Abstract
Obesity in older adults is ubiquitous in many developed countries and is related to various negative health outcomes, making it an important public health target for intervention. However, treatment approaches for obesity in older adults remain controversial due to concerns surrounding the difficulty of behavior change with advancing age, exacerbating the age-related loss of skeletal muscle and bone, and the feasibility of long-term weight maintenance and related health consequences. This review serves to systematically examine the evidence regarding weight loss interventions with a focus on obese (body mass index 30 kg/m2 and above) older adults (aged 65 years and older) and some proposed mechanisms associated with exercise and caloric restriction (lifestyle intervention). Our findings indicate that healthy weight loss in this age group can be achieved through lifestyle interventions of up to a one-year period. Most interventions reviewed reported a loss of lean body mass and bone mineral density with weight loss. Paradoxically muscle quality and physical function improved. Inflammatory molecules and metabolic markers also improved, although the independent and additive effects of exercise and weight loss on these pathways are poorly understood. Using our review inclusion criteria, only one small pilot study investigating long-term weight maintenance and associated health implications was found in the literature. Future research on lifestyle interventions for obese older adults should address the loss of bone and lean body mass, inflammatory mechanisms, and include sufficient follow up to assess long-term weight maintenance and health outcomes.
Keywords: older adults, obesity, weight loss, lifestyle intervention, weight maintenance
1. Introduction
Obesity in older adults is prevalent in many parts of the world and associated with a sequel of poor health outcomes. The prevalence of obesity has markedly increased in the elderly as more baby boomers become senior citizens (Flegal 2010). During the past 30 years, the proportion of obese older adults has doubled, and their prevalence in 2010 was estimated at 37.45% (Patterson 2004). This reflects both an increase in the total number of older persons and in the percentage of the older population that are obese (Villareal 2005). It also represents a significant increase from the 22.2% obese older adults reported in the 1988–1994 National Health and Examination Survey (NHANES) (Federal Interagency Forum on Aging-Related Statistics 2010). Currently, the per capita spending on obesity-attributable conditions are greater for Medicare recipients than for younger age groups (Finkelstein 2009). No doubt, the growing number of obese older adults in the population will present public health challenges unless actions are taken to reverse this trend.
Losing weight is difficult, and interventions that work in younger adults cannot be assumed to translate to older populations with co-morbidities, low muscle mass and frailty (Villareal 2004). The appropriate treatment approach for obesity remains highly contentious due to the lack of evidenced-based data demonstrating that long-term weight loss is net beneficial or harmful in this age group. There is evidence that successful weight loss is possible in adults 65 years and older (Villareal 2006a; Villareal 2006b; Villareal 2008; Frimel 2008; Lambert 2008; Shah 2009; Villareal 2011a; Armamento-Villareal 2012; Shah 2011; Kelly 2011). However, weight-loss trials have reported losses of lean body mass and bone mineral density, in addition to fat mass (Villareal 2006a; Villareal 2006b; Villareal 2008; Frimel 2008; Lambert 2008; Shah 2009; Villareal 2011a; Armamento-Villareal 2012; Shah 2011; Kelly 2011; Bales 2008). These negative outcomes discourage many geriatricians from advising weight loss to their obese older patients (Heiat 2001; Rossner 2001; Sorensen 2003; Villareal 2005; Zamboni 2005; Rolland 2006; Morley 2010), despite improvements in body composition, physical function, metabolic and cardiovascular parameters that accompany weight loss (Forsythe 2008; Anandacoomarasamy 2009; Cheung 2012; Erteck 2012). Given these positive functional and metabolic outcomes, it is somewhat surprising that advising weight loss in obese older adults is still shunned in the medical community (Houston 2009; Sommers 2011). Compounding the confusion surrounding risks versus benefits from intentional weight loss is the lack of human studies to elucidate the mechanisms associated with the loss of muscle and bone. Also lacking are trials with adequate follow-up to assess the behaviors associated with long-term maintenance of weight loss and health outcomes related to sustained weight loss.
In order to address these in a systematic review, we posed the research question: “Is there evidence that weight loss is achievable, safe, and maintainable in obese adults aged 65 years and older?” We hypothesized that weight loss would be achievable and safe despite some loss of lean body mass and bone. We also hypothesized that weight loss could be maintained in the long-term. Our primary aim was to systematically review the evidence on weight loss interventions in obese older adults, with a specific focus on changes in body composition, metabolic markers, and physical function, and also mechanisms associated with intentional weight loss through caloric restriction, exercise or both. We applied rigid criteria for defining older adults (≥65 years) and obesity (BMI ≥ 30 kg/m2) based on the position statement of the American Society of Nutrition and Obesity Society (Villareal 2005), and only included randomized controlled trials that used direct and precise methods for measuring body composition.
2. Literature Search Methods
A rigorous inclusion criterion as described above was employed. Only randomized controlled trials with a minimum weight loss intervention of three months, and body composition measured by DXA, MRI, CT, or hydrostatic weighing were included. Studies which targeted specific chronic diseases or conditions (e.g. diabetes mellitus, osteoarthritis), were excluded.
