Abstract
Background
The study investigated if tumor volume changes at 8 weeks of therapy are associated with outcomes in advanced NSCLC patients with sensitizing EGFR mutations treated with EGFR tyrosine kinase inhibitors (TKIs).
Patients and methods
In 56 advanced NSCLC patients with sensitizing EGFR mutations treated with first-line erlotinib or gefitinib, tumor volumes of dominant lung lesions were measured on baseline and follow-up CT and were analyzed for association with survival.
Results
Among 56 eligible patients, the median tumor volume was 17.8 cm3 (range: 1.3-172.7 cm3) on the baseline scans. 49 patients had follow-up CT at approximately 8 weeks; the median tumor volume at 8 weeks was 7.1 cm3 (range: 0.4-62.3 cm3), with the median proportional volume change of -59% (range: -90% to +91%) from baseline. The proportional volume change at 8 weeks was associated with survival (p=0.02). Using the cut-off value of 38% volume decrease (75th percentile) at 8 weeks, patients with volume decrease >38% (n=37) had a median overall survival of 43.5 months compared to 16.3 months among those with volume decrease ≤38% (n=12) (p=0.01). The median progression-free survival for patients with >38% volume decrease was 12.6 months, compared to 5.5 months for those with ≤38% volume decrease (p=0.2).
Conclusions
The proportional volume change at 8 weeks is associated with overall survival in EGFR-mutant advanced NSCLC patients treated with first-line EGFR-TKIs. The observation of the study, if confirmed in larger study cohorts, indicates that tumor volume analysis at 8 weeks may provide an early marker for survival, and contribute to therapeutic decision making by identifying patients who may benefit from additional anti-cancer therapy after 8 weeks of EGFR-TKI therapy.
Keywords: lung cancer, CT scans, tumor volume, epidermal growth factor receptor mutations, EGFR tyrosine kinase inhibitors
INTRODUCTION
Lung cancer is the leading cause of cancer death in the United States accounting for over 160,000 deaths per year [1-2]. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), erlotinib and gefitinib, are associated with a dramatic clinical response in patients with EGFR sensitizing mutations, with response rates greater than 70%, and progression-free survival (PFS) of 9.7-13.1 months [3-9]. However, nearly all patients with initial responses eventually relapse due to acquired resistance to EGFRTKIs [10-14].
Medical oncologists typically rely on changes in one-dimensional tumor size measured on computed tomography (CT) as the major determinant in defining tumor progression and deciding when to change therapy [15-17]. However, based on increasing clinical experience, the conventional RECIST-based assessment alone may not be sufficient to fully characterize response and progression in genomically-defined patients with specific tumor types, such as melanoma and lung cancer, receiving targeted therapies [18-21]. Given the increasing understanding of molecular mechanisms of NSCLC in response and resistance to EGFR-TKI, additional radiographic strategies for objective response assessment and determination of progression are needed to better guide therapeutic decisions in EGFR-mutant NSCLC patients [19-21].
Advancement of multidetector-row CT technology has enabled volumetric tumor measurements, which have attracted attention as a method to complement limitations of conventional size-based criteria [22-28]. Tumor volume measurement in lung cancer has been shown to be more reproducible than size measurement [22-28]. We have previously established a method of CT tumor volume measurement of advanced NSCLC using clinical chest CT and commercially available, FDA-approved software [28]. Variability of measurements in advanced NSCLC was investigated, demonstrating that tumor volume is more reproducible than size [28].
Although radiological investigations have been performed to assess tumor volume changes in lung cancer [29-30], few studies have assessed volumetric changes of advanced NSCLC with specific genomic mutations during targeted therapy. Moreover, the association between tumor volume changes and survival has not been systematically studied in genomically-defined NSCLC patients receiving targeted therapy.
The purpose of the present study is to determine if the tumor volume changes at 8 weeks of therapy is associated with survival in advanced NSCLC patients harboring sensitizing EGFR mutations treated with the first-line EGFR-TKI. If the greater initial decrease in tumor volume is associated with longer survival, the decrease in tumor volume can serve as an early predictor of survival and help optimize the therapeutic approaches. Eight-week landmark was chosen since it was when the first follow-up CT is performed in trials of EGFR-TKIs [31-33], and was used as a landmark time-point in BATTLE trial where disease control at 8 weeks was the primary endpoint [34-35].
PATIENTS AND METHODS
Patients
The original cohort included 101 consecutive patients with stage IV NSCLC or stage I-IIIA NSCLC with systemic relapse and sensitizing EGFR mutations who were treated with gefitinib or erlotinib as their initial systemic therapy for advanced NSCLC at the Dana-Farber Cancer Institute between February 2002 and May 2010 [36-37].
