Abstract
Opportunities for advances in the neurobiology of alcohol dependence have been facilitated by the development of sophisticated neurophysiological and neuroimaging techniques that allow us to have a window on developmental changes in brain structure and function. The search for genes that may increase susceptibility to alcohol dependence has been greatly facilitated by the recognition that intermediate phenotypes, sometimes referred to as endophenotypes. may be closer to the genetic variation than is the more complex alcohol dependence phenotype. This chapter will review the evidence that the brain is highly plastic, exhibiting major postnatal changes, especially during adolescence, in neural circuits that appear to influence addiction susceptibility. This chapter will suggest that heritable aspects of brain structure and function that are seen developmentally may be an important endophenotypic characteristic associated with familial risk for developing alcohol dependence. Finally, a review of studies showing associations between brain structural and functional characteristics and specific genes will be offered.
I. Introduction
Alcohol dependence (AD) is a major health problem in many parts of the world including the United States (Murray and Lopez, 1997). Data from the National Comorbidity Survey, a survey of respondents aged 15–54, find that AD is a common condition in the United States, with 20.1% of men and 8.2% of women affected (Kessler et al, 1997).
The scientific study of AD began to take shape when Jellinek (1960) first described the variants of alcoholism subtypes he had observed. As with any disorder for which we seek to find improvements in prevention, treatment, and intervention, defining the characteristics that typify the “alcoholic” person and delineating the major subtypes is a critical part of identifying the genetic and environmental determinants of the condition. As an example, identification of variants of diabetes (type I and type II) was an important step in better understanding the genetic underpinnings of both, and providing guidelines for treatment that led to identification of genes conferring susceptibility to type I diabetes (see Permutt et al., 2005, for review).
The recognition that genes have pleiotropic effects so that behavioral manifestations of the disorder at one age or stage in a person’s life may be quite different than they are at another stage has also enhanced the scientific study of AD. The relationship between particular child and adolescent disorders and later development of AD and related phenotypes makes it possible to identify risk factors useful in targeted intervention and prevention efforts. As one example, conduct disorder of adolescence has been identified as a precursor of adult substance use disorders (SUDs) in a number of studies (Robins, 1966; Hill and Muka, 1996; Hill et al., 1999a, 2008; Ohannessian et al., 2004; Kuperman et al., 2005).
A. Family Studies and the Search for Genes
One historical antecedent of the scientific study of AD occurred when family studies began to emerge. Among the earliest family studies that showed increased likelihood of developing AD if one’s relatives were alcohol dependent was conducted in St. Louis, Missouri, USA (Winokur and Clayton, 1968). This study along with many other more recent family studies (Merikangas et al., 1998; Hill et al., 2008) established that transmission of susceptibility to developing AD occurs within families. Based on the evidence that AD runs in families, along with more recent sophisticated statistical analyses of family data including segregation analysis (Yuan et al., 1996) and biometrical modeling of covariance among relatives (Kendler et al., 1992), most researchers in the addiction field now view AD as a complex, heritable disorder. Heritability in males has been reported to range between 0.49 (Caldwell and Gottesman, 1991) and 0.64 (Heath et al., 1997). For women, heritability has been reported to be in the same general range (0.56–0.59) (Prescott et al., 1999; Kendler et al., 1992).
B. Clinical Heterogeneity and Alcohol Dependence
An intensive genome-wide search for genes that alter susceptibility for AD and related phenotypes has been ongoing in the past 20 years facilitated by marked improvements in genetic technologies (Reich et al., 1998; Hill et al., 2004). However, determining the genetic underpinnings of this complex disorder is a daunting task because there are multiple clinical subtypes and variable expression across a lifespan. Existence of subtypes occurs because of the presence of comorbid conditions (mood disorders, drug dependence, anxiety disorders) that introduce clinical heterogeneity, which can lead to reduced statistical power to uncover a significant association between a genetic variation and the AD phenotype. Therefore, increased attention has been focused on finding biological variation associated with the AD phenotype that can be used to identify genes conferring increased susceptibility to the disorder. Recent advances in neuroscience have made it possible to study endophenotypes, sometimes referred to as intermediate phenotypes, that are potentially closely linked to AD and presumably closer to the genetic variation that is sought. The initiation of studies focusing on endophenotypes was an important breakthrough because most brain disorders show developmental trajectories. This plasticity of the human brain probably explains why some endophenotypes of childhood are such salient predictors of adult addictive disorders.
C. Genetic Heterogeneity: Implications for Gene Finding
AD is considered to be a complex disorder because its development is thought to be controlled by multiple genes. In contrast, there are many human diseases such as cystic fibrosis that are thought to be the result of mutations in a single gene, though even the expression of this single-gene disorder is influenced by additional modifier genes (Merlo and Boyle, 2003). Complex diseases often display allelic and locus heterogeneity (Botstein and Risch, 2003; Goldstein et al., 2003). In the case of allelic heterogeneity, multiple mutations within the same gene lead to the illness, while in the case of locus heterogeneity one sees multiple genes with mutations that increase risk for the disease. AD is undoubtedly the result of both processes and other more elusive mechanisms as well. These include germ line mutations, which are the result of the parent of origin and influenced by parental age (Crow, 2000). Additionally, epigenetic effects or heritable changes in DNA expression that are not the result of changes in the DNA are of increasing interest (Bird, 2007).
As noted by McClellan et al. (2007), current research in the genetics of schizophrenia has been guided primarily by the “common disease–common alleles” model advanced by Chakravarti (1999). This view as originally stated by Gottesman and Shields (1982) argues that illness results from the cumulative impact of multiple common genetic variants each of small effect interacting with environmental exposure that exceeds some disease threshold. McClellan et al. (2007) argue that a “common disease–rare allele” model may explain many cases of schizophrenia. This would appear to be equally true for AD.
Although AD is a common disorder in the population, there are families with multiple family members with the disorder (multiplex families) where it appears likely that a few large-effect alleles may be segregating. As recently noted by McClellan and King (2010), it is now recognized that large-effect mutations are causal in many common medical conditions. Evidence is offered that rare severe alleles have been implicated in all forms of inherited susceptibility to cancer, are present in inherited hearing loss, extreme levels of lipids, and severe mental illness such as schizophrenia. They note that this is a major theoretical shift in human genetics because the last decade has focused on the common disease/common variant model promulgated by the Risch and Merikangas (1996) publication which presumed that additive or multiplicative effects of combinations of common risk variants of small effect contributed to the emergence of a disease. No one variant was thought to cause the disorder.
McClellan et al. (2007) also persuasively argue that if many cases of schizophrenia arise from individually rare large-effect alleles, then current approaches will fail to identify critical genes. This is the result of the fact that most current psychiatric genetic research is designed to identify common alleles or haplotypes associated with an increased risk of disease and shared by large numbers of unrelated patients (cases) in comparison to unrelated controls (Merikangas and Risch, 2003). This approach may yield limited information based on the results of a large number of genome-wide association (GWAS) studies of nonpsychiatric complex disorders, where the majority of variants identified have no established biological relevance to the disease of interest or its outcome (see McClellan and King, 2010 for review). In contrast, studies of families that harbor informative genomic events may reveal multiple mutations, some of a more modest effect as well as those having more severe consequences for disease likelihood and progression. In short, rare severe mutations may occur in genes that also harbor more common genetic variants with modest effects on disease risk. The foregoing review will focus on data collected for endophenotypes of brain plasticity as they are related to AD susceptibility with special emphasis on studies that have collected such information in families where rare and important mutations may be occurring.
In this chapter, I will (1) review the evidence that the brain is a highly plastic organ that exhibits major postnatal changes in structure and function, especially during adolescence, in specific circuits that appear to influence addiction susceptibility; (2) present findings suggesting that salient endophenotypes for AD have been identified; (3) provide a review of the evidence that brain morphology is heritable and that developmental changes in morphology during adolescence and young adulthood is an important endophenotypic characteristic associated with familial risk for developing AD; (4) summarize the evidence showing that brain functional differences have been identified in those with familial risk for AD; and (5) provide a review of studies showing associations between brain structural and/or functional characteristics and specific genes.
II. Brain Plasticity
A. Developmental Issues
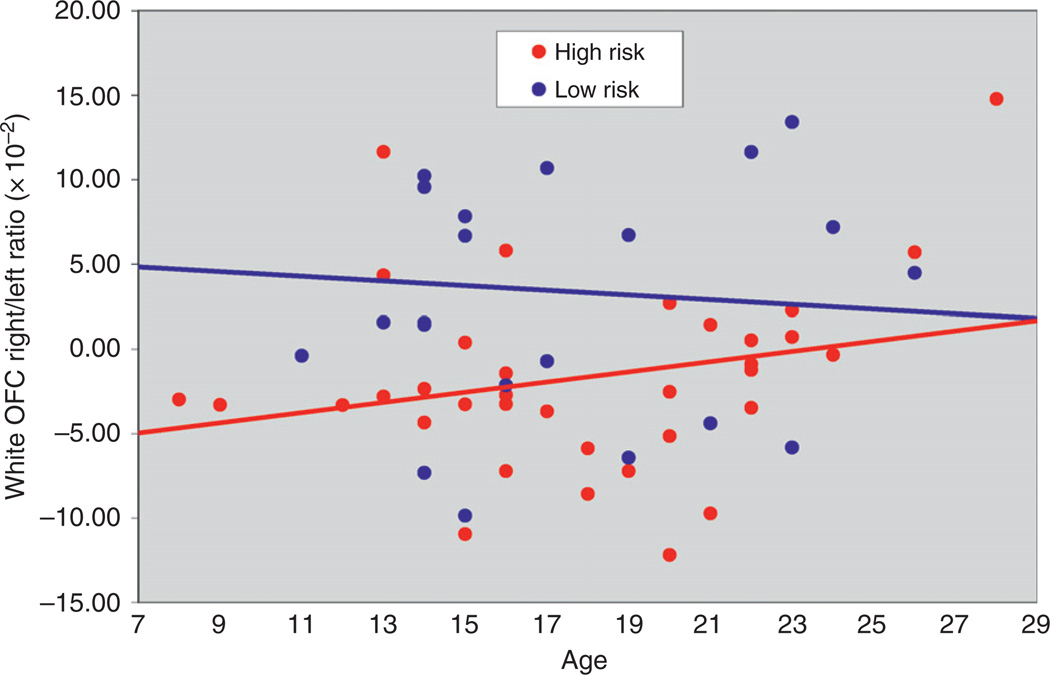
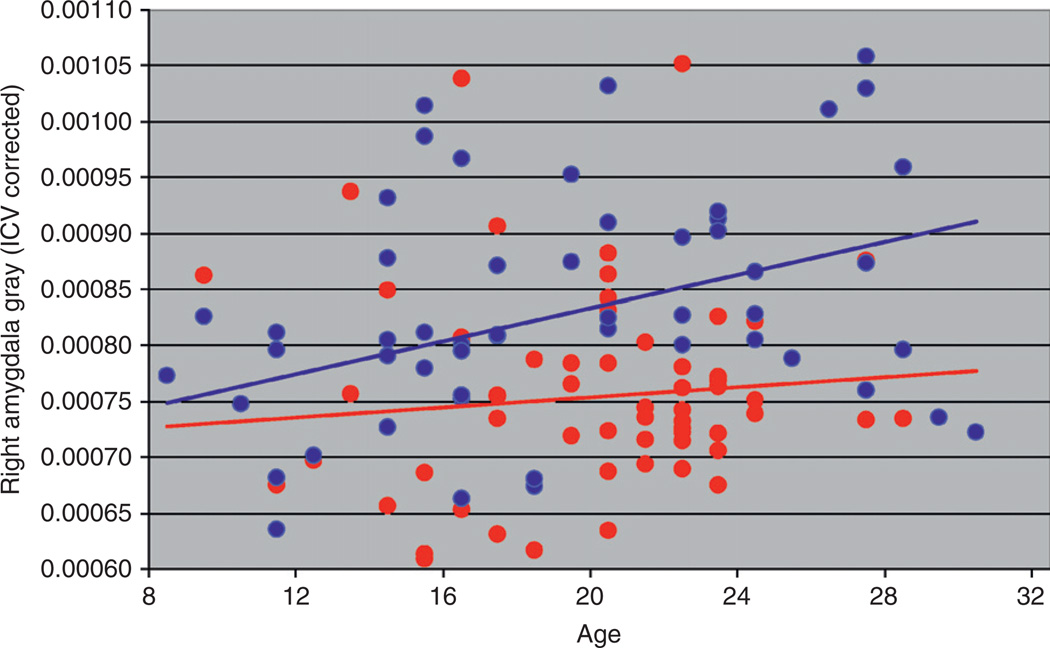
During adolescence and young adulthood, marked changes in brain structure occur, which are accompanied by refinement of brain organization, which in turn leads to changes in cognitive, social, and emotional behavior (Casey et al. 2005; Yurgelun-Todd, 2007). Cortical development including that seen during adolescence and young adulthood generally follows a pattern that supports the changing needs of the organism with primary motor, sensory, and visual areas maturing earlier than those supporting more complex cognitive functions such as the association areas (Gogtay et al., 2004). Accompanying the changes in regional volumes are changes in white and gray matter volumes. White matter (WM) increases well into adulthood while gray matter volume tends to increase in childhood and adolescence followed by a decrease (Giedd et al., 1996a,b;Jernigan et al., 1991; Pfefferbaum et al., 1994; Sowell et al., 2004), with females reaching their peak 1–2 years earlier than males (Lenroot and Geidd, 2006). These morphological changes during adolescence and young adulthood appear to vary by familial risk group status. Results for WM volume of the orbitofrontal cortex (OFC) in the right hemisphere differ by familial risk group (Hill et al., 2009b) (Fig. 1). Similarly, gray matter volume appears to vary by risk group for the amygdala (Fig. 2) (Hill et al., unpublished). These morphological differences, which vary by whether or not one has multiple relatives with AD (multiplex families) or no first-degree relatives with AD (control families), suggests that brain regions showing differing developmental trajectories by familial risk are the result of genetic variation.