2.1 Data source
An electronic database search was conducted on MEDLINE and PubMed (both clinical and general) for English language articles, with no cutoff dates. Searches were conducted on 20, 23 and 26–27 January 2012, and again on 18 April, 24 May 2012 and 2 July 2012 to capture newly published material. Two broad search areas were categorized: (1) weight loss through caloric restriction, exercise or both; and (2) long-term maintenance of weight loss, feasibility and safety among older adults. In order to cast the widest net for these two areas of interest, five separate overlapping searches were performed, using the keywords: obese, obesity, older adults, elderly, weight loss, body composition, caloric restriction, lifestyle intervention, diet, exercise, function, long-term feasibility, maintenance, and safety.
2.2 Data synthesis
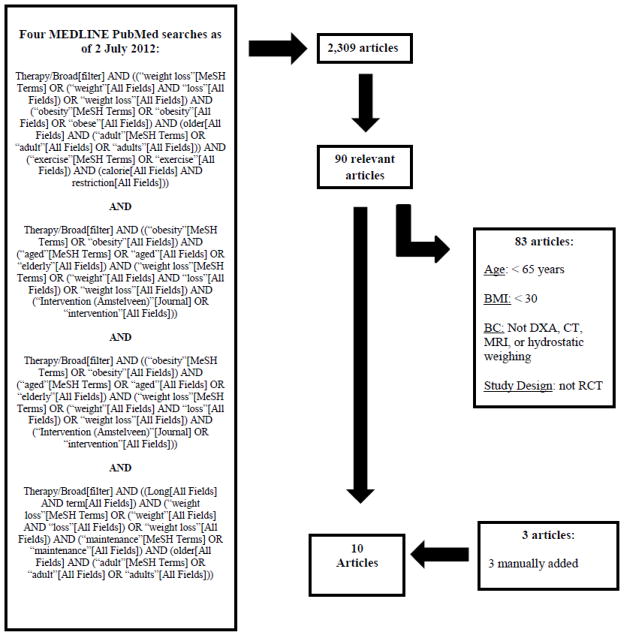
A total of 2,309 prospective articles were initially identified. After removing duplicates and irrelevant studies, 90 articles were retained. Of these 90 articles, 83 were excluded for not meeting the inclusion criteria outlined previously. Three articles were manually added. The selection of articles was agreed upon by two authors (DLW and DTV). The final analysis yielded a total of ten articles meeting all established criteria (Figure 1). These articles are listed in Table 1. They are not ordered chronologically, but instead grouped by similarities between study design and intervention, for ease of discussion. Only one small pilot study was found under the category feasibility/maintenance of long-term weight loss in older adults that satisfied our study selection criteria. This study is not included in Table 1, but is discussed under the subheading 3.2 Feasibility and Long-term Maintenance of Weight Loss, in the Discussion of the Systematic Review section.
Figure 1.
Schematic of the systematic selection process to identify relevant studies (Abbreviations: BC= body composition; DXA = dual energy x-ray absorptiometry; CT= computed tomography; MRI=magnetic resonance imaging; RCT = randomised control trial)
Table 1.
Weight loss interventions in obese adults: 65 years of age and older
| Reference | Health Outcomes (primary/secondary) | Study Design & Intervention | Sample/Follow up Measures | Summary of Findings | |
|---|---|---|---|---|---|
| Villareal et al. (2006a) | Physical function/Body composition | RCT: 6 months 2 groups: control (C); wt loss plus exercise (WL+Ex) |
n=27 women & men Age ≥ 65 years BMI ≥ 33 Follow up: 6 months |
DXA, Physical Performance Tests, VO2 peak | Body weight (−8.4% ± 5.6% WL+Ex vs + 0.5% ± 2.8% C†). Fat mass (−6.6 ± 3.4 WL+Ex vs +1.7 ± 4.1 kg C†). Fat free mass (−1.2 ± 2.1 WL+Ex vs −1.0 ± 3.5 kg C). Change in Physical Performance Test score (2.6 ± 2.5 WL+Ex vs 0.1 ± 1.0 C†), peak oxygen consumption (1.7 ± 1.6 WL+Ex vs 0.3 ± 1.1 mL/min per kg C*). |
| Villareal et al. (2006b) | Metabolic coronary heart disease/Body composition | See Villareal, et al. 2006a | See Villareal, et al. 2006a | DXA, serum FFA, C-reactive protein, IL-6 | Body weight, fat mass and fat free mass – see Villareal 2006a. Metabolic syndrome (−59% WL+Ex vs 0%*). Serum FFA (−99 WL+Ex vs +10 mol/L*) C-reactive protein (−2.5 WL+Ex vs 0.8 mg/L) and IL-6 (1.6 WL+Ex and −2.4 vs 1.6 pg/mL*. |
| Villareal et al. (2008) | Bone metabolism/Body composition | RCT: 1 year 2 groups: diet + exercise (D+Ex); control (C) | See Villareal et al. 2006a | DXA, insulin growth factor-1 (IGF-1), cortisol, parathyroid hormone (PTH), osteocalcin and bone-specific alkaline, bone mineral density (BMD), phosphatase, leptin | Body weight (−10 ± 2% D+Ex vs +1 ± 1% C†). Total hip BMD (0.1 ± 2.1 D+Ex vs. −2.4 ± 2.5% C), trochanter (0.2 ± 3.3 vs. −3.3 ± 3.1% C), and intertrochanter (0.3 ± 2.7 vs. −2.7 ± .3.0% C). |