Baseline CT and at least one follow-up CT during EGFR-TKI therapy were available in 70 patients. In 29 patients of the remaining 31 patients, baseline and/or follow-up CT scans were not available in our system; these studies were performed at other institutions. The remaining 2 patients had no follow-up CT during TKI therapy; one patient discontinued EGFR-TKI therapy at 2 weeks due to toxicity, and the other patient discontinued TKI at 2 weeks and died 2 weeks later, due to progressive disease.
The baseline chest CT scans of the 70 patients were reviewed by a thoracic radiologist (M.N.) to identify patients with at least one measurable lung lesion (≥ 10 mm) [28]. Among 70 patients, 56 patients had at least one measurable lung lesion. The remaining 14 patients had no measurable lung lesions, while they had non-measurable lesions in the lung (such as small nodules<10 mm or effusion) and/or lesions outside of the lungs such as hepatic or osseous lesions. Therefore, the study population consisted of 56 advanced NSCLC patients with sensitizing EGFR mutations treated with first-line erlotinib or gefitinib.
Thirty patients were treated in prospective trials of gefitinib or erlotinib [4, 31-33, 38], and 26 patients were treated as a part of the standard clinical care. The collection of clinical information on patients with somatic EGFR mutations was approved by the Institutional Review Board.
Mutation analysis
Tumor specimens were obtained from diagnostic or surgical procedures. Samples consisted of frozen tumor specimens or paraffin embedded material. EGFR exons 18 to 21 were amplified by PCR and analyzed bidirectionally by direct sequencing for the presence of somatic mutations [39-41]. Following EGFR mutations were considered sensitizing: deletions, duplications, and deletion-insertions of exon 19, L858R point mutation, L861Q point mutation, and G719 missense point mutations [37, 41].
CT Tumor volume and size measurement
Baseline and follow-up chest CT scans were performed to determine response to EGFR-TKI using the clinical chest CT protocol. The follow-up CT scans were performed after every 8 weeks (n=29) or every 6 weeks (n=1) in patients treated in trials, and per discretion of treating providers in patients treated off protocol (n=26).
A thoracic radiologist measured the volume and size (the longest diameter) of a dominant measurable lung lesion (one lesion per patient) on baseline and follow-up CT scans during EGFR-TKI therapy, using FDA-approved volume analysis software (Vitrea®2, Vital Images, Minnetonka, MN)[28].
Proportional tumor volume and size changes compared to baseline
The proportional changes of tumor volume and size at each follow-up were calculated. At time t(i), the tumor volume and size were defined as v(i) and x(i), respectively. t(0) was when baseline CT was obtained. At a time t(i), the percent volume and size changes (%v(i), %x(i)) comparing to baseline volume (v(0)) and size (x(0)) were calculated:
The proportional volume and size changes at 8 weeks, as well as at the nadir (the smallest tumor volume/size since baseline to TKI termination), were obtained. Since not all patients had follow-up CT exactly at 8 weeks, 6-10 weeks were allowed for the 8-week scan. Forty-nine patients had follow-up CT at 8±2 weeks; the subsequent analysis at 8 weeks was performed in these 49 patients, excluding 7 patients without scans at 8±2 weeks.
Statistical Analysis
The association between the 8-week measure of tumor volume/size and outcomes was assessed using landmark analyses defining 8 weeks as the landmark time. Overall survival (OS) was defined as the time from the date of the 8-week follow-up scan until death from any cause. PFS was defined as the time from the date of the 8-week follow-up scan until the date of progression or death. Progression was based on RECIST for patients in trials, and was defined clinically for non-trial patients based on sufficient tumor growth to discuss alteration of therapy and/or a new site of disease on imaging [21]. Patients not experiencing the event by the time of analyses were censored at the last known date of follow-up. The log-rank test was used to assess differences in the OS and PFS distributions between groups. Cox proportional hazards models were used to estimate hazard ratios and multivariate analyses were performed using a stepwise regression. Differences in demographics and disease characteristics were tested using Fisher's exact test for categorical data and Wilcoxon test for continuous data. All p-values are two-sided at the 0.05 level and no adjustments have been made for multiple comparisons.
RESULTS
Tumor volume and size at baseline and during EGFR-TKI therapy
Table 1 summarizes the demographics and disease characteristics of the 56 patients. The median follow-up time from the initiation of EGFR-TKI was 35.2 months.
Table 1.
Patient demographics and disease characteristics of 56 patients
| Variables | Category | Total (n=56) |
|---|---|---|
| Sex | Female | 48 (86) |
| Male | 8 (14) | |
| Age | Median (range) | 63 (35-84) |
| Race | White | 50 (89) |
| Asian | 4 (7) | |
| Black | 2 (4) | |
| Smoking Status◇ | Never | 26 (46) |
| Former | 28 (50) | |
| Current | 2 (4) | |
| Pathology | Adenocarcinoma | 49 (88) |
| NSCLC NOS | 7 (12) | |
| ECOG PS | 0 | 22 (39) |
| 1 | 31 (55) | |
| 2 | 2 (4) | |
| 3 | 1 (2) | |
| Extrathoracic Metastasis» | Present | 32 (57) |
| Absent | 24 (43) | |
| EGFR-TKI | Erlotinib | 51 (91) |
| Gefitinib | 5 (9) | |
| Sensitizing EGFR mutations | exon 19 del | 28 (50) |
| exon 19 del and L861Q | 1 (2) | |
| L858R | 19 (34) | |
| L861Q | 4 (7) | |
| L861Q and G719 | 1 (2) | |
| G719 | 3 (5) |
The values in parenthesis represent percentages unless otherwise specified.
