Abstract
Background
Low physical activity is associated with depression, which may in turn, negatively impact antiretroviral therapy (ART) adherence among HIV-infected individuals; however, prior studies have not investigated the relationships between physical inactivity and ART non-adherence.
Purpose
To examine the association of physical inactivity, depression, ART non-adherence, and viral load in HIV-infected men who have sex with men.
Methods
The sample (N = 860) was from a large, multicenter cohort of HIV-infected patients engaged in clinical care.
Results
Across time, depression mediated the relationship between physical inactivity and ART non-adherence, γ = .075, and the relationship between physical inactivity and viral load, γ = .05. ART non-adherence mediated the relationship between depression and viral load, γ = .002, and the relationship between physical inactivity and viral load, γ = .009.
Conclusions
Low levels of physical activity predicted increased depression and poor ART adherence over time, which subsequently predicted higher viral load.
Keywords: HIV/AIDS, physical activity, depression, adherence, viral load
Among the general population, increased physical activity is associated with decreased risk of developing cardiovascular disease, type 2 diabetes, osteoporosis, obesity, and some forms of cancer (1). Physical activity may be even more salient for those living with a chronic illness, such as HIV, given that HIV-infected individuals are at an increased risk for developing cardiovascular disease, (2) type 2 diabetes, (3) osteoporosis (4) and multiple types of cancer (5). Symptoms of depression significantly decline with increased physical activity (6–9). However, the impact of physical activity on disease outcomes such as HIV plasma RNA (i.e., viral load) is less clear, as the majority of studies have failed to find significant associations, (10–13) although one cross-sectional study (14) revealed a significant inverse relation between viral load and physical activity, and additional evidence suggests aerobic exercise training has a positive effect on immunologic functioning in HIV (15).
As HIV has moved from a terminal condition, to a manageable, chronic illness, understanding and addressing the modifiable factors associated with antiretroviral therapy (ART) adherence and biomarkers of disease progression (e.g., viral load) are important for both the treatment and prevention of HIV. Indeed, evidence has suggested that psychological interventions can have a powerful effect on HIV disease markers, including viral load, neuroendocrine hormone regulation, and immune status (16–17). This is a particularly important consideration for men who have sex with men (MSM), who represent the largest group of individuals living with HIV in the United States, constituting nearly 60% of the population (18). MSM with suppressed viral loads have a significantly diminished risk of morbidity and mortality (19) as well as lower risk of transmitting the disease to others (20–21). Understanding modifiable factors affecting ART non-adherence and poor disease progression among MSM has important public health implications.
One such behavior which has received limited attention in the context of disease progression and ART adherence is physical activity. A review of the literature suggests that studies have not examined the relationship between physical activity and ART adherence. This is a notable limitation given that the association between increased physical activity and improved biomarkers of disease progression may be at least partially accounted for by increased adherence. It is also possible that increased physical activity predicts improved biomarkers via the mechanism of reduced depression, which itself could be immunoenhancing (22). Increased physical activity is predictive of a reduction in depressive symptoms, (6–9) and reductions in depressive symptoms are predictive of improved ART adherence, (23) which has been shown to be predictive of lower viral load (22, 24–30). However, this has not yet been tested in a comprehensive mediational model. Thus, the aim of the current study was to assess, within a large multicenter sample, longitudinal mediation models involving physical inactivity, depression, ART non-adherence and viral load (see Figure 1 for the conceptual framework). The following hypotheses were generated:
Figure 1.
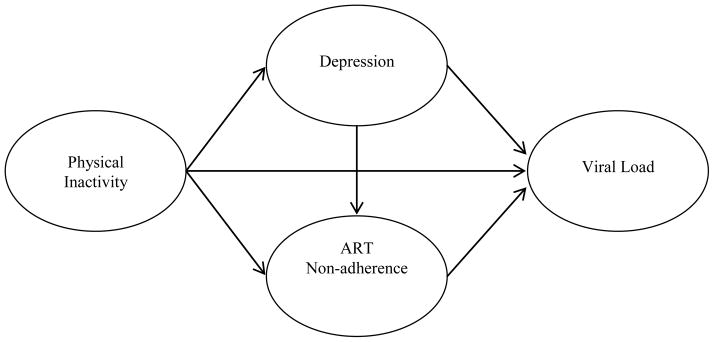
Conceptual model
-
Hypothesis 1
The relationship between physical inactivity and ART non-adherence will be mediated by depression.