| Frimel et al. (2008) | Body composition | RCT: 6 months 3 groups: control (C); diet + behaviour therapy (D+BT); diet + behaviour therapy + PRT (D+BT+PRT) |
n=30 frail women & men Age ≥ 65 years BMI ≥ 34 Follow up: 6 months |
DXA with focus on upper extremity (UE) and lower extremity (LE). | Body weight in C and D+BT+PRT (−10.7 ± 4.5 vs −9.7 ± 4.0 kg*) and fat mass (−6.8 ± 3.7 vs −7.7 ± 2.9 kg). D+BT+PRT lost less fat free mass (−1.8 ± 1.5 vs −3.5 ± 2.1 kg), D+BT+PRT greater increase % weight as fat free mass than D+BT (+7.9 ± 3.3 vs +5.4 ± 3.7%†). Lean mass in UE was retained in all groups. Not LE. |
| Lambert et al. (2008) | Inflammation/Body composition | RCT: 12 weeks 2 groups: exercise (Ex); wt loss (WL) |
n=16 frail women & men Age ≥ 65 years BMI ≥ 36 Follow up: 12 weeks |
DXA, vastus lateralis biopsy for total mRNA, serum IL-6, TNF-α, TLR-4 and MGF | Body weight (−7.5 ± 1.2 kg WL† vs −0.3 ± 0.8 kg Ex). Fat mass (−2.9 ± 0.6 kg WL† vs 1.6 ± 0.6 kg, Ex*). TLR-4 mRNA −37% in Ex* with no change in WL. IL-6 and TNF α mRNA −50% in Ex* with no change in WL. |
| Shah et al. (2009) | Metabolism/Body composition | RCT: 6 months 2 groups: diet (D); diet + exercise (D+Ex) |
n=18 women & men Age ≥ 65 years BMI ≥ 30 Follow up: 6 months |
DXA, VO2 peak, intra hepatic fat (IHF), oral glucose tolerance test; plasma glucose and insulin. | Body weight (−9 ± 1% D vs −10 ± 2% D+E) and fat mass (−13 ± 3% D vs −16 ± 3% D+Ex). VO2peak (–0 ± 1% D vs 9 ± 2% D + E). IHF (−46 ± 11% D vs −45 ± 8% D+Ex†), insulin sensitivity (66 ± 25% D, 68 ± 28% D+Ex†). |
|
| |||||
| Villareal et al. (2011a) | Physical function/Body composition | RTC: 1 year 3 groups: control (C); diet (D); exercise (Ex); diet + exercise (D+Ex) |
n=107 women & men Age ≥ 65 years BMI ≥ 30 Follow up: 6 and 12 months |
DXA, Physical Performance Tests. | Body weight (−10% D and −9% D+Ex, no change Ex or C†). Lean mass and hip BMD (−3% and −1% D+Ex, vs −5% and −3% D†). Physical Performance Test (D+Ex +21% vs +12% D vs +15% Ex†). |
| Armamento-Villareal et al. (2012) | Bone metabolism/Body composition | See Villareal, et al. 2011a | See Villareal, et al. 2011a | DXA, serum sclerostin, hip geometry | Body weight (−9.6% D vs −9.4% D+Ex; weight stable in Ex and C). Lean mass declined less in D+Ex group (mean±SE; −3.2% ± 0.5%) than in D group (−5.3% ± 0.7%), but increased in Ex group (2.4% ± 0.5%). Sclerostin increased in D (6.6% ± 1.7% and 10.5% ± 1.9% at 6 and 12 mo, respectively*), and was unchanged in C, Ex, D+Ex. Hip geometry is discussed in the text. |
| Shah et al. (2011) | Bone metabolism/Body composition | See Villareal, et al. 2011a | See Villareal, et al. 2011a | DXA, BMD, C-terminal teleopeptide of type collagen I (CXT), osteocalcin, N-terminal propeptide of type 1 procollagen, serum estradiol, IGF-1, Vit D, serum PTH | Body weight (−9.6% D vs −9.4% C). Lean mass (mean±SD; −3.2% ± 3.1% D+Ex vs −5.3% ± 3.4% D vs 2.4% ± 2.5% Ex). Total hip BMD (D+Ex −1.1% vs D −2.6% vs, Ex +1.5%Ex†). Osteocalcin, serum CTX, serum procollagen propeptide, serum leptin, estradiol, Vit D and PTH discussed in text. |
| Kelly et al. (2011) | Inflammation/Metabolism/Body composition | RCT: 12 weeks 2 groups: low-glycemic index diet + exercise (LGI+Ex); high-glycemic index diet + exercise (HGI+Ex) |
n=28 women & men Age ≥ 65 years BMI ≥ 30 Follow up: 12 weeks |
DXA, plasma glucose, MNC isolation, plasma insulin, plasma and MNC-derived TNFa, plasma IL-6 and MCP-1 | Weight, fat mass, fat free mass and truncal fat decreased in both groups. LGI+Ex and HGI+Ex decreased BMI, fasting plasma glucose*, and insulin *. Glycemic response reduced only in LGI+Ex *. Plasma and MNC-derived TNFα, IL-6, and MCP-1 discussed in the text. |
DXA=dual energy x-ray absorptiometry; LE=lower extremity; PRT=progressive resistance training; RCT=randomised control trial; UE=upper extremity.