ECOG = Eastern Cooperative Oncology Group, NSCLC NOS = Non-small-cell lung cancer not otherwise specified
Never: <100 lifetime cigarettes; Former: quit ≥ 1 year prior to start of EGFR-TKI; Current: smoked ≤1 year prior to start of EGFR-TKI [34]
Extrathoracic metastasis at diagnosis of advanced disease
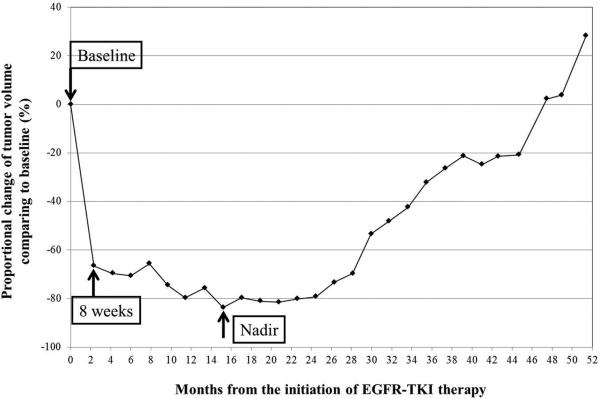
The median baseline tumor volume was 17.8 cm3 (range: 1.3-172.7 cm3), was 7.7 cm3 (range: 0.4-62.3 cm3) at 8 weeks of therapy, and was 4.8 cm3 (range: 0.2-62.3 cm3) at nadir. The median time from the initiation of TKI to nadir volume was 5.5 months. The median proportional volume change at 8 weeks was -59% (range: -90% to +91%). The proportional volume change at nadir had a median of -71% (range -99% to 0%). The tumor volume continued to increase throughout therapy in 2 patients, whose nadir volume was at baseline. A representative tumor volume changes during TKI therapy is shown in Fig. 1.
Fig. 1.
A representative tumor volume changes during EGFR-TKI therapy in a 56-year-old female with lung adenocarcinoma harboring exon 19 deletion treated with erlotinib. A significant initial volume decrease was noted on the 8-week scan (67% decrease comparing to baseline). The tumor volume further decreased gradually, and reached the nadir at 15 months with 83% decrease compared to baseline. Subsequently, tumor volume gradually increased during TKI therapy, which was terminated at 51 months.
The median baseline tumor size was 3.7 cm (range: 1.2-9.1 cm); was 2.8 cm (0.9-9.0 cm) at 8 weeks, and was 2.0 cm (0.7-8.4 cm) at nadir. The median time from the initiation of therapy to nadir size was 5.0 months. The median proportional change in size at 8 weeks was -22% (range: -69% to +50%); the proportional size change at nadir had a median of -40% (range -81% to 0%). The tumor size continued to increase throughout therapy in 5 patients, whose nadir size was at baseline.
Association between tumor volume and size versus survival
At baseline
Neither baseline volume nor size was a predictor of OS or PFS, when fitted as continuous variables in the Cox models (OS: p=0.57, 0.50, respectively; PFS: p=0.84, 0.57, respectively).
At 8 weeks of therapy
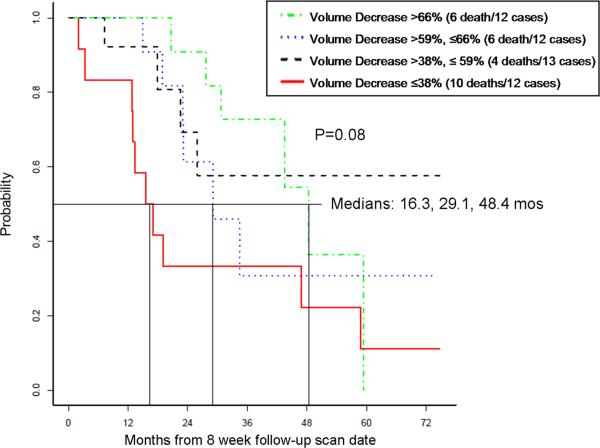
In 49 patients with 8-week scan, the proportional volume decrease at 8 weeks had significant association with OS, when fitted as a continuous variable in a Cox model (p=0.02). The association was further explored by categorizing patients according to the 8-week volume decrease and by plotting Kaplan-Meier curves. When patients were categorized into 4 groups using the quartiles (-66%, -59%, -38%), the group with the least volume decrease had the shortest OS (10 death in 12 patients, the median OS 16.3 months, 95%CI for the median: 13.0 months-NR), while OS was longer in groups with greater volume decrease (Fig. 2,3).