-
Hypothesis 2
The relationship between physical inactivity and viral load will be mediated by depression.
-
Hypothesis 3
The relationship between physical inactivity and viral load will be mediated by ART non-adherence.
-
Hypothesis 4
The relationship between depression and viral load will be mediated by ART non-adherence.
Method
The sample was taken from a large, national HIV clinical cohort (the Center for AIDS Research—Network of Integrated Clinical Systems), which has been previously described in detail (31). The cohort consists of 8 sites across the United States, and includes data from more than 25,000 HIV-infected individuals, dating back to 1995. Approximately every two months, participating sites upload longitudinal patient data into a data repository. The repository integrates clinical data from both inpatient and outpatient visits, including standardized HIV-relevant information assessed at the initial clinic visit. Data includes demographics, clinical, laboratory, and socioeconomic data obtained from each site’s electronic health record and other institutional data sources. In addition, participants complete a computer assisted self-interview twice a year that asks them about their medication adherence and depression symptoms, along with a variety of other behavioral data. The cohort protocol was approved by each collaborating site’s respective institutional review board.
For the current study, patients were eligible for inclusion if they were 1) MSM, 2) reported current ART, and 3) had completed the cohort clinical assessment (see below for more details; 32–34). The clinical assessment was integrated into clinical care between 2005 and 2008 at four participating sites (the University of Washington, the University of Alabama at Birmingham 1917 HIV/AIDS Clinic, Fenway Community Health Center, and the University of California, San Diego). Patients completed the assessment on touch-screen tablets or computers at the time of routine clinic appointments using a web-based platform designed for collecting patient reported outcomes. Clinical data (e.g., viral load) were obtained from the data repository, focusing on values around the time patients completed the self-reported measures. Data from September 2005 to July 2011 were included.
Measures
Viral load
Viral load values were obtained via routine clinical practice at the respective sites. Given the highly skewed nature of the raw viral load data, a log10 transformation was calculated for viral load analyses. The most commonly used viral load assays were HIV-1 RNA Roche Ultrasensitive, HIV-1 RNA Abbott m2000 real-time, and HIV-1 RNA Branched DNA version.
Depression
Symptoms of depression were measured using the continuous total score from the 9-item Depression Severity Scale of the Patient Health Questionnaire (35) a self-report instrument designed to detect symptoms of major depressive disorder in primary care settings through diagnostic and symptom severity assessments. Participants reported the frequency in which they experienced each symptom with response options ranging from 1 “not at all” to 4 “nearly every day.” For the current sample, internal consistency was α = .90.
Physical inactivity
Physical inactivity was measured via responses to two self-report items, from the Lipid Research Clinics Physical Activity questionnaire (36). The first item, “What was your highest level of physical activity?”, was answered with response options that ranged from 1 “none” to 3 “vigorous.” While the second item, “Thinking about things you do outside of work, how would you rate the amount of physical activity you get compared with others of your age and sex?” was answered with a response scale that ranged from 1 “much more active” to 5 “much less active.” Given the different response scales used, individual items were converted to z-scores (after reverse scoring the first item), and were subsequently averaged into a total score, with higher scores denoting lower physical activity. For the current sample, internal consistency was α = .70.
ART non-adherence
Non-adherence to ART was measured via responses to two self-report items. The first item, “In the past 4 weeks, how was your ability to take all of your anti-HIV medications that were prescribed by your doctor?”, was answered via a response scale that ranged from 1 “very poor” to 6 “excellent”. Studies have advocated for the use of this item, (37) and within the cohort, the validity of its use within routine clinical care for patients with HIV has recently been examined (38). The second item, “How many doses of your medications did you miss in the last 7 days?” (39) was answered with a response scale that ranged from 1 “zero” to 6 “more than four” and was included to increase the validity of the non-adherence variable. Given the different response scales used, individual items were converted to z-scores (after reverse scoring the first item), and subsequently averaged into a total score, with higher scores denoting greater non-adherence. For the current sample, internal consistency was α = .76.