=P<.05,
=P<.001
3. Discussion of the Systematic Review
3.1 Randomized Controlled Trials
Table 1 summarizes the ten trials that met our inclusion criteria (Villareal 2006a; Villareal 2006b; Villareal 2008; Frimel 2008; Lambert 2008; Shah 2009; Villareal 2011a; Armamento-Villareal 2012; Shah 2011; Kelly 2011). Figure 2 is a schematic representation of the inter-relationships of the mechanisms discussed in these trials. Three papers by Villareal et al. (two in 2006 and one in 2008) reported on the same cohort of 27 participants. The participants were sedentary (≤ 2 exercise sessions per week); with stable body weight (± 2kg) during the preceding year; unchanged medications regimes for at least six months; and mild to moderate frailty as measured by the Physical Performance Test (Brown 2000). The intervention consisted of both diet and exercise (lifestyle intervention). Energy deficit was 500–700 kcal/day supplemented with a daily multivitamin and counseling to consume adequate dietary calcium and vitamin D. The goal was 10% weight loss over the six-month intervention and weight maintenance for an additional six months. Exercise sessions consisted of 90 minutes of aerobic and resistance exercises, three days per week, at a moderate intensity (~75% peak heart rate) and progressed to 80–90% of peak heart rate. Resistance exercise started at 65% of one repetition maximum (1RM) and progressed to ~80% of 1RM.
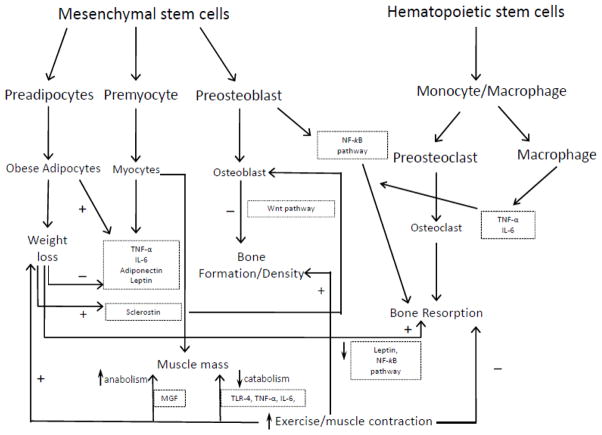
Figure 2. Schematic representation of presented pathways and proposed mechanisms.
Adipocytes, myocytes and osteoblasts are derived from mesenchymal stem cells, whereas osteoclasts and macrophages are derived from hematopoietic stem cells. Inflammatory cytokines (e.g. Interleukin 6 [IL-6] and tumor necrosis factor-alpha [TNF-α]) originate from obese adipocytes, myocytes, and macrophages.
Weight loss results in decreased inflammatory cytokines, leptin, and increased adiponectin which lead to improved metabolic profile. Exercise/muscle contraction results in decreased muscle catabolism through decreased toll-like receptor 4 (TLR-4) and inflammatory cytokines, and increased muscle anabolism through increased mechano growth factor (MGF). Exercise also exerts positive impact on bone mineral density through increased bone formation/decreased bone resorption via mechanical loading. Sclerostin increases in response to unloading by weight loss resulting in decreased bone formation through the Wnt and NF-kB pathways, an effect prevented by exercise. Thus, exercise attenuates the weight loss-induced reduction of muscle and bone mass and lifestyle therapy (weight loss + regular exercise) significantly improves physical function and metabolic profile in obese older adults.
As designed, body weight and fat mass (FM) decreased significantly in the intervention group. Fat free mass (FFM) decreased in both groups but the difference was not statistically significant. Physical performance test score, peak oxygen consumption, and functional status all significantly improved in the diet and exercise group. Increases in strength were equal to or greater than reported in earlier trials in non-obese older adults completing a similar exercise program (Binder 2002; Villareal 2003; Villareal 2004). The investigators stressed that it was not difficult to change the behavior of these older sedentary adults, showing that it was a feasible intervention, which also provided important social interactions that enhanced compliance.
In the second paper, all CVD risk factors significantly improved in the diet and exercise group (Villareal 2006b). Specific mechanisms were not proposed, but the discussion focused on medical care costs related to metabolic coronary heart disease (CHD) risk factors that were ameliorated by the intervention (Table 1). In the third paper (Villareal 2008), bone turnover was measured by type 1 collagen C-terminal telopeptide (CTX), osteocalcin, and bone-specific alkaline phosphatase. There was a marked increase in serum CTX (~100-fold) and osteocalcin (~60-fold) concentrations in response to weight loss indicating that bone resorption and formation, respectively, were stimulated. Moreover, the increases in both CTX and osteocalcin concentrations correlated with decreases in hip bone mineral density (BMD), suggesting that weight-loss induced bone loss was due to increased bone turnover, with greater stimulation of bone resorption than bone formation. However, the clinical significance of the decrease in BMD was not clear as all participants had high baseline BMD Z-scores, and none had evidence of osteoporosis following weight loss. The investigators argued that BMD was not lost in the spine, which implies that the exercises were more effective in preserving BMD at this site. Exact mechanisms for loss of BMD with weight loss are not currently elucidated, but it was suggested that weight loss decreases the mechanical stress on the hip, without negatively impacting the spine or wrist. Weight loss was also associated with a 25% reduction in serum leptin that was highly correlated with decreased hip BMD. No such relationship was found between decreasing estradiol and changes in BMD. Leptin was discussed in the context of its inhibiting action on the expression of receptor activator of nuclear factor κB (NF-κB) ligand levels (Burguera 2001) and osteoblast differentiation (Cornish 2002). Levels of insulin-like growth factor 1 (IGF-1), cortisol, and parathyroid hormone (PTH) did not change in response to weight loss, which suggests that these bone-active hormones were not involved with the loss of BMD in the hip. Vitamin D supplementation during the trial did not reach optimal serum concentrations and whether higher dose Vitamin D supplementation could have slowed bone loss, was raised by the investigators. It was also noted that bone quality was not measured and could have been positively impacted by the exercise training intervention.