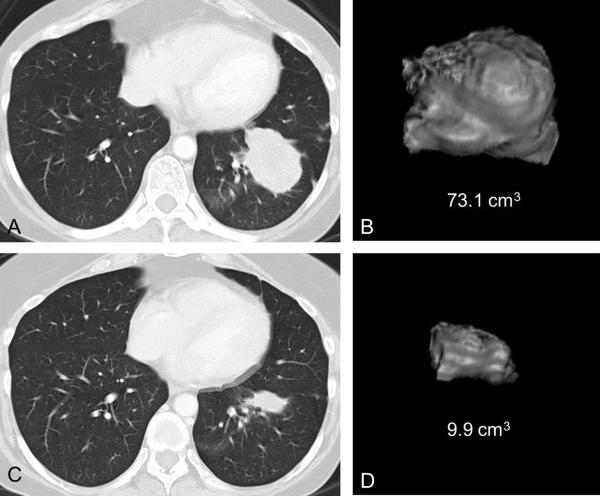
Fig. 2.
An example of marked proportional tumor volume decrease at 8 weeks associated with longer survival in a 54-year-old female with lung adenocarcinoma harboring exon 19 deletion treated with erlotinib.
A, B. Baseline CT scan of the chest of demonstrates a dominant lung mass in the left lower lobe, with a volume of 73.1 cm3.
C, D. Follow-up CT at 8 weeks of therapy demonstrated marked decrease of tumor volume, measuring 9.9 cm3, with the proportional volume change of -86% compared to baseline. The overall survival of the patient since the initiation of erlotinib was 45.4 months.
Fig. 3.
Overall survival in 4 groups of patients categorized using the quartiles (-66%, -59%, -38%) of the proportional volume change at 8 weeks of therapy.
Upon visual inspection of the Kaplan-Meier estimates of the OS distribution, we proposed that dichotomizing the patients into those with >38% volume decrease versus those with ≤38% volume decrease may serve as an appropriate cutpoint. This cutpoint was further studied using a recursive partitioning model. Patients with volume decrease >38% (n=37) had significantly longer OS (median OS: 43.5 months, 95%CI for the median: 29.1 months-NR) compared to those with volume decrease ≤38% (n=12, median OS: 16.3 months) (p=0.01) (Fig. 4,5). The median PFS for patients with >38% volume decrease was 12.6 mos (95% CI 9.4-15.0 mos), compared to 5.5 mos (95% CI 3.3-NA) for those with ≤38% volume decrease (p=0.2) (Fig. 6). Although not statistically significant, the longer PFS in the patients with volume decrease of >38% was consistent with the observation of longer survival with this degree of tumor shinkage. Table 2 summarizes the demographics and the disease characteristics of the 37 patients with >38% volume decrease and versus 12 patients with ≤38% volume decrease at 8 weeks.
Fig. 4.
Overall survival in patients dichotomized using the cut-off value of -38% volume change at 8 weeks of therapy.
Fig. 5.
Waterfall plot demonstrating the proportional volume change at 8 weeks in 49 patients. Overall survival was significantly longer in patients with >38% volume decrease at 8 weeks (n=37, light gray bars) than in patients with ≤38% volume decrease (n=12, dark gray bars) (median OS: 43.5 months versus 16.3 months, respectively.)
Fig. 6.
Progression-free survival in patients dichotomized using the cut-off value of -38% volume change at 8 weeks of therapy.
Table 2.
Patient demographics and disease characteristics of 49 patients with 8-week follow-up scans, dichotomized at the 38% cutpoint for volume decrease
| Variables | Category | 8-week tumor volume decrease ≤38% (n=12) | 8-week tumor volume decrease >38% (n=37) |
|---|---|---|---|
| Sex | Female | 10 (83) | 31 (84) |
| Male | 2 (17) | 6 (16) | |
| Age* | Median (range) | 69 (48-84) | 56 (35-78) |
| Race | White | 12 (100) | 32 (87) |
| Asian | 0 (0) | 3 (8) | |
| Black | 0 (0) | 2 (5) | |
| Smoking Status* | Never | 3 (25) | 21 (57) |
| Former | 8 (67) | 16 (43) | |
| Current | 1 (8) | 0 (0) | |
| Pathology* | Adenocarcinoma | 8 (67) | 35 (95) |
| NSCLC NOS | 4 (33) | 2 (5) | |
| ECOG PS | 0 | 5 (42) | 16 (43) |
| 1 | 6 (50) | 21 (57) | |
| 2 | 1 (8) | 0 (0) | |
| Extrathoracic Metastasis | Present | 6 (50) | 22 (60) |
| Absent | 6 (50) | 15 (40) | |
| EGFR-TKI | Erlotinib | 12 (100) | 33 (89) |
| Gefitinib | 0 (0) | 4 (11) | |
| Sensitizing EGFR mutations* | exon 19 del | 3 (25) | 22 (60) |
| exon 19 del and | 1 (8) | 0 (0) | |
| L861Q | |||
| L858R | 4 (34) | 13 (35) | |
| L861Q | 3 (25) | 0 (0) | |
| G719 | 1 (8) | 2 (5) |
The values in parenthesis represent percentages unless otherwise specified.