Statistical analyses
The main analyses of the study, longitudinally examining the relationship between physical inactivity, depression, ART non-adherence, and viral load, were conducted via linear mixed-effects modeling in PASW 18 (MIXED Procedure). The level-one variables represented the time variable (i.e., baseline, 11, and 14 months post-baseline), in addition to other time-variant predictor variables: physical inactivity, depression, ART non-adherence, and viral load, along with the time-variant covariate CD4. Level-two variables included the time-invariant covariate of participant age (see preliminary analyses for more details) along with participants. Participants were treated as random effects, with random slopes and intercepts, while all remaining variables were fixed effects. In doing so, each participant was allowed to have a unique growth trajectory. Further, the scaled identity covariance structure was chosen based on the best goodness-of-fit (as evaluated by the Akaike Information Criterion), compared to competing covariance structures. The restricted maximum likelihood estimation method was chosen in lieu of maximum likelihood estimation, as the former approach tends to result in unbiased estimates of the variances and covariances (40).
Longitudinal mediation was tested via the monte carlo method of assessing mediation (41–42) which is a form of bootstrapping. Using the parameter estimates and the associated standard errors, random draws from the a and b distributions are simulated and the product of these values is computed. This procedure is repeated a large number of times (i.e., 2,000) and the resulting distribution of the a*b values are used to estimate a 95% confidence interval (CI) around the observed value of a*b. The a*b path, or the indirect effect, allows one simple calculation to determine the presence of a mediated effect. The indirect effect is interpreted as “statistically significant” if 0 is not contained between the lower and upper CIs.
Bootstrapping methods are generally preferred over traditional methods of studying mediation (i.e., the causal steps approach and the product of coefficients approach; 43). In the widely known causal steps approach, (44) multiple regression analyses are conducted, testing the various paths of the mediational model; however, this approach does not directly test the indirect effect (45). Rather, determination of mediation is based on the presence of a significant total effect, and a drop, or non-significance in the direct effect. Additionally, the causal steps approach requires unnecessary power to conduct separate tests on the paths to and from the proposed mediator variable. The causal steps approach also requires a significant c path (i.e., a significant bivariate relationship between the predictor and outcome variable—the total effect). However, this requirement has routinely been viewed as superfluous, and current statistical thinking recommends that a significant total effect not be required to test mediational models (43, 45). The product of coefficients approach (46) also directly tests the existence of mediation, with one simple comparison; however, assumptions of the test (i.e., normal distribution) are rarely met (47). Thus, preference is generally given to the monte carol method of assessing mediation when conducting mediation due to assumptions of normality not being required. In a simulation study, the monte carlo method was superior to the product of coefficients approach (42). In the current study, the monte carlo method was employed via software created by Selig and Preacher (48).
Finally, κ2 was calculated for each indirect effect. As an estimate of effect size, κ2 is the magnitude of the indirect effect relative to the maximum possible indirect effect (49). Similar to other measures of effect size (i.e., r2), κ2 is bounded between 0 and 1, and is interpreted with the same benchmarks provided by Cohen (50); small, medium, and large effect sizes as .01, .09, and .25 (49). The MBESS package for R was utilized to calculate κ2 (51–53).
Results
Preliminary analyses
Participants and demographics
The current analyses focused on 860 HIV-infected MSM, who met inclusion criteria (noted above). The mean age of the sample was 43.8 years (SD = 9.9), and the racial/ethnic make-up was: 79.7% White, 10.5% Black, 2.3% Asian, and 7.5% other; 28.4% identified as Hispanic. The mean viral load(log10) at baseline was 2.37 (SD = 1.17), and 574 (67%) were virologically suppressed (see Table 1). Demographic characteristics were examined by predictor, mediator and outcomes variables. Participant age significantly positively correlated (p < 0.05) with physical inactivity and negatively correlated with viral load, while CD4 count significantly negatively correlated with ART non-adherence and viral load; thus, in all subsequent analyses, age and CD4 count were controlled (see Table 2).
