-III Hyperlipoproteinaemia ("remnant removal disease"). Insight into the pathogenetic mechanism. Lancet. 1977 Jun 4;1(8023):1176–1178. doi: 10.1016/s0140-6736(77)92717-9. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C. Y., Davidson J. M. Effects of testosterone implants and hypothalamic lesions on luteinizing hormone regulation in the castrated male rat. Endocrinology. 1977 Feb;100(2):292–302. doi: 10.1210/endo-100-2-292. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Kern F., Jr, Showalter R., Sutherland E., Sinensky M., Simon F. R. Alterations of hepatic Na+,K+-atpase and bile flow by estrogen: effects on liver surface membrane lipid structure and function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4130–4134. doi: 10.1073/pnas.75.9.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Aten R. F., Eisenfeld A. J. An unusual sex steroid-binding protein in mature male rat liver cytosol. Endocrinology. 1978 Nov;103(5):1636–1646. doi: 10.1210/endo-103-5-1636. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Huttunen J. K., Kinnunen P. J., Miettinen T. A., Nikkilä E. A. Effect of oxandrolone treatment on the activity of lipoprotein lipase, hepatic lipase and phospholipase A1 of human postheparin plasma. N Engl J Med. 1975 Jun 19;292(25):1314–1317. doi: 10.1056/NEJM197506192922503. [DOI] [PubMed] [Google Scholar]
- Forker E. L. The effect of estrogen on bile formation in the rat. J Clin Invest. 1969 Apr;48(4):654–663. doi: 10.1172/JCI106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D. Receptors for gastrointestinal hormones. Gastroenterology. 1979 Jan;76(1):202–214. [PubMed] [Google Scholar]
- Glueck C. J., Brown W. V., Levy R. I., Greten H., Fredrickson D. S. Amelioration of hypertriglyceridaemia by progestational drugs in familial type-V hyperlipoproteinaemia. Lancet. 1969 Jun 28;1(7609):1290–1291. doi: 10.1016/s0140-6736(69)92225-9. [DOI] [PubMed] [Google Scholar]
- Hillman L., Schonfeld G., Miller J. P., Wulff G. Apolipoproteins in human pregnancy. Metabolism. 1975 Aug;24(8):943–952. doi: 10.1016/0026-0495(75)90086-4. [DOI] [PubMed] [Google Scholar]
- Kekki M., Nikkilä E. A. Plasma triglyceride turnover during use of oral contraceptives. Metabolism. 1971 Sep;20(9):878–889. doi: 10.1016/0026-0495(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kalkhoff R. K. Sex steroid influence on triglyceride metabolism. J Clin Invest. 1975 Oct;56(4):888–896. doi: 10.1172/JCI108168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973 May;5(3):184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- Knopp R. H., Warth M. R., Carrol C. J. Lipid metabolism in pregnancy. I. Changes in lipoprotein triglyceride and cholesterol in normal pregnancy and the effects of diabetes mellitus. J Reprod Med. 1973 Mar;10(3):95–101. [PubMed] [Google Scholar]
- Kudzma D. J., St Claire F., DeLallo L., Friedberg S. J. Mechanism of avian estrogen-induced hypertriglyceridemia: evidence for overproduction of triglyceride. J Lipid Res. 1975 Mar;16(2):123–133. [PubMed] [Google Scholar]
- Kushwaha R. S., Hazzard W. R., Gagne C., Chait A., Albers J. J. Type III hyperlipoproteinemia: paradoxical hypolipidemic response to estrogen. Ann Intern Med. 1977 Nov;87(5):517–525. doi: 10.7326/0003-4819-87-5-517. [DOI] [PubMed] [Google Scholar]
- Legan S. J., Coon G. A., Karsch F. J. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975 Jan;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Luskey K. L., Brown M. S., Goldstein J. L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J Biol Chem. 1974 Sep 25;249(18):5939–5947. [PubMed] [Google Scholar]
- O'Doherty P. J., Kuksis A. Stimulation of triacylglycerol synthesis by Z protein in rat liver and intestinal mucosa. FEBS Lett. 1975 Dec 15;60(2):256–258. doi: 10.1016/0014-5793(75)80725-3. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Burnett D. A., Lysenko N., Manning J. A. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. J Clin Invest. 1979 Jul;64(1):172–181. doi: 10.1172/JCI109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid binding protein. Role in esterification of absorbed long chain fatty acid in rat intestine. J Clin Invest. 1976 Sep;58(3):632–641. doi: 10.1172/JCI108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Poppenhausen R. B., Ho W. K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972 Jul 7;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977 Jan 6;265(5589):69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Heimberg M. Comparison of metabolism of free fatty acid by isolated perfused livers from male and female rats. J Lipid Res. 1976 Nov;17(6):605–615. [PubMed] [Google Scholar]
- Warth M. R., Arky R. A., Knopp R. H. Lipid metabolism in pregnancy. II. Altered lipid composition in intermediage, very low, low and high-density lipoprotein fractions. J Clin Endocrinol Metab. 1975 Oct;41(4):649–655. doi: 10.1210/jcem-41-4-649. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Kenney F. T. Regulation of yolk protein synthesis in amphibian liver. I. Induction of lipovitellin synthesis by estrogen. Biochim Biophys Acta. 1972 May 29;269(3):485–492. doi: 10.1016/0005-2787(72)90136-0. [DOI] [PubMed] [Google Scholar]