ECOG = Eastern Cooperative Oncology Group, NSCLC NOS = Non-small-cell lung cancer not otherwise specified
Significant imbalances were observed in age (p=0.02), smoking status (p=0.05), pathology (p=0.03), and EGFR mutations (p=0.005).
Multivariable analysis was performed for OS, adjusting for 8-week volume decrease using the 38% cutpoint, sex, age, race, smoking status, pathologic type, ECOG performance status, distant metastasis, and EGFR mutations. Stepwise regression was conducted, removing variables if they were not significant at the p value of 0.2. The final model was adjusted for four factors: 8-week volume decrease >38% (HR= 0.23, p=0.002), distant metastasis (HR=2.4, p=0.05), age >50 ( HR=0.15, p<0.001), and EGFR exon19 deletion (HR=0.28 , p=0.01).
Proportional size change at 8 weeks was not associated with OS in a Cox model (p=0.22). When patients were dichotomized at the median size change of -22%, no significant difference was observed in OS (p=0.11) or PFS (p=0.06).
At nadir
Neither the proportional volume change at nadir nor the time to nadir volume was associated with OS (p=0.23, 0.25, respectively), when fitted as time-varying covariates in a Cox model. The tumor size change at nadir was associated with OS (p=0.03), as was the time to nadir size (p=0.04), with patients with longer time to nadir having longer survival.
DISCUSSION
The present study demonstrated proportional tumor volume decrease at 8 weeks of therapy was associated with prolonged survival in advanced NSCLC patients with sensitizing EGFR mutation treated with first-line gefitinib or erlotinib. To our knowledge, this is the first report demonstrating that tumor volume can serve as an early predictor of prolonged survival in EGFR-mutant advanced NSCLC patients treated with first-line EGFR-TKIs.
Increasing attempts have been made to utilize tumor volume in response assessment and provide an imaging-based marker for survival [25-29]. Tumor volume changes after therapy have shown to be associated with survival in different solid tumors, including locally-advanced rectal adenocarcinoma treated with chemoradiotherapy and surgery [42], cervical cancer receiving radiotherapy [43], and malignant pleural mesothelioma treated with chemotherapy, extrapleural pneumonectomy, and radiation [44]. In lung cancer, Zhao et al reported that semiautomated tumor segmentation identified a larger number of patients with absolute tumor volume changes, compared with unidimensional or bidimensional techniques [23]. The reproducibility of tumor volume measurements in lung cancer has been extensively studied, demonstrating that tumor volume is more reproducible than size [24, 27-28]. Volumetric tumor change during neoadjuvant gefitinib better distinguished tumors with sensitizing EGFR mutation compared to unidimensional tumor change [29].
However, only a few reports evaluated the association between tumor volume and survival in NSCLC patients, mostly during chemotherapy plus chest radiotherapy [45-47]. In inoperable stage I-IIIB NSCLC treated with chemotherapy plus chest radiotherapy, larger primary tumor volume and primary plus nodal tumor volume before therapy were associated with shorter survival [45]. In stage III NCSLC treated with concurrent chemotherapy and chest radiation, larger primary tumor volume and nodal volume were associated with shorter survival [46]. In locally advanced NSCLC treated with trimodality therapy, larger primary tumor volume was associated with shorter PFS; pre- and post-treatment PET uptake did not correlate with survival [47].
In the present study, proportional tumor volume change at 8 weeks of EGFR-TKI therapy was significantly associated with prolonged survival. The result is similar to the report of malignant pleural mesothelioma, which demonstrated that percent change of tumor volume after two cycles of chemotherapy was associated with OS (HR: 1.94, p=0.04) [44]. Furthermore, 8-week volume decrease remained significant after adjusting for other factors. Our results indicate that tumor volume change can be an early predictor of survival in EGFR-mutant NSCLC treated with first-line EGFR-TKIs. Further investigation to establish volumetric response criteria is needed to better guide treatment decisions in patients with sensitizing EGFR mutation.