Frimel et al. (2008) reported on a cohort of 30 community-living frail older adults. The participants were sedentary (≤ 2 exercise sessions per week); had stable medications and stable weight (± 2 kg over the past year); and met two out of three criteria for mild–moderate physical frailty. The intervention used was similar to the previously included Villareal studies (Villareal 2006a; Villareal 2006b; Villareal 2008) with a slightly higher daily energy deficit (750 kcal per day versus 500–700 kcal/day). The goal was 10% loss of body weight over six months. Combined aerobic and resistance exercise sessions were 90 minutes three times per week and resistance exercises focused on upper extremity (UE) and lower extremity (LE) muscle groups. The loss of lean body mass was completely prevented in the UE, but not LE. Despite LE lean body mass loss, strength improved. It was proposed that muscle quality improved due to a decrease in muscle fat infiltration and inflammation due to weight loss, as previously reported (Goodpaster 2001; Nicklas 2004). It was also suggested that retention of lean body mass in the UE but not the LE was attributed to UE muscle being more responsive to high-intensity training because these muscle groups are not used regularly for daily activities such as walking and climbing stairs.
Lambert et al. (2008) recruited 16 obese frail, older men and women, and also used a similar inclusion criteria and interventions to Villareal (2006a), although the intervention was only three months. A vastus lateralis biopsy was used to quantify mRNA expression of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), Toll-like receptor 4 (TLR-4) and mechano growth factor (MGF). Serum IL-6 and TNF-α were also obtained. Body weight and FFM significantly decreased in the weight loss group, while FFM increased in the exercise group. Toll-like receptor 4 mRNA significantly decreased in the exercise group, but did not change in the weight loss group. IL-6 and TNF-α mRNA decreased and MGF mRNA increased in the exercise group, but not in the weight loss group. It was concluded that cytokine gene expression appeared to be derived from muscle, as serum concentrations of TNF-α and IL-6 did not change with exercise. The investigators proposed that exercising muscle contraction decreased muscle inflammatory cytokine expression, whereas weight loss had no effect. The proposed mechanism was down regulation of TLR-4 mRNA (Flynn 2006). The study investigators also suggested that the lack of a significant effect of weight loss on TNFα-mRNA may indicate that a threshold of weight loss is needed. They concluded that exercise, but not weight loss, down regulates mRNA expression of TLR-4, TNF-α and IL-6, which is related to increased muscle catabolism, while up-regulating mRNA expression of MGF in skeletal muscle.
Shah et al. (2009) recruited 18 obese older adults. The participants were sedentary (≤ 2 exercise sessions per week), and were weight and medication stable. The intervention energy deficit was 500–1000 kcal per day, with three exercise sessions per week progressing to moderate intensity (~85% of peak heart rate). Intra hepatic fat (IHF) content was measured by Occipital Proton Magnetic Resonance Spectroscopy (1H-MRS). There was a 50% reduction in IHF with 9% weight loss that was consistent with findings in younger subjects (Petersen 2005; Sato 2007). The investigators reported that the liver appeared to readily mobilize intrahepatic triglycerides in response to negative energy balance. However, exercise training plus diet did not have an additive effect, consistent with previous reports (Tamura 2005; Larson-Meyer 2006).
Three papers (Villareal 2011a; Armamento-Villareal 2012; Shah 2011) reported on a cohort of 107 frail obese adults using similar inclusion criteria and interventions to their earlier trials. In Villareal 2011a, weight loss plus exercise improved physical function and ameliorated frailty more than either weight loss or exercise alone, and sarcopenic-obesity was reduced in all intervention groups. However, there was a loss of both lean body mass and hip BMD. These loses were attenuated by the addition of exercise but not stopped. It is currently unknown what additional intervention(s) are needed to totally mitigate these loses. The investigators suggested higher doses of calcium and vitamin D, or performing only aerobic or resistance exercise individually, or including anti-resorptive therapy during active weight loss might be effective in mitigating the losses of BMD and LBM during active weight loss.