Table 1.
Baseline Sociodemographic Characteristics of Participants
| Variable | N | % |
|---|---|---|
| Virologically Suppressed | 574 | 67 |
| Race | ||
| African American/Black | 95 | 10 |
| White | 685 | 80 |
| Asian | 17 | 2 |
| Other | 69 | 8 |
| Ethnicity | ||
| Hispanic or Latino | 241 | 28 |
|
| ||
| M | SD | |
|
| ||
| Age | 43.8 | 9.9 |
| Viral Load(log10) | 2.37 | 1.2 |
| CD4 | 488.4 | 272.6 |
| ART Non-Adherence (z score) | −.01 | .98 |
| Physical Inactivity (z score) | −.02 | .88 |
| Depression | 15.5 | 6.4 |
Table 2.
Correlations among Study Variables at Baseline
| Variable | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|
| 1. ART Non-Adherence (z score) | 1 | |||||
| 2. Physical Inactivity (z score) | .09* | 1 | ||||
| 3. Depression | −.007 | .34** | 1 | |||
| 4. CD4 | −.10** | −.002 | −.03 | 1 | ||
| 5. Viral Load(log10) | .13** | .01 | .09** | −.35** | 1 | |
| 6. Age | −.06 | .08* | −.01 | .03 | −.21** | 1 |
Note.
p < .05,
p < .01.
Prospective analyses
Changes in each variable over time
Before mediational analyses were conducted, unconstrained mixed-effects models were analyzed, for each study variable independently, to assess for temporal changes over the course of the study period. Results indicated that depression, γ = −.73, SE = .29, 95% CI [−1.3, −.14], t(188.9) = −2.5, p = .02, and viral load, γ = −.25, SE = .06, 95% CI [−.37, −.14], t(413.9) = −4.3, p < .0001, declined over time. Conversely, ART non-adherence, γ = −.03, SE = .06, 95% CI [−.13, .08], t(543.1) = −.47, p = .64, and physical inactivity, γ = .05, SE = .04, 95% CI [−.03, .13], t(199.1) = 1.2, p = .25, remained stable over time.
Tests of main hypotheses
The relationships between physical inactivity and depression, γ = 2.5, SE = .21, 95% CI [2.1, 2.9], t(1050.1) = 11.6, p < .0001, depression and ART non-adherence, γ = .03, SE = .005, 95% CI [.02, .04], t(900.9) = 5.3, p < .0001, depression and non-adherence (controlling for physical inactivity), γ = .03, SE = .005, 95% CI [.01, .04], t(884.1) = 4.6, p < .0001, and the total effect of physical inactivity on non-adherence, γ = .10, SE = .04, 95% CI [.03, .17], t(879.1) = 2.6, p = .009, were significant.
When depression was controlled for, the direct effect of physical inactivity to non-adherence became non-significant, γ = .04, SE = .04, 95% CI [−.04, .12], t(882.7) = 1.0, p = .30. Further, ART non-adherence significantly predicted viral load (while controlling for depression), γ = .08, SE = .03, 95% CI [.02, .14], t(880.4) = 2.7, p = .008, and while controlling for physical inactivity, γ = .09, SE = .03, 95% CI [.04, .15], t(865.4) = 3.2, p = .002. The total effect of depression to viral load was also significant, γ = .02, SE = .005, 95% CI [.008, .03], t(1098.3) = 3.5, p < .0001; however, when non-adherence was controlled for, the direct effect of depression to viral load became non-significant, γ = .009, SE = .005, 95% CI [−.0003, .02], t(869.2) = 1.9, p = .057. Conversely, when physical inactivity was controlled for, the effect of depression to viral remained significant, γ = .02, SE = .005, 95% CI [.009, .03], t(1049.9) = 3.5, p < .0001. Additionally, the total effect of physical inactivity on viral load emerged as non-significant, γ = .02, SE = .04, 95% CI [−.05, .10], t(1030.5) = .61, p = .54., and when ART non-adherence was controlled for, the direct effect of physical inactivity on viral load reduced and remained non-significant, γ = .015, SE = .03, 95% CI [−.05, .08], t(855.9) = .47, p = .64. It should also be noted that analogous models were analyzed with baseline values of physical inactivity, and the results were unchanged.