The cut-off value of 8-week volume change that best differentiated the survival duration in our patients was -38%. In our previous study using the same technique, the 95% limits of agreements for volume measurements were -26.0% and 18.6% [28]; -38% is beyond this range, and therefore likely reflects true change rather than measurement variability. The cut-off values of tumor volume change predicting outcome differ significantly among different tumor types, stages, and treatments. In rectal cancer receiving preoperative chemotherapy and radiation, patients with ≥70% tumor volume reduction had longer disease-free survival [42]. In mesothelioma, tumor volume “increase” versus “decrease” after chemotherapy differentiated patients with shorter and longer survival [44]. It appears that the cut-off values are specific to tumor types and therapeutic regimen. The cutoff value of 38% volume decrease, determined using recursive partition analysis in the present study, needs to be validated. We propose tumor volume change of -38% as an initial value for validation in a larger independent cohort of sensitizing EGFR-mutant NSCLC treated with EGFR-TKI, which is an important next step to establish practical volumetric response criteria.
The 12 patients with 8-week volume decrease ≤38% had significantly shorter survival. These 12 patients were significantly older, more frequently smokers, and had NSCLC NOS. Four of the 12 patients with ≤38% volume decrease had a L861Q mutation. It may be worthwhile to further investigate detailed clinical and genomic characteristics of tumors between groups divided by volume change in a larger population, to understand the differences in response to EGFR-TKI among EGFR-mutant NSCLC patients.
The longer survival is likely related to the therapeutic benefit of the EGFR-TKI administered to these patients with sensitizing mutations of EGFR. Median PFS was longer in the >38% volume decrease group than the other group (12.6 vs. 5.5 mos), demonstrating a similar trend as in OS, though statistically non-significant. Since radiographic tumor increase is one of the determinants of progression, tumor volume change and PFS may not be independent from each other. OS may be a more objective measure of outcome for the purpose of the study.
Eight-week size changes were not associated with survival, which is consistent with prior reports [48-49]. We did not further explore the cutpoints for size, because 1) size was not a significant predictor in a Cox model; 2) 95% limits of agreement for size were -23.1% and 24.4% from our prior study [28], and other cutpoints such as the 75th percentile of size change (-12%), or the size change equivalent to -38% volume change (-15%, based on a mathematical conversion proposed in RECIST [15]) were within the range of measurement error and therefore cannot be used reliably. In addition, our objective is not to compare volume and size; each measure has its advantages and disadvantages, and should be used jointly to aid therapeutic decisions in clinical practice.
Baseline tumor volume or size did not correlate with survival. The result is somewhat counterintuitive, since the baseline tumor volume and size are considered as functions of the T status of the TNM staging, which is associated with overall survival in NSCLC patient population in general. In the cohorts of NSCLC patients (stage I-IIIB in one study, and stage III in the other) treated with chemotherapy plus chest radiation, baseline primary tumor volume was associated with survival [45-46]. The result of the present study can be due to a small sample size with advanced disease patients alone, and can also due to a specific cohort of a genomically selected NSCLC patients harboring sensitizing EGFR-mutations, who demonstrated dramatic initial tumor shrinkage with a volumetric response (median change of -59%) to EGFR-TKIs. Association between tumor volume and survival appears to be dependent on cohorts and treatments given.
Neither the volume change at the nadir or the time to volume nadir was associated with survival. The results do not discourage the attempts to utilize tumor volume in response assessment; the nadir can only be determined retrospectively after completing tumor measurements until therapy termination, and therefore cannot serve as a useful marker to guide therapeutic options in patients with ongoing therapy. We observed that nadir size and time to nadir size were associated with survival. However, with a small sample size and a relatively low event rate, these results may be skewed by outliers,.
The study has several limitations, including a retrospective design and a small number of patients treated at a single institution. Although 101 patients met oncologic eligibility criteria, only 49 patients were eligible for 8-week volume analysis, and others were excluded due to non-availability of CT scans (n=31), lack of measurable lung lesions (n=14), and lack of 8-week scan (n=7). The volumetric analysis requires the actual CT data, which is not always available in patients scanned at other institutions. Our cohort of 49 patients is larger than most previous volumetric studies of NSCLC [23-24, 28-29, 47-48], and is the first genomically-defined cohort of EGFR-mutant NSCLC patients whose tumor volume and survival were analyzed. We are planning to further expand the cohort, by identifying more patients as well as by obtaining outside scans, and assess the validity of the 38% cutpoint.
Tumor volume measurement included one dominant lung lesion per patient, and smaller lung lesions or extrapulmonary lesions were not taken into account. We chose this approach because we believe that tumor volume analysis should be additive to RECIST, which is simple and practical, and designed to evaluate systemic tumor burden [19]. The single lesion approach is commonly used in studies of response assessment in NSCLC utilizing advanced imaging techniques [50-53]. Several report demonstrated that reducing the number of target lesions does not affect response assessment by RECIST in NSCLC and other malignancies [54-55]; the single largest lesion measurement yielded the same treatment-response classification as measuring up to five lesions in >90% of metastatic colon cancer patients treated in a phase 3 trial [54]. In our cohort of 49 patients, RECIST1.1 assessment has been performed as a part of our previous study [21], and 48 patients demonstrated PR (n=28) or SD (n=20) at 8 weeks. One patient had PD at 8 weeks due to a new lung nodule, while target lesions had no significant change. The 8-week volume decrease of this patient was ≤38% cutpoint. It should be noted that the results of tumor volume analysis of one representative lung lesion need to be interpreted in conjunction with the RECIST-based assessment to capture the systematic tumoral behavior.