Fig. 1.
White matter volume by age is shown for high-risk males. White matter volumes show a statistically significant correlation with Multidimensional Personality Questionnaire (MPQ) Control scale scores (reduced white matter being associated with greater impulsivity).
Fig. 2.
Gray matter volume of the amygdala in the right hemisphere is shown by age and risk status.
Morphological changes in brain development during childhood and adolescence are accompanied by changes in cognitive abilities including development of mature decision-making strategies that have been associated with electrophysiological (Crone and van der molen, 2007) and neurophysiological concomitants (Hill et al., 1999a). Changes in cognitive abilities are often assessed using neuropsychological tests though cognitive tasks are frequently administered while participants are monitored for changes in electroencephalographic (EEG) or event-related potential (ERP) activity. Studies using these methods provide dimensions of inquiry that are not possible using neuropsychological testing alone. These include the ability to determine if the participant is attending to the task and often whether this may have influenced their performance of the task.
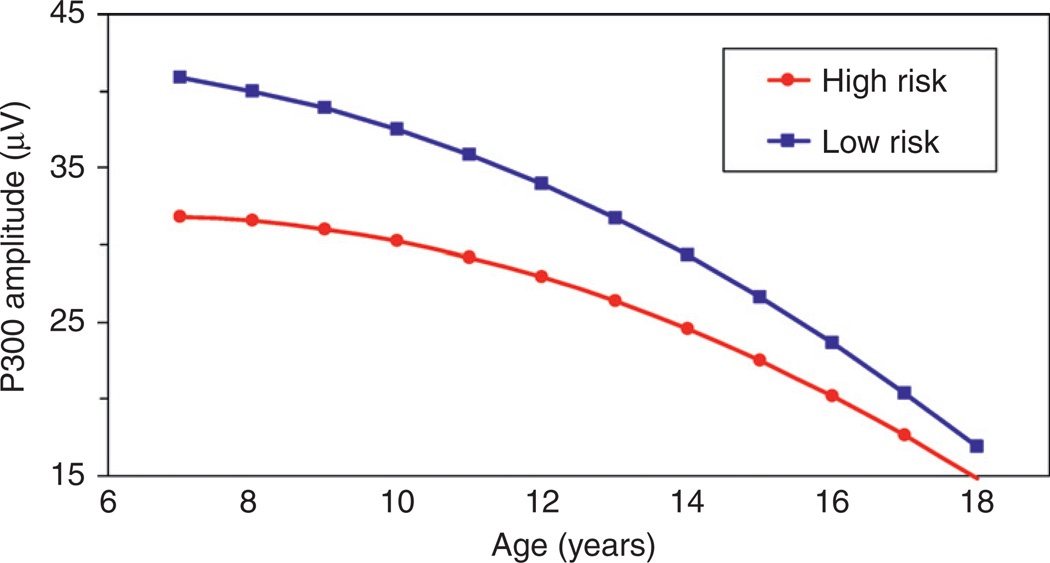
One component of the ERP that has received increased interest in the alcoholism field is the P300 component. P300 is a positive wave that occurs approximately 300 ms after the onset of a stimulus and is maximal over the parietal electrode in paradigms designed to measure the P3b variant of this wave. Although paradigms used to elicit the P300 component vary across studies, many utilize an oddball paradigm in which the participant is asked to attend to a “target” stimulus and ignore a “nontarget.” Both auditory and visual modalities are used. Many early cross-sectional studies had shown that P300 amplitude is reduced in children from alcoholic families (Begleiter et al., 1984; Berman et al., 1993; Hill et al., 1990a; Hill and Steinhauer 1993; Steinhauer and Hill, 1993; Hill et al., 1995). However, the author’s laboratory has the only large-scale follow-up using repeated measures of P300 across childhood, adolescence, and young adulthood. Using data acquired for offspring seen through childhood and adolescence, we performed growth curve modeling for 635 P300 assessments in which two-thirds of the sample were tested five or more times (Fig. 3). This study established that P300 was not a static quantity but was related to development. These developmental changes in P300 are most likely due to brain morphological changes associated with child/adolescent development, though the specific region is currently unknown. These data also showed that high-risk children exhibit altered patterns of age-appropriate P300 amplitude (Hill et al., 1999b).
Fig. 3.
Quadratic growth curves of visual P300 amplitude obtained for high- and low-risk males. High-risk males had lower P300 amplitude than low-risk males and displayed a slower rate of change with age than did the low-risk males.
Clearly, the human brain is a highly plastic organ that undergoes considerable postnatal change extending into young adulthood. With such notable changes being the rule, the search for genes that may control these processes requires considerable effort. Significant progress has been made in identifying genetic variation that is associated with regional differences in brain volume. A future challenge is identifying alterations not only in specific structures but also in the entire circuit that is involved in addiction (see Tessner and Hill, 2010, for review). Coupled with this challenge is a need to identify genetic variation associated with altered brain circuitry. This is a challenge not only for the addiction field but also for psychiatric disorders in general (AMI et al., 2010).
B. Plasticity of Neural Circuits Potentially Involved in Dependence
It has been hypothesized that drug addiction involves dysfunction of brain circuitry that leads to alterations in both impulsive and compulsive behavior (Koob and Kreek, 2007). It has further been hypothesized that the process of addiction initially involves behavioral characteristics that are more impulsive and in time the more compulsive aspects of addiction appear to take over. In this view, a transition from more positively reinforced behavior becomes behavior that is driven by negative reinforcement or avoidance of an aversive state such as drug withdrawal.
In the Koob and Kreek model, two distinct circuits are proposed as the underlying substrate for negative and positive reinforcement. One circuit, characterized as the “reward neurocircuit,” includes connections between the extended amygdala and lateral hypothalamus with input to the ventral medial ventral pallidum and output to the medial dorsal thalamus with feedback to the dorsal prelimbic cortex and basolateral amygdala. The other circuit, termed the “OCD neurocircuit,” by Koob and Kreek (2007) includes the dorsal prefrontal cortex (DPFC), cingulate and OFC, which provide feedback to the striatum, and, in turn, to the pallidum and thalamus with returning loop to the OFC, and dorsolateral prefrontal cortex (DLPFC) and cingulate. A fronto-striato-cerebellar network has also been proposed to account for developmental differences in response inhibition (Rubia et al., 2007) that overlaps the OCD circuit described by Koob and Kreek.
III. The Case for Intermediate Phenolypes or Endophenolypes
Because psychiatric disorders including AD and its related phenotypes such as substance use disorders (SUDs) may represent more distal and variable effects of genes, it has been suggested that molecular genetic studies not be performed on psychiatric diagnoses alone but rather include quantitative biological markers that reflect more proximal effects of genes that may be involved in such complex disorders (Tsuang and Faraone, 2000). If one is interested in relating genetic variation directly to the AD phenotype, candidate endophenotypes can also be used as covariates to reduce heterogeneity and better define the trait (Gottesman and Gould, 2003). This approach was utilized in our laboratory to perform a genome-wide linkage analysis in which P300 and personality variants were used as covariates in models in which genetic polymorphisms were related to the AD phenotype (Hill et al., 2004). A number of endophenotypes have now been recognized by the alcoholism field as valid intermediate phenotypes. Some of these show developmental trajectories that make them especially interesting in relation to brain plasticity.
A. Age of Onset to Begin Regular Drinking as an Intermediate Phenotype
The age of onset to begin regular drinking during adolescence is an important predictor of age of first alcohol problem and subsequent AD (Hawkins et al., 1997; Grant and Dawson, 1997), and greater severity and persistence of problems with illicit drugs (Kandel et al., 1992). Using a large-scale population sample, Grant and Dawson (1997) showed that age of onset of regular drinking predicted the likelihood of adult AD. For those individuals younger than 14 years the rate was 40%; for those aged 20 and older, it was only 10%. Using data from offspring studied longitudinally from a pedigree series identified through familial loading for male alcoholism, we have modeled variables across multiple domains that could be potentially influential in this process. We find that temperament (greater extraversion), familial density of alcoholism (number of alcohol-dependent members in the family), and markers of altered neurodevelopment (P300 amplitude trajectories and acquisition of age-appropriate postural sway) are important mediators of the relationship between age of onset and familial loading for AD (Hill and Yuan, 1999; Hill et al., 2000). These studies illustrate how a clinical phenotype that is related to familial risk for AD such as the age of onset to begin regular drinking can be related to biological endophenotypes and, in turn, to genetic variation.
B. Disinhibition as an Intermediate Phenotype for AD and Other SUDs
Although age at first drink (AFD) has been repeatedly associated with significant elevations in AD, the mechanism underlying this relationship continues to remain unclear. Importantly, McGue and colleagues (2001a,b) found that disinhibited behavioral problems at age 11, including oppositionality, hyperactivity/impulsivity, and inattention, predict earlier onset of drinking and numerous other indicators of externalizing problems (early onset for cigarette use, sexual activity, and drug use). Kuperman et al. (2005) also report greater externalizing problems (conduct symptoms) indicative of a behavioral orientation for disinhibition related to AFD. Although the connection between disinhibition and SUD is now well established, the neurobiological underpinnings of this trait are just beginning to emerge.
C. P300 Amplitude and Disinhibition
A consensus seems to have emerged that the amplitude of the P300 component of ERP is a measure of the disinhibited temperament that is related to the risk for SUD (Iacono and McGue, 2006; Porjesz and Rangaswamy, 2007). The P300 component of ERP reflects active stimulus processing that is affected by attention and memory and is genetically mediated (Van Beijersterveldt and Boosma, 1994). A lower amplitude of P300 appears to be associated with generalized disinhibition, which is associated with the early onset of a number of deviant behaviors (McGue et al., 2001a,b).