To quantify the mediational pathways, four indirect effects were tested, all of which emerged significantly. Additionally, the κ2 statistic was calculated for all indirect effects as a measure of effect size. See Table 3 for details.
Table 3.
Indirect effects and effect size estimates
| Indirect Effect | Point Estimate | 95% C.I. | κ2 |
|---|---|---|---|
| PI-Dep-Adh | .075 | .03, .13 | .051 |
| Dep-Adh-VL | .002 | .0006, .004 | .017 |
| PI-Dep-VL | .05 | .02, .08 | .035 |
| PI-Adh-VL | .009 | .001, .02 | .014 |
Note. PI = physical inactivity; Dep = depression; Adh = ART non-adherence; VL = viral load(log10). C.I. = confidence intervals. Small effect: κ2 = .01, medium effect: κ2 = .09, large effect: κ2 = .25
Discussion
Prior to the present study, there have been mixed findings regarding the role of physical activity on ART non-adherence and HIV disease progression (11, 14, 54); and the mechanisms driving bivariate associations found in prior research had yet to be studied. Additionally, these effects had not been examined longitudinally. Findings from the current longitudinal meditational analyses suggest that decreased physical activity is associated with worse depression and poor ART adherence, thereby leading to decreased virological suppression (see Figure 1 and Table 3).
Hypothesis 1 proposed that the path of physical inactivity to ART non-adherence would be mediated by depression. Although previous research has found significant negative associations between physical activity and depression, (6) as well as negative relationships between depression and ART adherence, (23) this was the first study to examine a meditational model of these variables. Thus, increased physical activity leads to increased ART adherence through the mechanism of reducing depressive symptomatology. It should also be noted that this indirect effect represented a small to medium effect size, indicating that there are additional comediators in this relationship, and that depression accounts for only a modest amount of variance.
Hypothesis 2, which stated that the relationship between physical inactivity and viral load would be mediated by depression, was also supported. Past research has revealed mixed findings regarding the association between physical activity and viral load (11, 14); however, to date, no known studies have examined potential mechanisms of this relationship. The findings from the current study indicated that increased physical activity predicted lower viral load through the mechanism of decreased depression. Hypothesis 3 proposed that ART non-adherence may also mediate this relationship, and indeed, results supported this proposal. Here too, the magnitude of the effects must be considered given that small to medium effect sizes emerged, suggesting that additional sources of variance in this relationship should continue to be explored.
Hypothesis 4 was developed to further understand the significant indirect effect found in hypothesis 2 (i.e., depression mediated the relationship between physical inactivity and viral load), and proposed that the relationship between depression and viral load would be significantly mediated by ART non-adherence. The results revealed that decreases in depression predicted decreased viral load through the mechanism of increased ART adherence; however, unmeasured biological variables, such as cortisol and norepinephrine may also play important roles in this association (22).
Despite the novel aspects of the current study, it is not without limitations. Most notably was the subjective assessment of physical inactivity and ART non-adherence. Empirical evidence suggests that assessing ART adherence via self-report can be a valid measurement method, (39) especially when not part of an adherence intervention trial per se. For instance, self-report adherence is highly correlated with unannounced pill counts, viral load, and pharmacy refill data. However, future research in this area may be enhanced by the use of electronic monitoring of medication utilization and pharmacological assessments (55). Objective measures of ART adherence would likely reduce concerns regarding social desirable responding, as self-report adherence items may be biased in the direction of greater adherence. Similarly, physical inactivity was also assessed subjectively. Additional research may benefit from including objective measures of physical activity, such as pedometers and accelerometers (56). Further, the sample was exclusively MSM by design, and largely White, thus inferences to heterosexual, female, racial/ethnic minority, and/or international HIV-infected individuals should be done with caution. Examining these relationships in other diverse HIV-infected groups is an important future direction. Lastly, it should be noted that although this longitudinal model builds upon cross-sectional designs, causal sequencing cannot be inferred. To truly determine the temporal ordering of the variables of interest, experimental manipulation of the independent variable and secondary manipulation of the mediator(s) are needed (57).