In conclusion, proportional tumor volume decrease at 8 weeks of EGFR-TKI therapy predicted overall survival in advanced NSCLC patients with sensitizing EGFR mutation. Further investigation is warranted to validate the observation and establish volumetric criteria of response assessment to better guide therapeutic decisions in this genomically-defined subset of NSCLC patients. If the observation of the study is reproduced in larger study cohorts, tumor volume analysis at 8 weeks of EGFR-TKI therapy may serve as an early objective marker of survival in advanced NSCLC patients harboring EGFR sensitizing mutations, and help identifying patients who may benefit from additional therapy in therapeutic decisions after 8 weeks of EGFR-TKI therapy.
Acknowledgments
The investigators were supported by 1K23CA157631 (NCI) (M.N.), Grants 1RO1CA114465-01 (B.E.J. and P.A.J.) and 5R21 CA11627-02 (H.H.) from the National Institutes of Health, Grant No. 2P50CA090578-06 (B.E.J. and P.A.J.) from the National Cancer Institute Specialized Program of Research Excellence in Lung Cancer, and a grant from Genentech Inc, as well as by the Doris and William Krupp Research Fund in Thoracic Oncology and American Society of Clinical Oncology Translational Research Professorship.
Footnotes
Disclosures:
David M. Jackman: Consultant, Foundation medicine; Advisory board, Genentech; Honararium, Chugai Pasi A. Jänne: Consultant: Genentech, Roche, OSI pharmaaceuticals; Post-marketing royalties from DFCI owned IP on EGFR mutations Bruce E. Johnson: Consultant: Bristol Myers, Astrazeneca, Genentech, Millenium, Pfizer, Sanofi, Chugai; Postmarketing royalties from DFCI for EFGR mutation testing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer facts and figures, 2012. www.cancer.org/Research/CancerFactsFigures/ACSPC-031941.
- 3.van Zandwijk N, Mathy A, Boerrigter L, et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer. Ann Oncol. 2007;18:99–103. doi: 10.1093/annonc/mdl323. [DOI] [PubMed] [Google Scholar]
- 4.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 5.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;233:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Nishino M, Jagannathan JP, Ramaiya N, et al. Pictorial review of the new Response Evaluation Criteria in Solid Tumors: revised RECIST guideline version 1.1 – What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–9. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 19.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized Tumor Response Assessment in the Era of Molecular Medicine: Cancer-specific and Therapy-specific Response Criteria to Complement Pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino M, Jackman DM, Hatabu H, Johnson BE, Van den Abbeele AD. Imaging of Lung Cancer in the Era of Molecular Medicine. Acad Radiol. 2011;18:424–36. doi: 10.1016/j.acra.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Cardarella S, Dahlberg SE, et al. Radiographic Response Assessment & Therapeutic Treatment Decisions at the Time of RECIST Progression in EGFR-mutant NSCLC. Lung Cancer. doi: 10.1016/j.lungcan.2012.11.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B, Schwartz LH, Moskowitz CS, et al. Pulmonary metastases: effect of CT section thickness on measurement--initial experience. Radiology. 2005;234:934–9. doi: 10.1148/radiol.2343040020. [DOI] [PubMed] [Google Scholar]
- 23.Zhao B, Schwartz LH, Moskowitz CS, Ginsberg MS, Rizvi NA, Kris MG. Lung cancer: computerized quantification of tumor response--initial results. Radiology. 2006;241:892–8. doi: 10.1148/radiol.2413051887. [DOI] [PubMed] [Google Scholar]
- 24.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–72. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hricak H. Oncologic imaging: a guiding hand of personalized cancer care. Radiology. 2011;259:633–40. doi: 10.1148/radiol.11110252. [DOI] [PubMed] [Google Scholar]
- 26.Mozley PD, Schwartz LH, Bendtsen C, Zhao B, Petrick N, Buckler AJ. Change in lung tumor volume as a biomarker of treatment response: a critical review of the evidence. Ann Oncol. 2010;21:1751–5. doi: 10.1093/annonc/mdq051. [DOI] [PubMed] [Google Scholar]
- 27.Mozley PD, Bendtsen C, Zhao B, et al. Measurement of Tumor Volumes Improves RECIST-Based Response Assessments in Advanced Lung Cancer. Transl Oncol. 2012;5:19–25. doi: 10.1593/tlo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino M, Guo M, Jackman DM, DiPiro PJ, Yap JT, Ho TK, Hatabu H, Jänne PA, Van den Abbeele AD, Johnson BE. CT Tumor Volume Measurement in Advanced Non-small-cell Lung Cancer: Performance Characteristics of Emerging Clinical Tool. Academic Radiology. 2011;18:54–62. doi: 10.1016/j.acra.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Oxnard GR, Moskowitz CS, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res. 2010;16:4647–53. doi: 10.1158/1078-0432.CCR-10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 31.Jackman DM, Cioffredi LA, Lindeman N, et al. Phase II trial of erlotinib in chemotherapy-naïve women with advanced pulmonary adenocarcinoma. J Clin Oncol. 2009;27(suppl):15s. abstr 8065. [Google Scholar]