D. Developmental Trajectories of P300 Amplitude as an Intermediate Phenotype
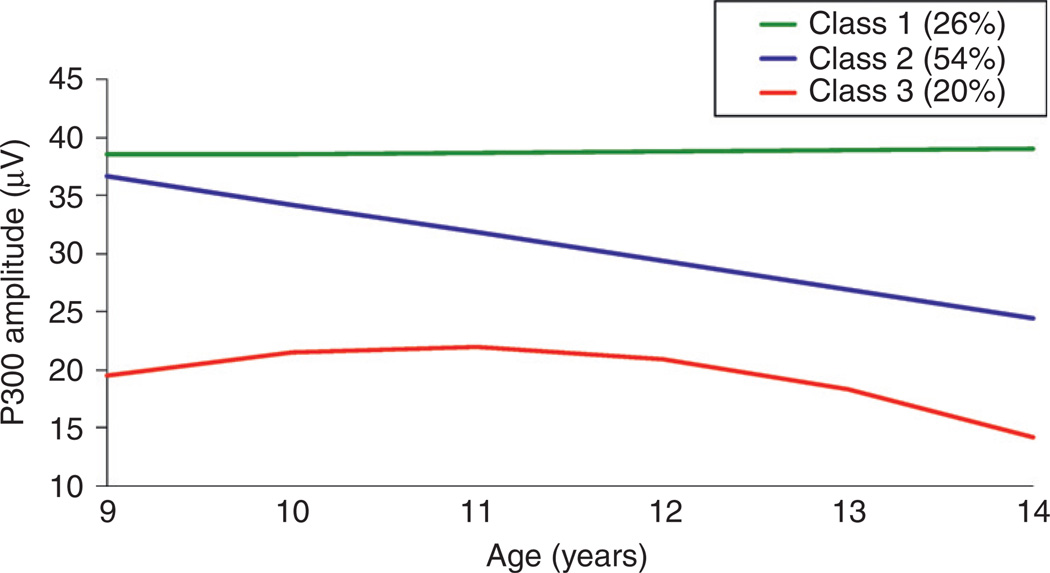
Because developmental trajectories of the P300 component of ERP show marked change during childhood and adolescence (Hill et al., 1999b; Hill and Shen, 2002), and differ by familial risk for AD, this suggests that these trajectories are indicators of brain plasticity, which may explain why some individuals have a greater risk for developing AD and related phenotypes. Trajectory class membership is of interest in attempting to find risk and protective factors among those with familial risk to determine if P300 trajectories can predict individual cases with psychopathology including SUDs. When growth curve analysis is applied to P300 data obtained at regular intervals for youngsters followed longitudinally, one sees that there is a significant amount of variation among individuals. Nevertheless, application of a statistical technique known as mixture model analysis reveals that three distinct patterns occur (Hill and Shen, 2002) (Fig. 4). Each individual’s trajectory class placement is based on a probability estimate so that any given individual may have a lower or higher probability of being in one class than another. However, most individuals’ data can be placed in a particular class with reasonable certainty. These three trajectory patterns of P300 are linked to risk for childhood psychiatric disorders (Hill and Shen, 2002) (Fig. 5). Specifically, those with a pattern characterized by low P300 amplitude across childhood and adolescence have a greater risk for externalizing disorders.
Fig. 4.
Estimated growth curves of visual P300 are illustrated for the three-class solution that provided the best fit to the data obtained for children and adolescents who had each provided five annual assessments. Class 2 pattern is the most commonly occurring pattern, with Classes 1 and 3 less common.
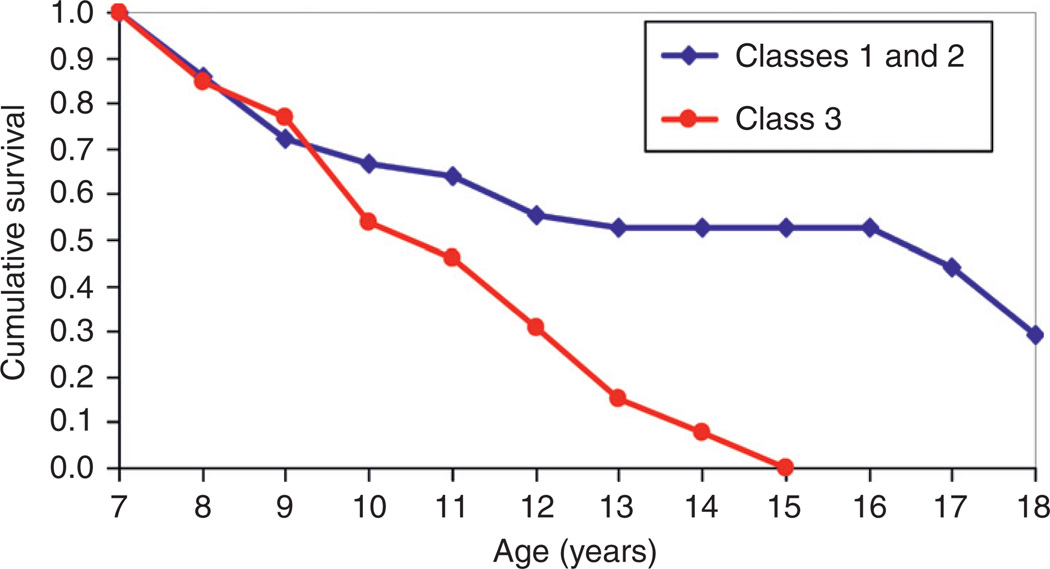
Fig. 5.
Survival curves for age of onset at first childhood psychiatric diagnosis among high-risk offspring can be seen. Membership in the Class 3 developmental trajectory group increases the likelihood that a child with a familial/genetic risk for AD will exhibit a childhood diagnosis by a given age.
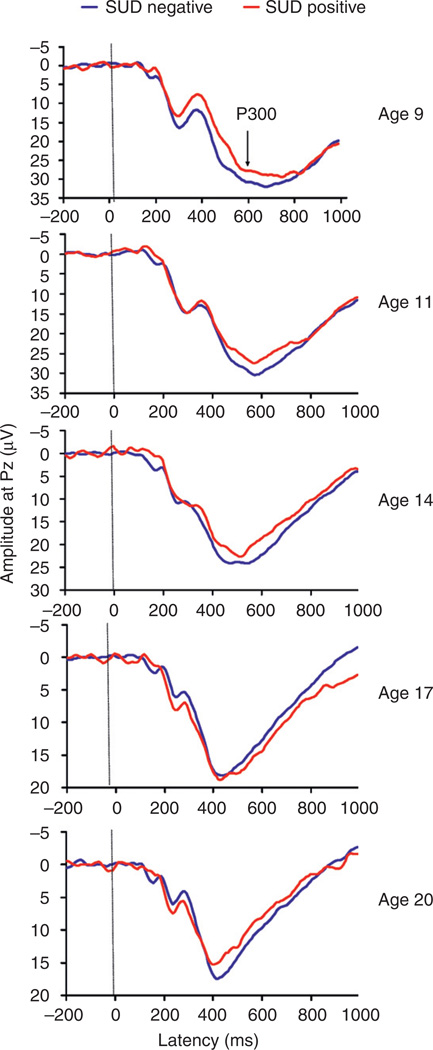
With the established link between risk for externalizing disorders and SUD outcome already well known, we followed youngsters to young adulthood to determine if P300 amplitude could predict SUD outcome at annual intervals for an average of 11 years, finding significant associations with outcome (Hill et al., 2009a). A similar finding has been reported with 7-year follow-up (Carlson et al., 2004). ERP tracings by age group for participants seen in our longitudinal follow-up may be seen in Fig. 6. What the figure shows is that those who later developed an SUD by young adulthood could be differentiated by P300 amplitude at age 9. When the child’s P300 amplitude was combined with assessment of body sway as measured by a computerized movement platform, we found an 8-fold increase in risk for developing SUD by young adulthood. Those in the lowest tercile for P300 amplitude and the highest tercile for body sway (children who were less capable of standing steady on the platform) had the highest risk for SUD outcome.
Fig. 6.
Grand averages for visual P300 amplitude by SUD outcome groups are illustrated by age at P300 recording. Only the amplitude difference at age 9 differentiates those who will later develop SUD.
In summary, intermediate phenotypes have been identified that aid in recognizing those who may be at the highest risk for developing AD by adulthood. These include having an early onset to begin drinking and indicators of disinhibition across several behaviors including initiation of smoking, sexual activity, and drug use. These disinhibited behaviors appear to be related to the amplitude of the P300 component of ERP. Because P300 amplitude changes during child and adolescent maturation, trajectories of amplitude change have been identified that appear to confer greater or lesser risk for developing AD and related phenotypes.
IV. Brain Morphology as an Endophenolype for Alcohol Dependence Risk
A. The Heritability of Brain Volumes
There is substantial evidence that the volume of specific brain structures is heritable (Pfefferbaum et al., 2004; Schmitt et al., 2007). Based on twin and family data, the heritability of large brain regions appears to be consistently high and suggests that genetic factors account for at least half of the phenotypic variance. This suggests that offspring from families where AD has a strong recurrence risk over multiple generations would be likely to have altered brain morphology that might predispose them to greater risk for developing SUD. Although numerous studies point to a relationship between atypical behavior and neuroanatomic phenotypes, few have attempted to understand how genetic and environmental effects on typical cognitive and behavioral measures are mediated through brain morphology.
B. Neurological Underpinnings of the Familial Diathesis for AD
The study of brain circuitry that may be specifically involved in addiction is still relatively uncommon (Volkow et al., 2004) and especially so in samples of adolescent/young adults at risk by familial/genetic background or personal alcohol/drug exposure. Also, it has been suggested that dysfunction of a neurologically based system designed for decision-making processes is the underlying factor in the profound drug/alcohol craving that leads to continued use in spite of adverse consequences (Goldstein and Volkow, 2002). Because brain morphology appears to be heritable, it would appear important to control for familial background in studies that address effects of alcohol and drug exposure and their effects on brain morphology.
C. The Amygdala and Hippocampus
In mammals, the amygdala has connections to the hypothalamus and brainstem that modulate visceral responses to emotional stimuli and connections with a fore-brain circuit that appears to be directly involved in emotional processing and mood (Price, 2003). A ventral system that includes the ventral striatum, ventral anterior cingulate gyrus, and the prefrontal (PFC), particularly the orbital and medial PFC, has received extensive attention for its role in the etiology and consequences of major depressive disorder (Drevets, 2000). The possible role of this circuit in addiction is beginning to be recognized (Hill et al, 2001; Glahn et al., 2007).
The hippocampus is of interest because of its well-known role in working memory. Based on data from animal studies, the hippocampus appears to be especially vulnerable to effects of alcohol (Lundqvist et al., 1995). Adults with AD have been reported to show reduction in hippocampal volume (Agartz et al., 1999). DeBellis and colleagues (2000) were among the first to perform MRI scans of adolescent alcohol-dependent participants, finding a reduced volume of the hippocampus in these individuals. However, the DeBellis study could not disentangle the possible effects of genetic predisposition to altered brain morphology and subsequent exposure to alcohol. Utilizing the offspring from our multiplex set of pedigrees, we investigated whether hippocampal volume might differ in those not exposed to alcohol and explored possible differences in the amygdala. We found a reduction in right amygdala volume in high-risk (HR) subjects relative to controls with no differences seen in the hippocampus (Hill et al., 2001). Recent results (Hill et al., unpublished) from a larger sample (N= 115) support the right amygdala finding in showing reduced volume among those with multiplex for AD familial background.
A more recent study of hippocampal volume performed in adolescents (ages 15–18) who were either users of alcohol, alcohol and marijuana, or controls without use of either reported that alcohol users had reduced volume of the left hippocampus compared to the other two groups (Medina et al., 2007). Also, the authors concluded that heavy alcohol use contributes to aberrations in hippocampal asymmetry, with left hippocampal volume being the most affected. However, this study did not address familial risk for AD, so it is unclear if the effects might have been present before heavy drinking was initiated.
D. Volume of the Orbitofrontal Cortex as an Intermediate Phenotype
The OFC has been a candidate region for addiction studies because it is thought to be involved in inhibitory decision-making processes (Bechara and Damasio, 2002). Methamphetamine abusers show reduced functional magnetic resonance imaging (fMRI) blood oxygen level–dependent (BOLD) response in the right OFC region (Paulus et al., 2002, 2003). Without task presentation, substance users in the period beyond acute withdrawal exhibit hypofunctionality of the OFC (Dom et al., 2005). Our work with offspring from multiplex families shows reduced volume in the right hemisphere (Hill et al., 2009b).