The findings from the current study have the potential to inform clinical practice. For instance, low levels of physical activity are linked with a host of poor health outcomes, including cardiovascular disease, diabetes, cancer, hypertension, obesity, and osteoporosis (58–59). Although this study focused exclusively on HIV disease management, current findings could easily be applicable to the management of these other chronic conditions and should be consideration for future work. In HIV specifically, low physical activity has been associated with depression, (6) and increased depression is related to poor ART adherence (23) and disease progression (24). Given that the association of physical inactivity to ART non-adherence and viral load is mediated, at least in part, by depression, perhaps integrative interventions which address physical inactivity, depression, and ART non-adherence could be particularly cost-effective and efficient to improve health outcomes for this population. Indeed, psychological interventions have shown to have a powerful impact on viral load and other immunologic outcomes (16–17). More specifically, integrative cognitive behavioral treatments for ART non-adherence and depression have proved to be efficacious at increasing adherence and reducing depression (60–61). Cognitive behavioral stress management interventions also have demonstrated significant effects on HIV immune markers, including CD4 count and viral load (62, 16). Similarly, stand-alone physical activity interventions are effective for improving not only physical, but also, mental health, including depression, (63) and in HIV specifically, aerobic exercise training has been shown to be associated with favorable psychological outcomes and improved immunologic functioning (15).
One intervention approach that may be particularly useful given the current findings is behavioral activation (64–66) which is a brief, empirically supported intervention for depression, which aims to increase engagement in value-driven activities to increase feelings of reward, enjoyment, and mastery. Although behavioral activation does not target increases in physical activity per se, it does provide a framework for increasing engagement in meaningful activities more broadly; in future trials, physical activity could be used as the primary treatment target, and further, increasing other forms of activation may also serve to increase physical activity indirectly. Additionally, behavioral activation has been considered useful for integration with other treatment approaches, (67–68) and has been integrated with physical activity and nutrition counseling, (69) as well as an intervention for ART adherence specifically (70). To date, we are unaware of any interventions that integrate treatment to increase physical activity and treat depression in order to increase ART adherence among HIV-infected individuals; however, behavioral activation may be a useful platform for an integrated treatment approach to facilitate increases in physical activity and ART adherence and reductions in depressive symptoms. With the need to disseminate evidenced-based interventions, promoting exercise as part of a treatment program for individuals with HIV and mood difficulties is worthy of further investigation.
Acknowledgments
Data collection and infrastructure support for this research is from the CFAR Network of Integrated Clinical Systems-CNICS, an NIH funded program (R24AI067039) that was made possible by the National Institute of Allergy and Infectious Diseases (NIAID). Some investigator time for analysis and authorship was supported by the National Institutes of Health [K24 MH094214 to S.A.S.], [R01MH084759 to H.C.], [K23MH096647 to A.J.B.], and the Harvard University Center for AIDS Research/National Institutes of Health [5P30AI060354-08 to A.J.B.].