- 32.Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients >or ¼ 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 33.Janne PA, Gurubhagavatula S, Yeap BY, et al. Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer. 2004;44:221–30. doi: 10.1016/j.lungcan.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Gold KA, Kim ES, Lee JJ, Wistuba II, Farhangfar CJ, Hong WK. The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila) 2011;4:962–72. doi: 10.1158/1940-6207.CAPR-11-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lara PN, Jr, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–7. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 36.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, Johnson BE. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, Costa DB, Rabin MS, Jackman DM, Johnson BE. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–14. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janne PA, Wang XF, Socinski MA, et al. Randomized phase III trial of erlotinib (E) alone or in combination with carboplatin/paclitaxel (CP) in never or light former smokers with advanced lung adenocarcinoma: CALGB 30406. J Clin Oncol. 2010;28(suppl) doi: 10.1200/JCO.2011.40.1315. abstr 7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 40.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 41.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–60. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nougaret S, Rouanet P, Molinari N, et al. MR Volumetric Measurement of Low Rectal Cancer Helps Predict Tumor Response and Outcome after Combined Chemotherapy and Radiation Therapy. Radiology. 2012;263:409–18. doi: 10.1148/radiol.12111263. [DOI] [PubMed] [Google Scholar]
- 43.Wang JZ, Mayr NA, Zhang D, et al. Sequential magnetic resonance imaging of cervical cancer: the predictive value of absolute tumor volume and regression ratio measured before, during, and after radiation therapy. Cancer. 2010;116:5093–101. doi: 10.1002/cncr.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Zhao B, Krug LM, Ishill NM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol. 2010;5:879–84. doi: 10.1097/JTO.0b013e3181dd0ef1. [DOI] [PubMed] [Google Scholar]
- 45.Dehing-Oberije C, De Ruysscher D, van der Weide H, et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1039–44. doi: 10.1016/j.ijrobp.2007.07.2323. [DOI] [PubMed] [Google Scholar]
- 46.Alexander BM, Othus M, Caglar HB, Allen AM. Tumor volume is a prognostic factor in non-small-cell lung cancer treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1381–7. doi: 10.1016/j.ijrobp.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 47.Kozak MM, Murphy JD, Schipper ML, et al. Tumor volume as a potential imaging-based risk-stratification factor in trimodality therapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:920–6. doi: 10.1097/jto.0b013e31821517db. [DOI] [PubMed] [Google Scholar]
- 48.Ding L, Bliss RL, Ingebrand M, et al. Computer-aided volumetry analysis in assessing pulmonary chemotherapy response in advanced NSCLC comparing with RECIST criteria. J Clin Oncol. 2011;29(suppl) abstr10593. [Google Scholar]
- 49.Birchard KR, Hoang JK, Herndon JE, Jr, Patz EF., Jr Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer. 2009;115:581–6. doi: 10.1002/cncr.24060. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol. 2009;193:1090–6. doi: 10.2214/AJR.08.1367. [DOI] [PubMed] [Google Scholar]
- 51.Lind JS, Meijerink MR, Dingemans AM, et al. Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol. 2010;20:2890–8. doi: 10.1007/s00330-010-1869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraioli F, Anzidei M, Zaccagna F, et al. Whole-tumor perfusion CT in patients with advanced lung adenocarcinoma treated with conventional and antiangiogenetic chemotherapy: initial experience. Radiology. 2011;259:574–82. doi: 10.1148/radiol.11100600. [DOI] [PubMed] [Google Scholar]
- 53.Yabuuchi H, Hatakenaka M, Takayama K, et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 261:598–604. doi: 10.1148/radiol.11101503. 201. [DOI] [PubMed] [Google Scholar]
- 54.Zacharia TT, Saini S, Halpern EF, Sumner JE. CT of colon cancer metastases to the liver using modified RECIST criteria: determining the ideal number of target lesions to measure. AJR Am J Roentgenol. 2006;186:1067–70. doi: 10.2214/AJR.05.0038. [DOI] [PubMed] [Google Scholar]
- 55.Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 2010;195:W221–8. doi: 10.2214/AJR.09.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]