E. The Possible Importance of Laterality of the Orbitofrontal Cortex
Because of the functional asymmetry of the cerebral cortex, Bechara and colleagues (2002) set out to determine if the decision-making deficit associated with damage to the ventromedial (VM) PFC is caused mostly by unilateral right or left lesions. Utilizing rare patients with either right or left VM lesions, the left VM patients performed well on the Iowa Gambling Task (IGT) and were not impaired in real-life decision making as all were gainfully employed. In contrast, right VM patients were severely impaired in real-life decision making and performed as poorly on the IGT as bilaterally damaged patients. These results suggest the importance of considering laterality in hypotheses concerning a possible role for the OFC in addiction. Our reduced right/left OFC ratios in HR males may provide a neurological basis for poor impulse control and poorer decision-making capacity (Hill et al., 2009b).
F. Cerebellum
Although the cerebellum has not been formally included in models of reinforcement such as that suggested by Koob and Kreek (2007), there is reason to believe that cerebellar involvement in compulsive behaviors such as those seen in OCD is quite likely. Neuroimaging of a consecutive series of adult OCD patients has revealed relative increases in gray matter volume in the anterior cerebellum (Pujol et al., 2004). Functional imaging studies have been consistent in showing increased resting cerebral blood flow in the cerebellum of OCD patients (Busatto et al., 2000; Nakao et al., 2005). We have reported increased gray matter for cerebellum in offspring from multiplex AD families (Hill et al., 2007b). Further comparison to the Pujol et al. (2004) study in which increased gray matter volume in anterior cerebellum were found shows that the OCD patients also exhibited smaller volumes of the amygdala in the right hemisphere. With similar findings in high-risk offspring for AD (Hill et al., 2001, 2007b), it is possible that a neural diathesis for compulsivity may be present before individuals develop an addiction. If so, it may suggest that individuals at highest risk for developing an addiction may be more prone to compulsive behaviors that set the stage for disinhibited behavior, which fuels the drive to ignore negative reinforcers and continue the addicted behavior.
G. White Matter Volume, Fractional Anistropy, and Limbic Connectivity
Robust increases in WM are seen in adolescence through early young adulthood (Pfefferbaum et al., 1994). This is also a period when age-related improvements in executive functioning are thought to occur. This is probably due to functional reorganization of the frontal cortex during this period along with improved functional WM connectivity within and between brain regions (Pfefferbaum et al., 1994). Increased axonal diameter, thicker myelin sheaths, or better organization of tracts can lead to better signal conduction. Improved connectivity may alter behavioral functioning as well, decreasing impulsivity with age. Changes in behavior have been documented in association with WM micro structure. WM micro structure of the corpus callosum has been shown to be related to impulsivity in adolescence (Silveri et al., 2006). Similarly, we have reported that WM volume of the OFC is related to impulsivity as measured by Multidimensional Personality Questionnaire (MPQ) scale scores (Hill et al., 2009b).
Although WM volume has been investigated across adolescence and young adulthood (Giedd et al., 1999; Paus et al., 1999; Jernigan et al., 1991; Pfefferbaum et al., 1994; DeBellis et al., 2001; Sowell et al., 2002, 2003; Lenroot and Geidd, 2006; Gogtay et al., 2004; Nagel et al., 2006), extension of these developmental studies of WM volume to those varying by familial risk group status has not been undertaken very often. As previously shown in Fig. 1, analysis of WM volume of the OFC by hemisphere does show differing patterns by age and family risk for AD (Hill et al., 2009b).
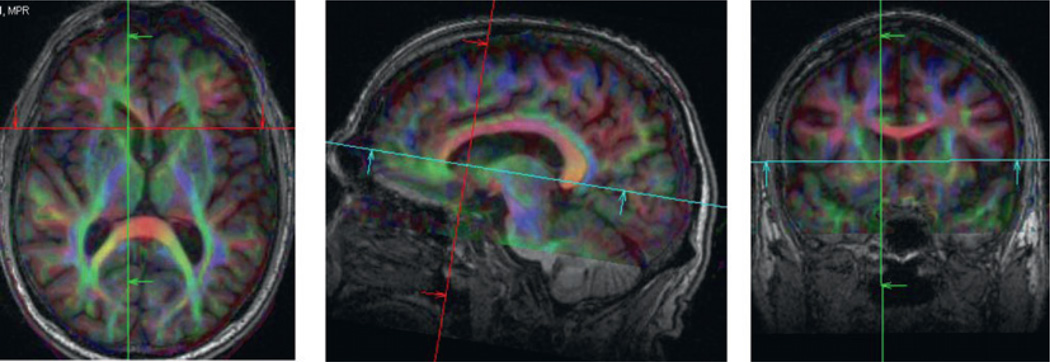
Magnetic resonance diffusion tensor imaging (DTI) is a method for quantifying brain WM microstructure using the sensitivity of DTI to the linear structure of WM (Sullivan and Pfefferbaum, 2006). Diffusion tensor image of a normal brain can be seen in Fig. 7. The scan shown illustrates the good resolution that is possible for collecting fractional anisotropy (FA) maps.
Fig. 7.
Diffusion tensor image of a normal adult brain depicting the white matter tracts. Thirty diffusion directions were used to obtain the data for the measurement of the components of the diffusion tensor. DTI can be used to assess differing patterns of connectivity in relation to familial/genetic predisposition to AD and related phenotypes.
The diffusion of water molecules in brain tissue is anisotropic because motion is restricted by fibers. By quantifying the magnitude and orientation of diffusion, DTI can provide information about a structure. Anistropy measures can estimate the extent to which water is diffusing unidirectionally, as it diffuses more along the length of axonal fibers than perpendicular to them. FA is an intra-voxel measure that yields values between 0 (equal diffusion in all directions) and 1 (all in one direction, e.g., along an axon). Using DTI techniques, greater WM anistropy is seen with increasing age (Klingberg et al., 1999; Schmithorst et al., 2002; Barnea-Goraly et al., 2005). Klingberg and colleagues reported greater WM anisotropy in adults than in children. Schmithorst et al. (2002) reported decreased trace (a measure of overall diffusivity) throughout WM and greater FA with increasing age (ages 5–18) in 33 subjects. Barnea-Goraly et al. (2005) found a linear increase in FA on a voxel-by-voxel basis in 6–19-year-old subjects (N= 30) in several regions including the prefrontal region, though many did not show concurrent increases in WM density. One significant cluster extended into the orbitofrontal gyrus. This suggests that the significant changes seen may indicate increased axonal diameter or axonal density, or increased myelination, all of which may indicate increased connectivity with other brain regions with development.
Although a number of developmental differences have been shown for WM volume and FA, few have considered familial risk group differences in their analyses. Studies of this type are essential to better understanding of altered functional connectivity between structures within specific circuits thought to influence addiction liability.
V. Brain Functional Differences—Familial Risk and Developmental Stage
Brain function has been addressed using neuropsychological tests, fMRI BOLD response, and electrophysiological response in those with exposure to a variety of substances. In understanding the underlying predisposition to AD susceptibility, the following review will focus on studies that have specifically contrasted those with and without a family history. Also, brain regions that have been implicated in addiction will be highlighted. Studies involving neuroimaging or neurophysiology will be the central focus of this section. Neuropsychological studies will be included in this review where specifically related to familial risk for AD.
A. fMRI Studies Related to Alcohol Dependence Susceptibility
1. Amygdala
Among the structures postulated to be part of the Reward and OCD circuits (Koob and Kreek, 2007), the amygdala, as part of the Reward circuitry, stands out as a structure with abundant fMRI paradigms that have variously been used to promote a BOLD response of the amygdala. These include presentation of stimuli designed to elicit an emotional response such as presentation of happy, sad, or angry faces (Hariri et al., 2002) or stimuli as simple as presentation of single-letter stimulus with informative positive feedback (presentation of the word CORRECT) (Bischoff-Grethe, 2009). However, only one study has specifically addressed amygdala activation specifically in relation to familial risk for addiction (Glahn et al., 2007). In that study, subjects viewed trios of faces (Ekman and Friesen, 1976) and geometric shapes as control stimuli. The family history negative (FHN) group showed greater percent signal change in response to faces than to geometric shapes, while the family history positive (FHP) individuals did not. Conclusions that can be drawn from that study must be tempered by the small number of subjects (N = 8 per group). Nevertheless, the findings suggest that amygdala hyporesponsiveness seen in those with a family history of addiction (AD) may have implications for signaling from the amygdala to the hypothalamus and PFC, potentially preventing the formation of aversive associations to the negative consequences of alcohol use.
2. Orbitofrontal Cortex
The OFC proposed by Koob and Kreek (2007) as an integral part of the OCD neurocircuit that maintains the negatively reinforced aspects of long-term effects of addiction has been studied using a variety of functional imaging techniques. These studies have addressed functional effects in various addicted subject groups (e.g., alcohol, cocaine, methamphetamine). Of the 18 studies using this methodology, only 3 were found to not show involvement of this region (see Dom, 2005, for review).
The OFC has been a candidate region for addiction studies because it is thought to be involved in inhibitory decision-making processes (Bechara and Damasio, 2002). However, only a few imaging studies have investigated BOLD activation during a decision-making task. These include studies of methamphetamine abusers showing reduced fMRI BOLD response in the right OFC region (Paulus et al., 2002, 2003). The Paulus studies used a two-choice prediction task in which participants did not know at the onset of the experiment which action would lead to the best outcome, and a neutral control task (two-choice response task). In comparison to controls, the methamphetamine abusers showed less task-related activation in the OFC (Brodmann areas (BA) 10 and 11), the DLPFC (BA 9), and the anterior cingulate (BA 32) during the prediction task relative to the response task. These results suggest that drug-dependent individuals may have OFC dysfunction, which is the most prominent in tasks where choices are associated with uncertain outcomes.
Without task presentation, substance users in the period beyond acute withdrawal exhibit hypofunctionality of the OFC (see Dom et al., 2005, for review). A few structural studies provide evidence of changes in OFC in cocaine-dependent subjects (Franklin et al., 2002; Lim et al., 2002; Matochik et al., 2003). Our work with offspring from multiplex alcohol-dependent families shows reduced volume of the OFC in the right hemisphere in offspring that is independent of alcohol or drug exposure.
3. Cerebellum
Studies of cerebellar activation in groups with drug reinforcement histories are relatively uncommon. Chang et al. (2006) utilized a set of nonverbal visual attention tasks with variable levels of difficulty to show that relative to active marijuana users, abstinent marijuana users had normalized activation in the frontal and medial portions of the cerebellum. Schneider et al. (2001) investigated cue-induced craving in alcoholic patients before undergoing treatment reported finding activation in the cerebellum and in the right amygdala/hippocampus. Interestingly, administration of alcohol suppresses activity in frontal and parietal regions along with cerebellar nuclei, all of which are believed to be involved in internal cognitive models of motor representation and action (Van Horn et al., 2006). Using a Go/No-Go response inhibition task, Hester and Garavan (2004) have demonstrated that cocaine-addicted individuals find it difficult to inhibit their behavior when the working memory load becomes greater and when cue-induced craving for the drug increases. Cerebellar activation identified during response inhibition showed increases in the cocaine users while controls showed decreases as the working memory load increased. Cerebellar vermis BOLD activation has been reported to be greater in crack cocaine users than comparison subjects (Anderson et al., 2006). This is consistent with earlier studies showing cerebellar activation in users exposed to morphine cues (London et al., 1990).
B. fMRI Studies Focusing on Traits Related to Alcohol Dependence
1. Theory of Mind Task
The clinical characteristics of alcohol-dependent persons suggest that many may have difficulties in emotional learning. It has been known for some time now that alcoholics show poorer social skills than nonalcoholics (Van Hasselt et al., 1978). Consistent with this observation, alcohol-dependent individuals show impaired performance on emotional facial expression tasks which may be seen as long as 3 months after achieving abstinence (Foisy et al., 2007).