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
References
- 1.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circ. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 2.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 3.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50:499–505. doi: 10.1097/QAI.0b013e31819c291b. [DOI] [PubMed] [Google Scholar]
- 4.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 5.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ciccolo JT, Jowers EM, Bartholomew JB. The benefits of exercise training for quality of life in HIV/AIDS in the post-HAART era. Sports Med. 2004;34:487–499. doi: 10.2165/00007256-200434080-00001. [DOI] [PubMed] [Google Scholar]
- 7.Dudgeon WD, Phillips KD, Bopp CM, Hand GA. Physiological and psychological effects of exercise interventions in HIV disease. AIDS Patient Care STDS. 2004;18:81–98. doi: 10.1089/108729104322802515. [DOI] [PubMed] [Google Scholar]
- 8.Neidig JL, Smith BA, Brashers DE. Aerobic exercise training for depressive symptom management in adults living with HIV infection. J Assoc Nurses AIDS Care. 2003;14:30–40. doi: 10.1177/1055329002250992. [DOI] [PubMed] [Google Scholar]
- 9.Wagner G, Rabkin J, Rabkin R. Exercise as a mediator of psychological and nutritional effects of testosterone therapy in HIV+ men. Med Sci Sports Exerc. 1998;30:811–817. doi: 10.1097/00005768-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Rigsby LW, Dishman RK, Jackson AW, Maclean GS, Raven PB. Effects of exercise training on men seropositive for the human immunodeficiency virus-1. Med Sci Sports Exerc. 1992;24:6–12. [PubMed] [Google Scholar]
- 11.Smit E, Crespo CJ, Semba RD, et al. Physical activity in a cohort of HIV-positive and HIV-negative injection drug users. AIDS Care. 2006;18:1040–1045. doi: 10.1080/09540120600580926. [DOI] [PubMed] [Google Scholar]
- 12.Smith BA, Neidig JL, Nickel JT, Mitchell GL, Para MF, Fass RJ. Aerobic exercise: Effects on parameters related to fatigue, dyspnea, weight and body composition in HIV-infected adults. AIDS. 2001;15:693–701. doi: 10.1097/00002030-200104130-00004. [DOI] [PubMed] [Google Scholar]
- 13.Stringer WW, Berezovskaya M, O’Brien WA, Beck CK, Casaburi R. The effect of exercise training on aerobic fitness, immune indices, and quality of life in HIV+ patients. Med Sci Sports Exerc. 1998;30:11–16. doi: 10.1097/00005768-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Bopp CM, Phillips KD, Fulk LJ, Dudgeon WD, Sowell R, Hand GA. Physical activity and immunity in HIV-infected individuals. AIDS Care. 2004;16:387–393. doi: 10.1080/09540120410001665385. [DOI] [PubMed] [Google Scholar]
- 15.LaPerriere AR, Antoni MH, Schneiderman N, et al. Exercise intervention attenuates emotional distress and natural killer cell decrements following notification of positive serologic status for HIV-1. Appl Psychophysiol Biofeedback. 1990;15:229–224. doi: 10.1007/BF01011107. [DOI] [PubMed] [Google Scholar]
- 16.Antoni MH, Carrico AW, Durán RE, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-positive gay men treated with HAART. Ann Behav Med. 2006;31:155–164. doi: 10.1207/s15324796abm3102_7. [DOI] [PubMed] [Google Scholar]
- 17.Carrico AW, Antoni MH. Effects of psychological interventions on neuroendocrine hormone regulation and immune status in HIV-positive persons: A review of randomized controlled trials. Psychosom Med. 2008:557–584. doi: 10.1097/PSY.0b013e31817a5d30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) [Accessed Aug 20 2012];CDC analysis provides new look at disproportionate impact of HIV and syphilis among U.S. gay and bisexual men. Available at http://www.cdc.gov/nchhstp/Newsroom/msmpressrelease.html.
- 19.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: A prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosioni J, Calmy A, Hirschel B. HIV treatment for prevention. J Int AIDS Soc. 2011;14:28. doi: 10.1186/1758-2652-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25:1113–1118. doi: 10.1097/QAD.0b013e3283463f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook JA, Cohen MH, Grey D, et al. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002;92:82–87. doi: 10.2105/ajph.92.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 27.Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 29.Patterson T, Shaw W, Semple S, Cherner M. Relationship of psychosocial factors to HIV disease progression. Ann Behav Med. 1996;18:30–39. doi: 10.1007/BF02903937. [DOI] [PubMed] [Google Scholar]
- 30.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 31.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: The Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: The first 100 patients. Curr HIV Res. 2007;5:109–118. doi: 10.2174/157016207779316369. [DOI] [PubMed] [Google Scholar]
- 33.Fredericksen R, Crane PK, Tufano J, et al. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. J Acquir Immune Defic Syndr. 2012;4:47–55. doi: 10.5897/jahr11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence ST, Willig JH, Crane HM, et al. Routine, self-administered, touch-screen, computer-based suicidal ideation assessment linked to automated response team notification in an HIV primary care setting. Clin Infect Dis. 2010;50:1165–1173. doi: 10.1086/651420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Jacobs DR, Jr, Leon AS. Validity and reliability of self-reported physical activity status: The Lipid Research Clinics questionnaire. Med Sci Sports Exerc. 1993;25:92–98. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 38.Feldman BJ, Fredericksen RJ, Crane PK, et al. Evaluation of the single-item rating adherence scale in routine clinical care of people living with HIV. AIDS Behav. doi: 10.1007/s10461-012-0326-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval Health Prof. 2009;32:207–228. doi: 10.1177/0163278709338554. [DOI] [PubMed] [Google Scholar]
- 41.Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: New procedures and recommendations. Psychol Methods. 2006;11:142–163. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- 42.Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 44.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 45.Hayes A. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- 46.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. Washington, DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- 47.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 48.Selig JP, Preacher KJ. [Accessed Aug 20 2012];Monte Carlo method for assessing mediation: An interactive tool for creating confidence intervals for indirect effects. Available at http://www.quantpsy.org/medmc/medmc.htm.