Hip geometry and serum sclerostin were the focus of the second paper (Armamento-Villareal 2012). Sclerostin is an inhibitor of bone formation and increases in states of unloading. It may mediate the changes in bone metabolism associated with weight loss and exercise by increased sclerostin production by the mechanostat in osteocytes. Sclerostin then inhibits signaling through the canonical Wnt pathway that results in an inhibition of osteoblastic differentiation, inhibiting bone formation. The resultant skeletal loading from exercise training increases BMD and improves bone geometry, and when added to caloric restriction, inhibits the weight-loss induced increase in sclerostin. This results in the attenuation of bone loss and preservation of bone geometry. The study investigators hypothesized a reduction in sclerostin with weight loss, but found no change. They suggested a floor effect of mechanical loading on the osteocyte’s response due to chronic overload in obese subjects. Additionally, the significant correlations between sclerostin and hip geometry parameters indicated that sclerostin may mediate the degradation in bone quality from unloading during weight loss, which is preserved with the addition of exercise.
The third paper, Shah et al. (2011), focused on bone loss measuring C-terminal telopeptide of type I collagen (CTX) as a marker of bone resorption, and osteocalcin and N-terminal propeptide of type I procollagen as a markers of bone formation. Bone-active hormones, serum estradiol, IGF-1, 25-hydroxyvitamin D, and serum PTH concentration were also obtained. Serum C-terminal telopeptide (CTX) and osteocalcin increased in the diet group, with bone resorption increasing more than bone formation. Both of these markers decreased in the exercise and control groups. Osteocalcin did not change with the combination of diet and exercise. Serum leptin and estradiol concentrations decreased more markedly in diet plus exercise than in diet alone (−38% and −13%, respectively). It was suggested that the decrease in leptin with weight loss could stimulate the receptor activator of NF-κB ligand and the receptor activator of NF-κB pathway leading to increased bone resorption and bone loss (Burguera 2001; Thomas 2002). There was no decrease in IGF-1 with weight loss and this was attributed to adequate protein intake during weight loss. Change in mechanical stress was cited as the mechanism behind BMD loss in the hip, but not in the spine or whole body. The most important finding was that in these obese older adults supplemented with calcium and vitamin D, exercise training added to weight loss offset increased bone turnover and loss of BMD. This was supported by changes in lean body mass, 1RM strength and osteocalcin, the only variables that remained in the final regression model predicting the changes in hip BMD, suggesting that exercise countered the unloading effect of weight loss on BMD.
Kelly et al. (2011) recruited 28 sedentary, obese adults who were weight stable for the previous six months. The group was randomly allocated to exercise plus either a low-glycemic index (LGI) or high-glycemic index (HGI) diet. Participants engaged in five exercise sessions per week for 60 minutes at 85% maximum heart rate. All food was provided and balanced for macronutrients and both groups lost similar amounts of weight over the six month intervention. Weight, FM, FFM, truncal fat, fasting plasma glucose and insulin decreased in both groups, but did not differ between groups. Glycemic response reduced only in the LGI group. Plasma and mononuclear cells (MNC)-derived TNFα reduced in the LGI group, but increased in the HGI group. Secretion of IL-6 from MNC and plasma IL-6 and monocyte chemotactic protein-1 (MCP-1) was reduced in the LGI group. Change in MNC-derived TNFα and plasma MCP-1 correlated with decreased glycemic response. It was concluded that a LGI diet plus exercise decreased inflammatory markers, whereas a HGI diet attenuated improvements in glycemia and inflammation that usually occur with exercise. One proposed mechanism was the production of nicotinamide adenine dinucleotide phosphate (NADPH), which results in reactive oxygen species that activates the NFkB pathway, and increases TNF-α (Mohanty 2000; Evans 2002; Ghanim 2004). Hypertrophied adipocytes in obesity are partially responsible for the secretion of IL-6, and are thus regulated by not only weight loss, but also hyperglycemia and physical activity. The decrease of MCP-1 in the LGI group associated with changes in plasma glucose was attributed to reduced stimulus to recruit MNC into adipose tissue, seen as improved glucose tolerance. This implies an improvement in adipose tissue function, and the investigators concluded that eating a LGI diet in combination with aerobic exercise can reverse the effects of obesity on proinflammatory cytokines, which appears independent of weight loss.
3.2 Feasibility and Long-term Maintenance of Weight Loss
Another aim of this review was to report on weight maintenance and long-term health outcomes to determine if weight loss can be maintained beyond one year. It was unexpected that only one small follow-up pilot study fit our inclusion criteria (Waters et al. 2013).