Based on these observations, we hypothesized (Hill et al., 2007a) that judgment of facial expression, being a neurobiologically based trait, might be altered in offspring from multiplex AD families. We expected that deficits observed in alcohol-dependent individuals might have preceded the onset of AD. Our hypothesis was based in part on the observation that adult alcoholics and their high-risk nonalcoholic adult relatives report higher levels of Alienation than do controls (Hill et al., 1990b; McGue et al., 1997). Persons scoring higher on the Alienation scale from the Multidimensional Personality Questionnaire (Tellegen, 1985; Tellegen et al., 1988) report believing that they are mistreated, that others wish them harm, and often feel betrayed and used by “friends.” Because there had been no fMRI studies of offspring from alcohol-dependent families to test for emotion recognition, we viewed this as an important goal. We chose the Reading the Mind in the Eyes Task modeled after the theory of mind (ToM) test to measure fMRI BOLD response to stimuli varying in emotional content. It was expected that differences in BOLD activation in regions previously identified by Baron-Cohen et al. (1999a) would show enhanced activation in response to processing of the Eyes Task. Regions that are typically activated by the Eyes Task include primarily the medial prefrontal cortex (mPFC), the temporal lobe, particularly the middle and superior regions, the left inferior frontal cortex, and amygdala (Baron-Cohen et al., 1999).
Participants included eight individuals with multiplex family history of AD (four female and four male) and eight normal controls without a personal or family history (at least first-degree relatives) of AD or other psychiatric illness (four female and four male). Following the method described by Baron-Cohen et al. (1999), two tasks were presented that required the participants to extract visual information considered to be socially relevant. In one task the participant simply judged the gender of faces presented in the scanner. In the other, the subject was asked to judge the inferred mental state of persons shown in photographs (24 faces of men and women). The experiment alternated between three conditions. In Task A (Gender) the subject indicated whether the photograph viewed was male or female, and in Task B (Emotion) the subject indicated the inferred mental state of the person in the photograph, followed by a rest condition. For Task A, the subject was instructed to decide for each stimulus which of two simultaneously presented words, “male” or “female,” best described the eyes presented. Response for the emotion condition (Task B) was a two-alternative forced choice (modal response vs. a randomly picked alternative) based on the original Baron-Cohen et al. (1999b) norms. For this task, the subject was required to make a forced-choice decision between two simultaneously presented words (Fig. 8).
Fig. 8.
An example of the eyes stimuli used in Task B. Participants were presented with a choice of mental state words that best described the mental state of the person whose eyes are depicted. The correct response is “worried” in the upper figure and “fantasizing” in the lower figure. Brain regions typically activated in the ToM task include middle temporal gyrus, superior frontal gyrus, and inferior frontal gyrus (see Baron-Cohen et al., 1999a).
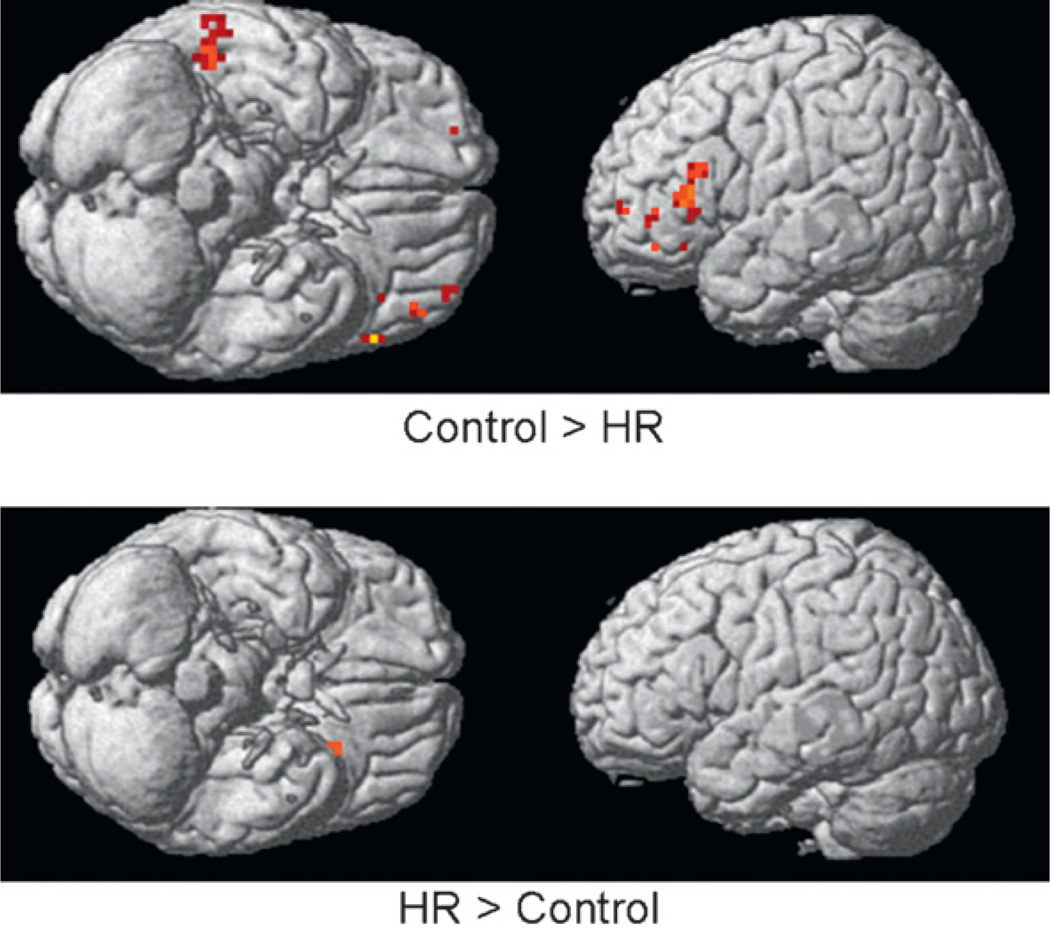
BOLD fMRI analysis of the high risk (n = 8) versus low-risk comparison subjects (n = 8) was conducted at a preset threshold of uncorrected p =.005 with no extent threshold. An activation difference between the BOLD response to Task B (emotion task) and the rest epoch defined the individual subject contrast. These contrast images were then used to perform a between-group analysis. Using SPM5 running on MATLAB version 7.0.4, small volume correction analysis was applied to the regions showing overall uncorrected values of 0.001 or less and for which an a priori reason could be justified such as reports of significant BOLD activation in controls relative to clinical populations. Masks were created using the Wake Forest Pick Atlas (Maldjian et al., 2003). The results may be seen in Fig. 9.
Fig. 9.
Blood oxygen level–dependent (BOLD) fMRI analysis of high-risk-for-AD participants from multiplex families (N = 8) conducted at a preset threshold of uncorrrected p = .005 and no extent threshold. Note reduced activations in high-risk subjects in the right middle temporal gyrus, left middle frontal gyrus, and left inferior frontal gyrus.
As may be seen, diminished BOLD response was seen in the high-risk offspring relative to control participants in the right middle temporal gyrus (RMTG), right superior frontal gyrus (RSFG), and the left inferior frontal gyrus (LIFG) (uncorrected significance). Small volume correction analysis was performed for the three regions showing diminished BOLD response in the uncorrected analysis and for which there was a priori evidence to suggest ToM-associated activation. This analysis revealed significant reduction in BOLD response in the RMTG that survived our small volume correction and corrections for multiple comparisons, along with a trend for reduction in the RSFG and the LIFG. Also depicted is the Control minus High-Risk comparison. Reduced activations may be seen in HR subjects in left inferior frontal and right temporal regions.
This pilot fMRI study suggests that adolescent/young adult individuals at high genetic risk for developing AD (members of a multiplex for AD families) may inherit deficits in social intelligence that are reflected in reduced BOLD response in brain regions previously associated with performance of theory of mind tasks. These results showing reduced activation in areas previously associated with performance of ToM tasks suggest that High-Risk offspring may experience deficiencies in emotion recognition that may make it more difficult for them to interpret the actions of others in terms of mental states, such as their thoughts or beliefs, to empathize with them, and to predict how others will react or feel. These deficits may have important implications for life satisfaction as it relates to successful social skill and absence of an alienated personality style.
Importantly, the reduction in activation seen in regions associated with performance of the ToM task occurred in individuals who showed equivalent IQ and educational attainment to the controls. Also, the High- and Low-Risk subjects performed equally well on the task administered. This further suggests that the high-risk individuals were processing emotional material differently than controls though clearly unimpaired in their cognitive performance. This dissociation between cognitive performance and social intelligence has been addressed by Baron-Cohen et al. (1999a, 2001), noting that diminished social intelligence, including capacity for ToM tasks, can often be seen among individuals with higher levels of general intelligence such as mathematicians and scientists.
2. Response Inhibition
Disinhibition may be a characteristic of those with a family history of AD and/or may be the result of long-term use of alcohol. Schweinsburg et al. (2004) studied 12 FHP and 14 FHN youth between the ages of 12 and 14 years and contrasted their response to a Go/No-Go task during fMRI acquisition. The FHP youth appeared to show less BOLD response in frontal regions than did the FHN youth. These results appear to differ from those of Hester and Garavan (2004) for cocaine-addicted individuals. When cocaine-addicted individuals begin to find it difficult to inhibit their behavior as the working memory load increases and when cue-induced craving for the drug increases, cerebellar activation increases. In contrast, the controls showed decreases as the working memory load increased. However, the studies are not directly comparable because Schweinsburg et al. (2004) studied frontal regions and Hester and Garavan (2004) the cerebellum. Nevertheless, these studies are important in emphasizing the value of using fMRI paradigms to investigate traits or conditions thought to be related to risk for developing alcohol abuse and dependence and their related phenotypes.
3. Alcohol Cue Reactivity
Reactivity to alcohol cues in adult alcohol-dependent individuals has shown that those with a history of alcohol abuse respond differently than those without such a history. Two fMRI studies in which alcohol-related cues have been administered have reported that adolescents and young adults with a history of alcohol use disorders (AUDs) show greater BOLD response to alcohol-related words (Tapert et al., 2004) and to pictures of alcoholic and nonalcoholic beverages (Tapert et al., 2003). In the task involving presentation of alcohol-related words, alcohol-dependent women (N= 8) and female light social drinkers (n = 9) aged 18–24 were administered the cue reactivity paradigm during an fMRI assessment. Comparison of the response to alcohol-related words versus neutral words for the two groups revealed significantly more BOLD response in several regions (subcallosal, anterior cingulate, left prefrontal, and bilateral insular regions) for the alcohol-dependent women. In contrast, controls showed greater response to alcohol words in some right cortical regions. Similarly, presentation of alcohol-related pictures to adolescents with an AUD show greater BOLD activation in left anterior, limbic, and visual system areas in comparison to adolescents without a history of AUD. The greatest response was found in youth who reported greater craving for alcohol and a greater number of drinks consumed per month.
These studies illustrate the plasticity of brain functioning by demonstrating that experience using alcohol alters the response of specific brain regions. This phenomenon appears to be related to gender and age and degree of exposure and/or craving for alcohol. An important addition to studies of this type would be to vary the familial/genetic loading for AD. As was the case for individuals with a family history of AD who showed diminished BOLD response in the RMTG in the ToM task involving emotionally cued faces (Hill et al., 2007a), possibly an even greater BOLD response to alcohol cues might be seen in those with a family history even before they begin drinking regularly.