- 49.Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communication indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- 51.Kelley K. Confidence intervals for standardized effect sizes: Theory, application, and implementation. J Stat Softw. 2007;20:1–24. [Google Scholar]
- 52.Kelley K. Methods for the behavioral, educational, and social sciences: An R package. Behav Res Methods. 2007;39:979–984. doi: 10.3758/bf03192993. [DOI] [PubMed] [Google Scholar]
- 53.Kelley K, Lai K. MBESS (Version 3.2.0) Computer software and manual. Available at http://www.cran.r-project.org/
- 54.Ogalha C, Luz E, Sampaio E, et al. A randomized, clinical trial to evaluate the impact of regular physical activity on the quality of life, body morphology and metabolic parameters of patients with AIDS in Salvador, Brazil. J Acquir Immune Defic Syndr. 2011;57:S179–S185. doi: 10.1097/QAI.0b013e31821e9bca. [DOI] [PubMed] [Google Scholar]
- 55.Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: Practical considerations. AIDS Behav. 2005;9:103–110. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- 56.Santos-Lozano A, Garatachea N. Physical activity measurements using accelerometers and pedometers in HIV-infected people. J AIDS Clinic Res. 2011;2:126. [Google Scholar]
- 57.Spencer SJ, Zanna MP, Fong GT. Establishing a causal chain: Why experiments are often more effective than mediational analyses in examining psychological processes. J Pers Soc Psychol. 2005;89.6:845–851. doi: 10.1037/0022-3514.89.6.845. [DOI] [PubMed] [Google Scholar]
- 58.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 59.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. J Consult Clin Psychol. 2012;80:404–415. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antoni MH, Baggett L, Ironson G, et al. Cognitive-behavioral stress management intervention buffers distress responses and immunologic changes following notifications of HIV-1 seropositivity. J Consult Clin Psychol. 1991;59:906–915. doi: 10.1037//0022-006x.59.6.906. [DOI] [PubMed] [Google Scholar]
- 63.Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: Returning to contextual roots. Clin Psychol. 2001;8:255–270. [Google Scholar]
- 65.Lejuez CW, Hopko DR, LePage J, Hopko S, McNeil D. A brief behavioral activation treatment for depression. Cogn Behav Pract. 2001;8:164–175. [Google Scholar]
- 66.Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the Brief Behavioral Activation Treatment for Depression: Revised treatment manual. Behav Modif. 2011;35:111–161. doi: 10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- 67.Mazzuchelli T, Kare R, Rees C. Behavioral activation treatments for depression in adults: A meta-analysis and review. Clin Psychol. 2009;16:383–411. [Google Scholar]
- 68.Sturmey P. Behavioral activation is an evidence-based treatment for depression. Behav Modif. 2009;33:818–829. doi: 10.1177/0145445509350094. [DOI] [PubMed] [Google Scholar]
- 69.Pagoto S, Bodenlos JS, Schneider KL, Olendzki B, Spates CR, Ma Y. Initial investigation of behavioral activation therapy for comorbid major depressive disorder and obesity. Psychotherapy. 2008;45:410–415. doi: 10.1037/a0013313. [DOI] [PubMed] [Google Scholar]
- 70.Daughters SB, Magidson JF, Schuster RM, Safren SA. Act Healthy: A combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cogn Behav Pract. 2010;17:309–321. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