This study was a follow up of a one-year lifestyle intervention (Villareal 2011a). The participants remained in the community, with no contact by study personnel, until the 30-month follow-up point. The investigators recruited the first half of the participants who were randomized to the weight loss group (n=13) and diet plus exercise group (n=13) from this previously reported life-style intervention (Villareal 2011a). Of the potential participants available for recruitment, ten (38%) were lost to follow-up. The remaining sixteen participants recruited into the study were representative of the original cohort with regard to age, gender, and other demographic characteristics. Outcomes of interest in the follow-up study were changes in body weight and composition, physical function, quality of life, insulin sensitivity, BMD, and renal and liver function. Participants also completed the Block Brief 2000 Food Frequency Questionnaire (FFQ) to quantify their average daily energy intake over the previous year. Participants were included if they completed at least three days of food records, submitted the FFQ, and had daily energy intakes of more than 500 kcal per day for women, and 800 kcal per day for men. At the 30-month follow-up compared to baseline, weight (101.5 ± 3.8 vs 94.5 ± 3.9 kg) and BMI (36.0 ± 1.7 vs 33.5 ± 1.7 kg/m2) remained significantly below baseline (all p<0.05). Fat free mass (56.7 ± 2.1 vs 56.9 ± 2.2 kg) and appendicular lean mass (24.1 ± 1.0 vs 24.1 ± 1.1kg) remained unchanged when compared to the 12-month point (end of trial) and the 30-month follow-up (all p>0.05). Improvements in the physical performance test (PPT 27 ± 0.7 vs 30.2 ± 0.6), insulin sensitivity (4.1 ± 0.8 vs 3.0 ± 0.6), and insulin area under the curve (12484 ± 2042 vs 9270 ± 1139 min.mg/dl) remained unchanged at 30 months compared to baseline (all p<0.05). Waist circumference and systolic blood pressure remained lower at 30 months compared to baseline (all p<0.05). Whole body and lumbar spine BMD did not change; however, total hip BMD progressively decreased from baseline to 30 months (0.985 ± .026 vs 0.941 ± .024 g/cm2; p<0.05). There were no adverse effects on liver or renal function. Thirteen participants met inclusion requirements for the dietary analysis. At baseline the average caloric intake was 2045 ± 178 kcal per day. At the 30-month follow-up, the FFQ estimated mean daily intake was 1427 ± 142 kcal per day. Overall, participants consumed an average of 619 ± 157 kcal per day less at 30 month follow-up compared to baseline (p<0.05).
The results of this pilot study suggest that changes in weight, body composition, dietary intake, physical function, and insulin sensitivity following an intensive lifestyle therapy may be sustained long-term even without contact. However, this study was limited by the small sample size, high potential for selection bias, lack of a control group, and potential for under-reporting food intake. In addition, the participants who did not return for follow-up may have had outcomes that were different from those participating in this pilot study. Moreover, without a non-weight loss control group, it was not possible to separate the effects of weight loss from the aging process, per se on the variables of interest.
4. Summary of findings
This systematic review focused on randomized controlled trails in obese adults aged 65 years and older. The authors acknowledge that of the ten studies, three reported on the same cohort of 27 participants (Villareal 2006a; Villareal 2006b; Villareal 2008), and three reported on the same cohort of 107 participants (Villareal 2011a; Shah 2011; Armamento-Villareal 2012). The remaining studies had small sample sizes, which although limiting statistical power and inference, do provide initial mechanistic findings in humans, of which few studies exist. Just one article was included that met our inclusion criteria and reported on long-term weight maintenance.
In summary, the evidence confirmed that weight loss of about 10% is achievable through caloric restriction and exercise in sedentary, frail, obese adults aged 65 years and older. However, there was loss of BMD and lean body mass, which can be attenuated, but not stopped, by the addition of exercise during the active weight loss period. The loss of skeletal muscle and bone is a common outcome in weight loss trials (Bales 2008) and one of the primary reasons that recommending weight loss for older adults remains controversial. However, the clinical relevance of this adverse effect remains to be determined due to high baseline BMD and improvements in physical function and metabolic parameters with weight loss. Although the notion that obesity is osteoprotective is now challenged by newer findings that excess adiposity could be detrimental to bone (Nielson 2011), it is possible that dietary-induced caloric restriction through its effect in reducing inflammation may preserve bone quality despite the reduction in BMD (Villareal 2011b). Moreover, it is unclear whether the beneficial effects of weight loss therapy on physical function lower the overall risk of falls and fractures, despite the low BMD.
Weight-loss trials with adults 65 years and older that include mechanisms are few. These studies demonstrate that volume of exercise (particularly resistance training) appears critical in attenuating the loss of bone and muscle, along with calcium and Vitamin D supplementation. Inflammatory molecules and pathways, bone active hormones, exercise, mechanical unloading, sclerostin, and diet composition (glycemic index) all appear to be mediators in the response to weight loss.
Inflammatory markers in particular have received much attention since the discovery in the 1990’s that adipocytes act as an endocrine organ (Forsythe 2008). It is now widely accepted that weight gain results in adipocyte hypertrophy, which leads to an increased in obesity-related inflammatory markers such as leptin, TNF-a, IL-6, while weight loss results in a decrease in these markers (Forsythe 2008). It is also known that adipocytes are not the only source of inflammatory molecules, with macrophages and muscle also secreting these molecules (Cao 2011). The complex interplay of weight loss and exercise with inflammatory cytokines, growth factors, and regulatory pathways discussed in this review are represented in Figure 2.
5. Conclusions and Future Directions
This systematic review has highlighted that there are relatively few randomized controlled trials on weight loss interventions in obese adults over the age of 65 years. From the studies in this review, our hypothesis that weight loss is achievable in this age group was upheld. Lifestyle interventions using a combination of diet and exercise were successful in achieving a 10% weight loss over three to twelve months, and led to positive changes in physical function, metabolic outcomes, and cardiovascular risks. This occurs in spite of the lean mass and BMD losses, although the clinical significance of these changes is unclear. A number of complex and interconnected molecules and pathways were put forward and discussed. With only one small pilot study published to date, the long-term maintenance of weight loss and long-term health implications remain unknown. However, the retention of lean body mass and maintained weight loss and function in this small pilot is encouraging; in particularly, regarding concerns of worsening sarcopenic-obesity with weight loss and weight regain (Zamboni 2005; Houston 2009; Lee 2010).