VI. Brain Structural and Functional Effects and Genetic Variation
A. Structural Characteristics and Genes
A number of genes have been associated with the AD phenotype (see Hill, 2000, for review), but only a few genes have been investigated with respect to brain structures that appear to be good intermediate phenotypes for AD or related phenotypes. The serotonin transporter (5-HTT) regulates the reuptake of serotonin to the presynaptic neuron for recycling or degradation after serotonin has been released. One polymorphism that has been studied frequently is the serotonin transporter–linked promoter region (5-HTTLPR). Two primary variants have been identified, a 44-base-pair insertion (L allele) or deletion (S allele). Variation in 5-HTTLPR alleles is associated with variation in 5-HTT expression. Lymphoblast cell lines homozygous for the L allele have been reported to have a 2-fold increase in serotonin reuptake (Lesch et al., 1996). Individuals who are homozygous for the L allele have been reported to have reduced hippocampal (Frodl et al., 2004, 2008) and amygdala volume (Scherk et al., 2009). Also, alterations in the microstructure of frontal limbic WM tracts have been reported to be associated with 5-HTTLPR polymorphic variation (Pacheco et al., 2009). Although associations between variation in 5-HTTLPR and structural aspects of the brain have been reported, studies involving alcohol-dependent persons or their offspring are rare.
Another gene that has been studied frequently with respect to brain morphometry is the brain-derived neurotrophic factor (BDNF) gene. BDNF is a member of the neurotrophin superfamily and plays a role in differentiation during development (Engelhardt et al., 2007) and survival of neurons in the adult brain (Morse et al., 1993). It has also been implicated in regulation of synaptic function (Lessmann et al., 2003). One frequently occurring single nucleotide polymorphism (SNP) (rs6265) at nucleotide 196 (G/A) has been identified in the human BDNF gene. This SNP produces an amino acid substitution of valine to methionine at codon 66 (val66met) in the 5’-region. This polymorphism is responsible for decreased distribution of BDNF val66met to neuronal dendrites.
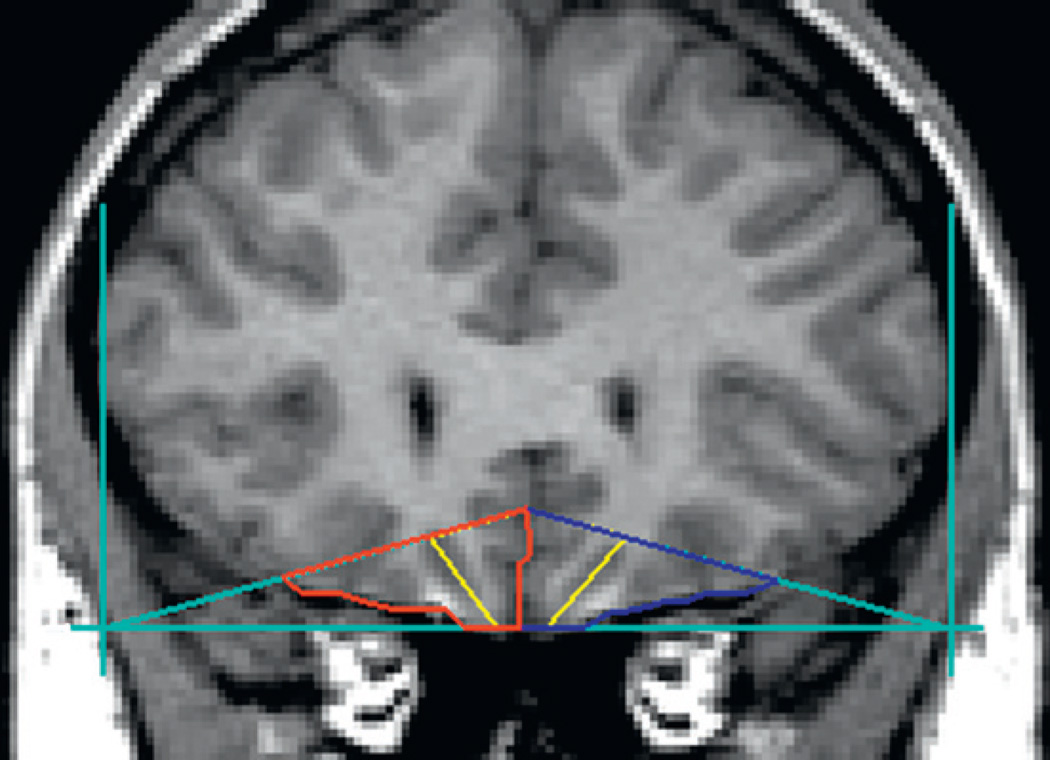
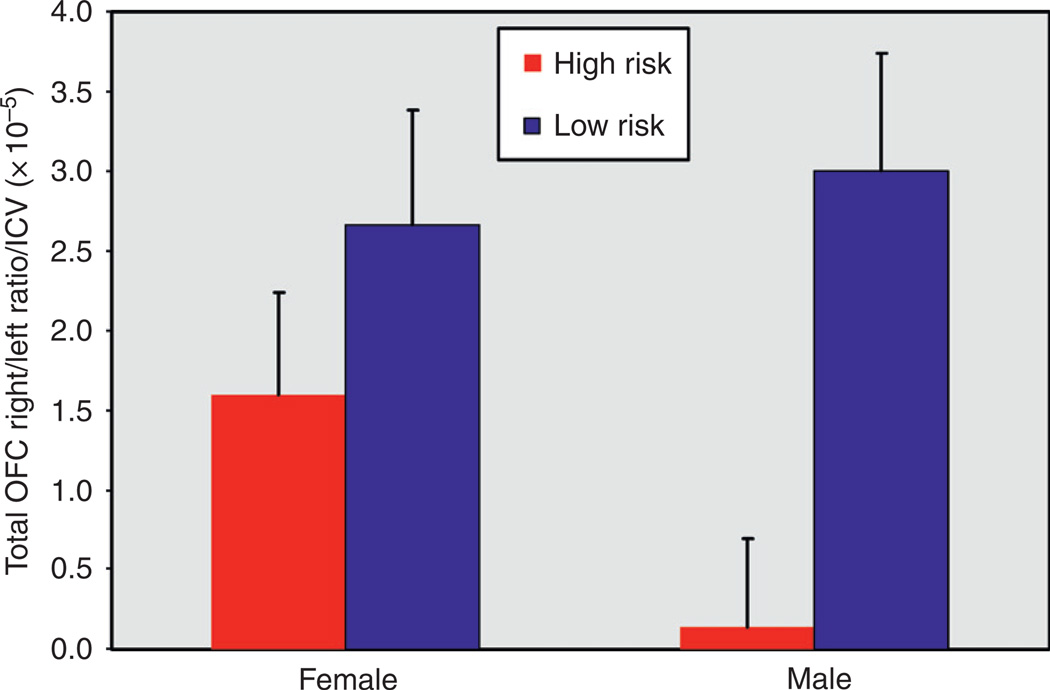
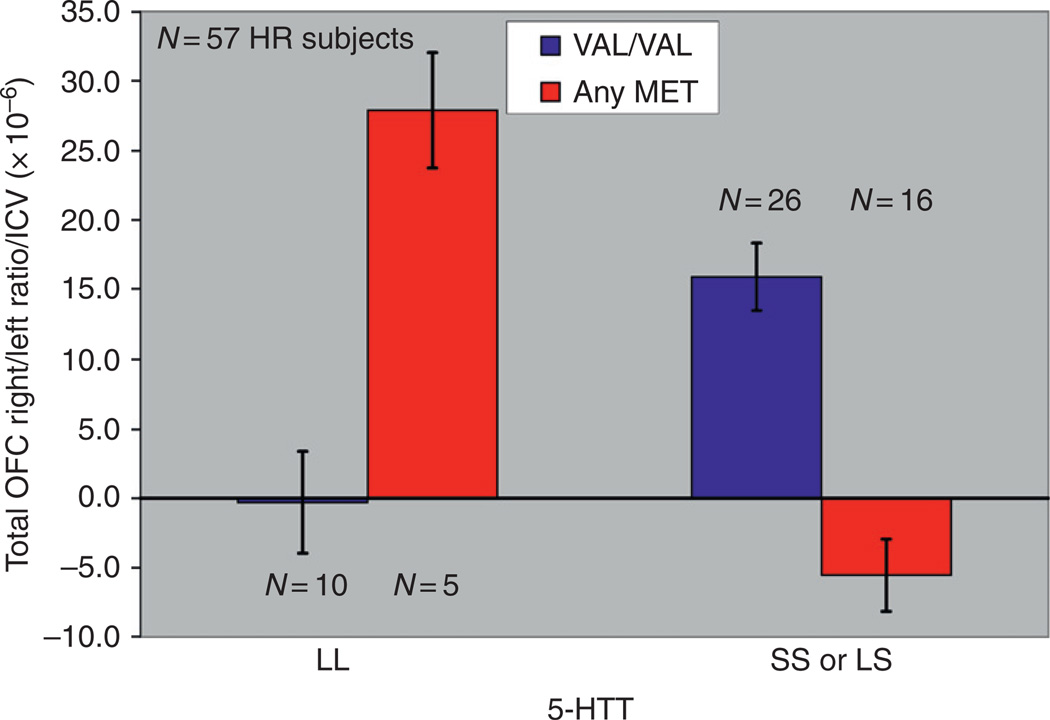
Variation in this polymorphism has been found to be related to volumetric differences in a number of brain structures. Reduced volume of the PFC (Pezawas et al., 2004), hippocampus (Pezawas et al., 2004; Bueller et al., 2006; Frodl et al., 2007), caudate and cerebellar vermis (Agartz et al., 2006), OFC in the right hemisphere (Hill et al., 2009b), and total gray matter of the temporal and occipital lobes (Ho et al., 2006) has been reported. This gene is especially interesting because variation in this gene influences the behavioral effects of alcohol in laboratory animals (Janak et al., 2006). As noted previously, a study in the author’s laboratory demonstrated that offspring from families with multiple cases of AD differed from controls by having lesser volume of the OFC in the right hemisphere. The outline of the structure and its right and left components can be seen in Fig. 10. The volume of this structure was determined by using manual tracings guided by a software program, BRAINS2, which allows the operator to consistently draw regions of interest for multiple brain slices to arrive at brain volumes. The lesser amount of OFC volume in the right hemisphere relative to the left for high-risk individuals may be seen in Fig. 11. The difference is especially pronounced in high-risk males. The morphological data obtained were then studied with respect to variation in the 5-HTT and BDNF genes. Results of the analysis may be seen in Fig. 12. Here, it can be seen that there was interaction between the two polymorphisms. Genotyping variation in the 5-HTT and BDNF were then evaluated with respect to these OFC ratios. As may be seen, there was a significant association between the presence of the S allele of the 5-HTT gene, the Met allele of the VAL/MET variation of the BDNF gene, and volume of the OFC in the right hemisphere (Right − Left)/(Right + Left), corrected for intracranial volume (ICV), for the 87 participants. For total volume, the BDNF by 5-HTT by risk interaction was significant (F=5.55, df= 1, 77, P=.021). This interaction was also significant for white (F =4.92, df = 1, 77, p =.03) and for gray (F =4.16, df = 1, 77, P=.045) volumes. An analysis restricted to the 57 High-Risk participants was also significant (F = 5.31, df = l,51, p = .025), without IGV correction, and with IGV correction (F = 6.32, df = 1, 51, p = .015).
Fig. 10.
The boundaries and landmarks for the OFC: right, left, and total. Outlines for the left OFC are seen in blue, with the right OFC in red. The yellow line depicts the lateral and medial portions of the OFC in each hemisphere (See color plate 1).
Fig. 11.
OFC ratios were determined for each participant using the formula (Right − Left)/(Right + Left). Volumes were adjusted for intra-cranial volume before statistical analyses were performed. Depicted here are the adjusted means (adjustment for age) and standard deviations for the male and female High- and Low-Risk participants.
Fig. 12.
A statistically significant association between the presence of the S allele of the 5-HTT gene, the Met allele of the VAL/Met variation of the BDNF gene, and volume of the OFC in the right hemisphere (Right − Left /Right + Left)(ICV corrected) was seen for the 87 participants genotyped.
B. Brain Function Assessed by fMRI Neuroimaging and Genes
Although there are numerous studies now suggesting functional differences among individuals who use psychoactive substances extensively or have a family history of AD and its related phenotypes, inclusion of genetic variation in these studies has not been addressed. This would appear to be a fruitful approach in future studies based on an emerging literature suggesting that genotypic variation is associated with fMRI BOLD response.
Functional neuroimaging studies have placed a major emphasis on two neurotransmitter systems (serotonin and dopamine). The emphasis on serotonin comes from animal and human studies which have implicated variation in serotonin (5-HT) neurotransmission as a major determinant in individual differences in trait characteristics, especially in the continuum of negative affect (Lucki. 1998). Pharmacological manipulation of the serotonergic system indicates a potential for modifying stress reactivity peripherally and for alteration of negative affect (Roth, 2006). Dopaminergic transmission is critical to motivational and reward-related functions of the brain including reinforcement learning (Tobler et al., 2003) and decision making (Montague et al., 2004). Because there is considerable variation among individuals with respect to these characteristics (Gonzalez and Wu, 1999), it has been suggested that genetic variation may be responsible (Trepel et al., 2005). Individual variation in dopaminergic functioning has been suggested as a major contributing factor to personality traits (Benjamin et al., 2000) and addiction (Volkow et al., 1997).
There is evidence that genetic variation may predict alterations in BOLD response in the ventral striatum and PFC (Yacubian et al., 2007), middle and inferior temporal gyrus, superior frontal gyrus and cerebellum (Boettiger et al., 2007), and amygdala (Fisher et al., 2009; Munafò et al., 2008). Using a guessing task that is sensitive to reward-related activation in the PFC and ventral striatum, Yacubian et al. (2007) found that the dopamine transporter (DAT) and catechol O-methyltransferase (COMT) influences reward processing, as reflected in changes in BOLD activation. Because of the established relationship between reduced reward sensitivity and addiction, these findings suggest that genetic variation in these two genes is related to fMRI BOLD activation in the PFC and ventral striatum.
Additionally, a relationship between threat-related amygdala reactivity and variation in the serotonin 2A receptor gene (5-HT 2A) has been shown (Fisher et al., 2009). This relationship was found using BOLD fMRI response and focusing on the density of 5-HT2A receptors in the mPFC. A remarkable 25–37% of variability in amygdala variability was explained by mPFC 2A density. Because there is feedback inhibition of the amygdala via the mPFC, finding genetic variation that can influence complex emotional behaviors that are influenced by this circuit is of some significance.
There are a number of studies that have related variation in the serotonin transporter (5-HTTLPR) genotype and amygdala activation (see Munafò et al., 2008, for review). Munafo and colleagues conducted a meta-analysis of 17 studies in which the 5-HTTLPR polymorphism was genotyped and either fMRI or positron emission tomography (PET) was used. They concluded that the 5-HTTLPR polymorphism may contribute up to 10% in phenotypic variance in the BOLD response. However, they also concluded that most studies reviewed were not of sufficient size to provide adequate statistical power.
In summary, there are clear indications that genetic variation contributes to functional effects as measured by fMRI and PET. The challenge now is to bring these observations together with studies designed to assess variations in familial risk for AD to begin to uncover how altered brain functioning may influence susceptibility to AD and related phenotypes.
C. Brain Function Assessed with Electrophysiological Variants—Genetic Variation
Genetic association has been found between the amplitude of visual P300 in high-risk children and a marker locus in the D2 dopamine receptor on chromosome 11 (Taq1 A restriction fragment length polymorphism (RFLP) near the DRD2 receptor locus) (Hill et al., 1998). Although this finding has not been replicated in normal young women (Lin et al., 2001) or in patients with depression (Chen et al., 2002), important differences across samples may have contributed to different outcomes. As noted previously in the discussion of P300 trajectories of childhood and adolescence, there are substantial differences in P300 amplitude by age and gender that can be expected to influence results. Search for genes associated or linked to P300 need to control for these important sources of variation.
Linkage analysis of P300 data from the Collaborative Study on the Genetics of Alcoholism (COGA) has revealed significant linkage on chromosomes 2, 5, 6, 13, and 17 using P300 recorded during a visual oddball task (Begleiter et al., 1998). In more recent studies, the COGA group has reported on specific candidate genes in their linkage and linkage disequilibrium analyses. These have included study of a cholingeric receptor gene (CHRM2), a gene that encodes the muscarinic acetycholine receptor M2 (Jones et al., 2006), a glutamate receptor gene (GRM8) (Chen et al., 2008), and GABRA2 (Porjesz and Rangaswamy, 2007).
Although earlier studies from the COGA group had looked for linkage to the P300 component of the ERP wave, more recent research from this group has focused on event-related oscillations (EROs). The basis for this shift has been in the emphasis on the superposition of multiple EROs that comprise the ERP rather than emphasis on the ERP as a unitary transient phenomenon (Basar, 1980; Gruber et al., 2005). This view continues to be open to debate (Yeung et al., 2004; Makinen et al., 2005). The P300 component is thought to be primarily composed of delta (1–3 Hz) and theta (4–7 Hz) band energy. The delta component is concentrated in more posterior regions while theta is more fronto-central (Basar-Eroglu et al., 1992). The underlying multiple source generators of P300 can be studied using time–frequency distribution analysis as an alternative measure of P300 amplitude. The important point is that studies of the P300 component of ERP and its associated amplitude and study of EROs are not fundamentally different; rather, they are alternative forms of the same brain process that can be utilized to measure aspects of brain functioning.
In summary, ERPs or EROs provide an important source for understanding functional differences in individuals with familial/genetic risk for AD and to elucidate mechanisms involved in alcohol exposure. These electrophysiological variants provide a complementary method to fMRI techniques and are especially useful in addressing response to stimuli within 1 s after presentation. The P300 response occurs approximately 300 ms after presentation of a stimulus, though this can be somewhat later in children. Nevertheless, the typical ERP occurs within 1 s. Although fMRI BOLD can be measured within a few seconds after a stimulus is presented, it requires sufficient time for blood flow to redistribute following presentation. The typical fMRI response signal changes above baseline at approximately 2 s, growing to maximum value at about 5 s for a short stimulus (Huettel et al., 2004).
VII. Conclusions
Alcoholism is a complex disease that is influenced by genetic and environmental factors and their interaction. The search for genes that may increase susceptibility to AD has been greatly facilitated by the recognition that intermediate phenotypes, sometimes referred to as endophenotypes, may be closer to the genetic variation than is the more complex AD phenotype.
Although the genetic makeup of the individual is fixed at birth, the response of the genome to environmental triggers (both external and internal) means that expression of genetic variation can be expected to vary across developmental stages. Consequently, major changes can be expected to occur by developmental stage, which in turn affect the brain structure and function. These structural and functional effects can be expected to have a major influence on the individual’s risk for developing a variety of psychopathological conditions including AD. Development of sophisticated neurophysiological and neuroimaging techniques over the past several years has allowed us to identify neural circuits involved in addictive behaviors and provided a window on developmental changes in brain structure and function. Identification of neural plasticity within specific components of these addiction circuits that are related to genetic variation will offer numerous opportunities for treatment and intervention.
We can expect to see a growth in the number of studies addressing genetic influences on brain function in the future. Because it is clear that environmental variation influences genetic mechanisms, the challenge will be in identifying the relevant environmental triggers and specifying these by developmental stage. It is quite likely that critical periods will be identified where environmental influences will be more salient than at others. In addition, defining the relevant genetic variation will be a challenge in future studies. Because there are over 30,000 genes in the human genome, and many of these show complex temporal and spatial expression in the brain, selection of the most appropriate candidate genes for study is a daunting task. Moreover, the number of gene–gene interactions that might be responsible for a given mechanism is beyond the number that could be realistically assessed. It has been suggested that an alternative approach to this problem might be to sequence the entire genome of individuals from families where there is evidence that extraordinary genetic vulnerability is segregating (McClellan et al., 2007). The idea behind this view comes from linkage studies where tracking genetic variants within families shows that some families show evidence that a given variant is highly linked to the psychiatric disorder of interest while others do not. In conventional linkage analysis, the linkage value for a study is the sum of the LOD scores obtained for each family. Experience with linkage studies shows that this variation is considerable even when endophenotypes are utilized rather than the more complex phenotype.
In the very near future, it is expected that the cost of sequencing and analyzing single personal genomes will be in the range of US$5000–10,000 (AMI et al., 2010). Nevertheless, it would appear prohibitively expensive to do a large-scale epidemiological investigation using case-control methodologies. Recent reports from collaborative efforts to identify genetic loci associated with type 2 diabetes required genotyping over 200,000 separate individuals to identify five loci (Dupuis et al., 2010; Saxena et al., 2010). Even if the extraordinary cost of sequencing that many individuals is not considered, there would undoubtedly be an even greater cost associated with acquiring endophenotypic data such as MRI scans on such a scale. Finally, the issue of whether or not this approach would lead to useful scientific data is in question if as McClellan et al. (2007) suggest the common disease–rare allele model is the correct model for studying psychiatric disorders. They note that “a current mantra in schizophrenia genetic research is the need for ever-larger sample sizes in order to detect common small-effect variants” (Devon et al., 2001). Quite possibly, this mantra has widened to include many psychiatric disorders including AD.
A highly appealing alternative is to target families with known ultrahigh loading for the disease of interest, such as those seen in multiplex families, a strategy that could provide an economical method for complete sequencing of targeted families, and to combine this data with structural and functional imaging and neurophysiological assessment.