Future trials need to address specific exercise training modalities, calcium, Vitamin D and protein supplementation, and/or prescribing anti-resorptive therapy (e.g. in patients with low BMD to start with) during active weight loss. Trials specifically designed to investigate the complex interplay between exercise, caloric restriction, weight loss, diet composition, hormones, growth factors, and inflammatory markers are also needed. Finally, trials need to have adequate sample size with appropriate controls, and long follow-up periods are needed to determine how best to achieve sustained lifestyle change associated with optimal health outcomes in frail, obese older adults. With the 65+ age group representing the fastest growing segment of the population, and with a high prevalence of obesity, these studies should become a priority for public health research.
Highlights.
Obesity in those >65 years of age is prevalent and linked to negative health outcomes
Our review found diet & exercise facilitates weight loss in frail obese older adults
Muscle quality and physical function improved with weight loss
Weight loss included bone & lean body mass, issues to be addressed in future studies
Studies need to address feasibility & health outcomes of long-term weight maintenance
Acknowledgments
Supported by grants R01-AG025501, R01AG31176, UL1TR000041, and resources at the New Mexico VA Health Care System and Dunedin School of Medicine.
Footnotes
6. Disclosure Statement
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Debra L. Waters, Email: debra.waters@otago.ac.nz.
Aimee L. Ward, Email: aimee.ward@otago.ac.nz.
Dennis T. Villareal, Email: dennis.villareal@va.gov.
References
- Anandacoomarasamy A, Fransen M, March L. Obesity and the musculosketal system. Curr Opin Rheumatol. 2009;21:71–77. doi: 10.1097/bor.0b013e32831bc0d7. [DOI] [PubMed] [Google Scholar]
- Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. JAMDA. 2008;9:302–312. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Ehsani AA, Steger MK, Brown M, Sinacore DR, Yarasheski KE, Holloszy JO. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- Burguera BL, Hofbauer C, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6 doi: 10.1186/1749-799X-6-30. open access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Giangregorio L. Mechanical stimuli and bone health: what is the evidence? Curr Opin Rheumatol. 2012;24:561–566. doi: 10.1097/BOR.0b013e3283570238. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci. 2012;8:794–804. doi: 10.5114/aoms.2012.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. Older Americans2010: Key Indicators of Well-Being. US Government Printing Office; Washington D.C: 2010. [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev. 2006;34:176–181. doi: 10.1249/01.jes.0000240027.22749.14. [DOI] [PubMed] [Google Scholar]
- Forsythe LK, Wallace JMW, Livingstone MBE. Obesity and inflammation: the effects of weight loss. Nutrition Research Reviews. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight- loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim HA, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc. 2009;109:1886–1895. doi: 10.1016/j.jada.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Haus JM, Solomon TPJ, Patrick-Melin AJ, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet- induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Visser M, Tylavsky FA, Kritchevshy SB, Schwartz AV, Sahyoun N, Harris TB, Newman AB. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation byleucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- Morley JE, Chahla E, AlKaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010;13:40–45. doi: 10.1097/MCO.0b013e3283331384. [DOI] [PubMed] [Google Scholar]
- Nielson CM, Marshall LM, Adams AL. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2011;26:496–502. doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BWJH, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- Ozcelik O, Ozkan Y, Karatas F, Kelestimur H. Exercise training as an adjunct to orlistat therapy reduces oxidative stress in obese subjects. Tohoku J Exp Med. 2005;206:313–318. doi: 10.1620/tjem.206.313. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Frank LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. Am J Prev Med. 2004;27:385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Kim MJ, Gammack JK, Wilson MMG, Thomas DR, Morley JE. Office management of weight loss in older persons. Am J Med. 2006;119:1019–1026. doi: 10.1016/j.amjmed.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Rossner S. Obesity in the elderly - a future matter of concern? Obes Rev. 2001;2:183–188. doi: 10.1046/j.1467-789x.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- Sato F, Tamura Y, Watada H, Kumashiro N, Igarashi Y, Uchino H, Maehara T, Kyogoku S, Sunayama S, Sato H, Hirose T, Tanaka Y, Kawamori R. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007;92:3326–3329. doi: 10.1210/jc.2006-2384. [DOI] [PubMed] [Google Scholar]
- Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, Napoli N, Qualls C, Villareal DT. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers A. US Congressional Research Service: Obesity among older Americans, CRS Report for Congress. 2011 http://www.legistorm.com/score_crs/show/id/104646.html.
- Sorensen TI. Weight loss causes increased mortality: pros. Obes Rev. 2003;4:3–7. doi: 10.1046/j.1467-789x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17:1563–1569. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Binder EF, Yarasheski KE, Williams DB, Brown M, Sinacore DR, Kohrt WM. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc. 2003;51:985–990. doi: 10.1046/j.1365-2389.2003.51312.x. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006a;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Miller BV, III, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006b;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011a;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Kotyk JJ, Armamento-Villareal RC. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell. 2011b;10:96–102. doi: 10.1111/j.1474-9726.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term weight maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging. 2013;17:3–7. doi: 10.1007/s12603-012-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]