References
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Agartz I, Sedvall GC, Terenius L, Kulle B, Frigessi A, Hall H, Jönsson EG. BDNF gene variants and brain morphology in schizophrenia. Am. J. Med. Gen. Part B. 2006;141:513–523. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- Akil H, Brenner S, Kandel E, Kendler KS, King M-C, Scolnick E, Watson JD, Zoghbi HY. The future of psychiatric research: Genomes and neural circuits. Science. 2010;327:1580–1581. doi: 10.1126/science.1188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Maas LC, Frederick BD, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autistic brain: An fMRI study. Eur. J. Meurosci. 1999a;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Stone V, Rutherford M. A mathematician, a physicist, and a computer scientist with Asperger syndrome: Performance on folk psychology and folk physics test. Neurocase. 1999b;5:475–483. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism spectrum quotient (AQ): Evidence from Asperger syndrome high functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Basar E. EEG–Brain Dynamics: Relation between EEG and Brain Evoked Potentials. New York, NY: Elsevier; 1980. [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: Possible psychophysiological correlates in delta and theta frequency channels. A review. Int. J. Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): Impaired activation of the somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science (Washington, DC, USA) 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li T-K, Conneally PM, Hesselbrock V, Rice J, Schuckit M, Cloninger R, Nurnberger J, Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr. Clin. Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH, Ebstein RP. Association between the tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: Dopamine receptor D4 (DRD4), sertonin transporter promoter region (5HTTLPR) and catechol O-methyltransferase (COMT) Mol. Psychiatry. 2000;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance abuse. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Hazeltine E, Bergren L, Ivry RB, Grafton ST. The influence of feedback valence in associative learning. Neuroimage. 2009;44:243–251. doi: 10.1016/j.neuroimage.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL. Immediate reward bias in humans: Fronto-parietal networks and a role for the catechol-O-methyltransferase 158 Val/Val genotype. J. Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: Past successes for Mendelian disease. Nat. Genet. 2003;33:229–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, Maia AF, Rosario-Campos MC, Campi Castro C, Furuie SS, Gutierrez MA, McGuire PK, Miguel EC. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatry Res. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Caldwell CB, Gottesman II. Sex differences in the risk for alcoholism: A twin study. Behav. Genet. 1991;21:563. [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn. Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chakravarti A. Population genetics — making sense out of sequence. Nat. Genet. 1999;21(Suppl.):56–60. doi: 10.1038/4482. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chen ACH, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Kuperman S, O’Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am. J. Med. Genet., Part B. 2008;150:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TJ, Yu YW, Chen JY, Wang YC, Chen MC, Hong CJ, Tsai SJ. Association analysis of two dopamine D2 receptor gene polymorphisms and p300 event-related potential in depressive patients. Meuropsychobiology. 2002;46:141–144. doi: 10.1159/000066395. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Development of decision making in school aged children and adolescents: Evidence from heart rate and skin conductance analysis. Child Dev. 2007;78:1288–1301. doi: 10.1111/j.1467-8624.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat. Rev. Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;57:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Devon RS, Anderson S, Teague PW, Burgess P, Kipari TM, Semple CA, Millar JK, Muir WJ, Murray V, Pelosi AJ, Blackwood DH, Porteous DJ. Identification of polymorphisms within disrupted in schizophrenia 1 and disrupted in schizophrenia 2, and an investigation of their association with schizophrenai and bipolar affective disorder. Psychiatr. Genet. 2001;11:71–78. doi: 10.1097/00041444-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, Van Den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. Br. J. Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornells M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martin ez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, ZiUikens MC, DIAGRAM Consortium, GIANT Consortium, global BPgen Consortium. Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan L, Wright AF, StumvoU M, Campbell H, Wilson JF, Anders Hamsten on behalf of Procardis Consortium MAGIC investigators. Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy ML, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetesrisk. Nat. Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Engelhardt M, Di Cristo G, Berardi N, Wahle P. Differental effects of NT-4, NGF and BDNF on development of neurochemical architecture and cell size regulation in rat visual cortex during the critical period. Eur. J. Neurosci. 2007;25:529–540. doi: 10.1111/j.1460-9568.2006.05301.x. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial prefrontal cortex 5-HT2a density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb. Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisy M-L, Kornreich C, Fobe A, D’Hondt L, Pelc I, Hanak C, Verbanck P, Phillippot P. Impaired emotional facial expression recognition in alcohol dependence: Do these deficits persist in abstinence. Alcohol. Clin. Exp. Res. 2007;31:404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal. cingulate, and temporal cortices of cocaine patients. Biol. Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, Rupprecht R, Bondy B, Reiser M, Moller H-J. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry. 2004;61:177–183. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Möller H-J, Meisenzahl EM. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch. Gen. Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Frodl T, Zill P, Baghai T, Schule C, Rupprecht R, Zetzsche T, Bondy B, Reiser M, Mdller HJ, Meisenzahl EM. Reduced hippocampal volumes associated with the long variant of the tri- and diallelic serotonin transporter polymorphism in major depression. Am. J. Med. Gen. Part B. 2008;147:1003–1007. doi: 10.1002/ajmg.b.30680. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb. Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala. and hippocampus in normal human development: Ages 4–18. J. Comp. Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Biol. Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Cavalleri GL, Ahmadi KR. The genetics of common diseases: 10 million times as hard. Cold Spring Harb Symp Quant Biol. 2003;68:395–402. doi: 10.1101/sqb.2003.68.395. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Wu G. On the shape of the probability weighting function. Cogn. Psychol. 1999;38:129–166. doi: 10.1006/cogp.1998.0710. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia: The Epigenetic Puzzle. Cambridge, UK: Cambridge University Press; 1982. [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J.Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb. Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J. Stud. Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol. Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY. Biologic phenotypes associated with individuals at high risk for developing alcohol-related disorders. Addict. Biol. 2000;5:5–22. doi: 10.1080/13556210071234. [DOI] [PubMed] [Google Scholar]
- Hill SY, DeBellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent/young adult offspring from families at high risk for developing alcoholism. Biol. Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott MD, Diwadkar V, Keshavan MS. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol. Clin. Exp. Res. 2007a;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Locke J, Lowers L, Connolly J. Psychopathology and achievement in children at high risk for developing alcoholism. J. Am. Acad. Child. Adolesc. Psychiatry. 1999a;38:883–891. doi: 10.1097/00004583-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer SR, Wipprecht G, Xu J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol. Psychiatry. 1998;43:40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Steinhauer S, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2007b;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muka DH. Childhood psychopathology in children from families of alcoholic female probands. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:725–733. doi: 10.1097/00004583-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka DH, Steinhauer SR, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biol. Psychiatry. 1995;38:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Patterns of visual P3b in association with familial risk and childhood diagnosis. Biol. Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke-Wellman J, Lowers L. Psychopathology in childhood and adolescence: A prospective study of offspring from multiplex alcoholism families. Psychiatry Res. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-relatcd disorders. Biol. Psychiatry. 1999b;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high-risk for developing alcoholism. Biol. Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome-wide search for alcoholism susceptibility genes. Am. J. Med. Genet. Part B. 2004;128:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J. Stud. Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2009a;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol. Clin. Exp. Res. 1990a;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. Reprinted in Ann. Rev. of Addict. Res. Treatment 177–192, 1992. [DOI] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2009b;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. J. Stud. Alcohol. 1999;60:7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Zubin J, Steinhauer SR. Personality resemblance in relatives of male alcoholics: A comparison with families of male control cases. Biol. Psychiatry. 1990b;27:1305–1322. doi: 10.1016/0006-3223(90)90501-r. [DOI] [PubMed] [Google Scholar]
- Ho BC, Millev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain neurotrophic factor val66met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch. Gen. Psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Wolf FW, Herberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: New findings on growth factor pathways BDNF, insulin, and GDNF. Alcoholism: Clin, and Exper. Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Jellinek EM. The Disease Concept of Alcoholism. New Haven, CT: Hillhouse; 1960. [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav. Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. J. Stud. Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony J. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1997;54:3123–3321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Chan G, Kramer JR, Bierut L, Bucholz KK, Fox L, Hesselbrock V, Nurnberger JI, Jr, Reich T, Reich W, Schuckit MA. Relationship of age of first drink to child behavioral problems and family psychopathology. Alcohol. Clin. Exp. Res. 2005;29:1869–1876. doi: 10.1097/01.alc.0000183190.32692.c7. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Benegal D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hammer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (Washington, DC, USA) 1996;29:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. (Amsterdam, Neth.) 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol. Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yu YW, Chen TJ, Tsa SJ, Hong CJ. Association analysis for dopamine D2 receptor Taq1 polymorphism with P300 event-related potential for normal young females. Psychiatr. Genet. 2001;11:165–168. doi: 10.1097/00041444-200109000-00010. [DOI] [PubMed] [Google Scholar]
- London ED, Broussolle EP, Links JM, Wong DF, Cascella NG, Dannals RF, Sano M, Herning R, Snyder FR, Rippetoe LR, Toung TJK, Jaffe JH, Wagner HN., Jr Morphine-induced metabolic changes in human brain. Studies with positron emission tomography and [fluorine 18] fluorodeoxyglucose. Arch. Gen. Psychiatry. 1990;47:73–81. doi: 10.1001/archpsyc.1990.01810130075010. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Ailing C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: Hippocampal cell loss after one month of treatment. Alcohol. 1995;30:737–748. [PubMed] [Google Scholar]
- Makinen V, Tiitinen H, May P. Auditory event-related responses are generated independently of ongoing brain activity. Neuroimage. 2005;24:961–968. doi: 10.1016/j.neuroimage.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: A magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McClellan JM, King M-C. Genetic heterogeneity in human disease. Cell. 2010;141:210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King M-C. Schizophrenia: A common disease caused by multiple rare alleles. Br. J. Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink: II. Familial risk and heritability. Alcohol. Clin. Exp. Res. 2001a;25:1166–1173. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink: I. Associations with substance-use disorders. disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol. Clin. Exp. Res. 2001b;25:1156–1165. [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcohol. Clin. Exp. Res. 1997;21:513–520. [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stevens DE, Fenton B, Stolar M, O’ Malley S, Woods SW, Risch N. Comorbidity and familial aggregation of alcoholism and anxiety disorders. Psychol. Med. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Risch N. Genomic priorities and public health. Science. 2003;302:599–601. doi: 10.1126/science.1091468. [DOI] [PubMed] [Google Scholar]
- Merlo CA, Boyle MP. Modifier genes in cystic fibrosis lung disease. J. Lab. Clin. Med. 2003;141:237–241. doi: 10.1067/mlc.2003.29. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morse JK, Wiegand SJ, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano PS, Alter CA, Lindsay RM. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transaction. J. Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biol. Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability and the contribution of risk factors. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, Togao O, Kanba S. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM, Kramer J, Kuperman S, Bucholz KK, Schuckit MA, Nurnberger JI. The relationship between parental alcoholism and adolescent psychopathology: A systematic examination of parental comorbid psychopathology. J. Abnorm. Child Psychol. 2004;32:519–533. doi: 10.1023/b:jacp.0000037781.49155.a6. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J. Neurosci. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-related-independent decrease in prefrontal and parietal activation. Biol. Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in me thamphetamine dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science (Washington, DC, USA) 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. J. Clin. Invest. 2005;115:1431–1439. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiol Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition. and risk for alcohol dependence and related disorders. Sri. World J. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population based sample of US twins. Alcohol. Clin. Exp. Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann. NT Acad. Sri. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li T-K, Conneally PM, Nurnberger JI, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genomewide search for genes affecting the risk for alcohol dependence. Am. J. Med. Genet. Part B. 1998;81:207–215. [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Robins LN. Deviant Children Grown Up – A Sociological and Psychiatric Study of Socio-patic Personality. Baltimore, MD: Williams [[amp]] Wilkins; 1966. [Google Scholar]
- Roth BL. The Serotonin Receptor: From Molecular Pharmacology to Human Therapeutics. Totowa, NJ: Humana Press; 2006. [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko L, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O’Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Kottgen A, Le Bacquer O, Pattou F, Taneera J, Steinthors-dottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proenca C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, Crawford G, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jorgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Levy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparso T, Swift AJ, Syddall H, Thorleifsson G, Tonjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, GIANT consortium MAGIC investigators. Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvanen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JL, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy ML, Boehnke M, Barroso L, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Mat. Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherk H, Gruber O, Menzel P, Schneider-Axmann T, Kemmer C, Usher J, Reith W, Meyer J, Falkai P. 5-HTTLPR genotype influences amygdala volume. Eur. Arch. Psychiatry Clin. Meurosc. 2009;259:212–217. doi: 10.1007/s00406-008-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EJ, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res. Hum. Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am. J. Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Brown SA, Tapert SF. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann. Nγ Acad. Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelin-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn. Reson. Imaging. 2006;24:833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, andjernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;38:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. J. Stud. Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci. Biobehav. Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict. Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Multidimensional Personality Questionnaire, unpublished manual 1985 [Google Scholar]
- Tellegen A, Lykken DT, Bouchyard TJ, Jr, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. J. Pers. Soc. Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Tessner K, Hill SY. Neural circuitry associated with risk for alcohol dependence. Neuropsychol. Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Tobler PN, Schultz W. Adaptive coding of reward value by dopamine neurons. Science (Washington, DC, USA) 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Res. Cogn. Brain Res. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV. The future of psychiatric genetics. Curr. Psychiatry Rep. 2000;2:133–136. doi: 10.1007/s11920-000-0057-8. [DOI] [PubMed] [Google Scholar]
- Van Beijersterveldt CEM, Boosma DI. Genetics of the human electroencephalogram (EEG) and event-related potentials (ERPs): A review. Hum. Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- Van Hasselt VB, Hersen M, Milliones J. Social skills training for alcoholics and drug addicts: A review. Addict. Behav. 1978;3:221–233. doi: 10.1016/0306-4603(78)90023-0. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Yanos M, Schmitt PJ, Grafton ST. Alcohol-Induced suppression of BOLD activity during goal-directed visuomoto performance. Neuroimage. 2006;31:1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl. 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Winokur G, Clayton PJ. Family history studies: IV. Comparison of male and female alcoholics. Q. J. Stud. Alcohol. 1968;29:885–891. [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Gläscher J, Kalisch R, Leuenberger B, Braus DF, Biichel C. Gene-gene interaction associated with neural reward sensitivity. Proc. Natl. Acad. Sci. USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the electroencephalogram: An evaluation of methods. Psychophysiology. 2004;41:822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Yuan H, Marazita SY, Hill SY. Segregation analysis of alcoholism in high density families: A replication. Am. J. Med. Genet: Neuropsychiatric Genet. 1996;67:71–76. doi: 10.1002/(SICI)1096-8628(19960216)67:1<71::AID-AJMG12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr. Opin. Neuorbiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]