Abstract
Human immunodeficiency virus (HIV) is capable of infiltrating the brain and infecting brain cells. In the years following HIV infection, patients show signs of various levels of neurocognitive problems termed HIV-associated neurocognitive disorders (HAND). Although the introduction of highly active antiretroviral therapy (HAART) has reduced the incidence of HIV-dementia, which is the most severe form of HAND, the milder forms have become more prevalent today due to the increased life expectancy of infected individuals. Pre-HAART era markers such as HIV RNA level, CD4+ count, TNF-α, MCP-1 and M-CSF are not able to clearly distinguish mild from advanced HAND. One promising approach for new biomarker discovery is the identification and quantitation of proteins that are post-translationally modified by oxidative and nitrosative species. The occurrence of oxidative and nitrosative stress in HIV-infected brain, both through the early direct and indirect effects of viral proteins and through the later effect on mitochondrial integrity during apoptosis, is well-established. This review will focus on how the reactive species are produced in the brain after HIV infection, the specific oxidative and nitrosative species that are involved in the post-translational modification of the brain proteome, and the methods that are currently used for the detection of such modified proteins. This review also provides an overview of related research pertaining to oxidative stress-related HAND using cerebrospinal fluid and human brain tissue.
Keywords: HIV, dementia, oxidation, nitration, biomarker, mass spectrometry
Introduction
The human immunodeficiency virus (HIV) is able to cross the blood brain barrier via various mechanisms soon after an individual is infected with the virus. The presence of the virus in the central nervous system (CNS) has been well established in human and macaque studies and is summarized elsewhere (Bell, 2004). Even in the era of highly active antiretroviral therapy (HAART), HIV finds sanctuary in the blood brain barrier protected brain where most antiretroviral drugs may not completely control viral replication. The virus may evolve in the brain and viral products are released from the infected glial cells. Through a multitude of direct and indirect effects they cause injury to the brain, leading to cognitive and motor dysfunction in the infected individuals (McArthur et al., 2010). Patients suffer from memory loss, personality changes, diminished mental capacity and inability to use acquired knowledge (McArthur et al., 2003). A combination of neurologic and neurophysiological clinical tests is required for the diagnosis of HAND (McArthur et al., 2005). In the post-HAART era, the early 1991 HAND nosology became insufficient for diagnosis and HAND was re-categorized in increasing severity as: HIV-associated asymptomatic neurocognitive impairment (ASI), HIV-associated mild neurocognitive disorders (MNDs), and HIV-associated dementia (HIV-D) (Antinori et al., 2007; Cherner et al., 2007). Although, the prevalence of severe forms of HAND has decreased in the post-HAART era, the milder ASI and MNDs have become more widespread with the evolving neuropathology of the virus (McArthur, 2004). In a recent study, aviremic patients with cognitive complaint were compared with those without cognitive complaint (Simioni et al., 2010). Even though the prevalence of the cognitive complaint was 27%, the milder forms of HAND were widespread in both groups. The prevalence of HAND was 84% in the group with cognitive complaints with the following distribution: 24% ASI, 52% MNDs, and 8% HIV-D. Interestingly, the prevalence of HAND was as high as 64% among the non-complaining group, 60% ASI and 4% MNDs. After HAART, the old biomarkers of HAND became incapable of distinguishing the milder forms (McArthur et al., 2004) and this necessitates the discovery of novel markers as tools for accurate diagnosis before extensive neuronal death takes place. Thus, it is important to understand the neuropathology of HAND and to develop the specific clinical assays for its diagnosis.
HIV mostly infects microglia, macrophages and astrocytes (Churchill et al., 2009) in the brain. Neurons are not considered to be infected despite an occasional report suggesting infection by gene amplification techniques (Torres-Munoz et al., 2001). Mechanisms of neuronal damage after HIV infection are active fields of research. One hypothesis for the mechanism of neuronal damage following HIV infection involves the downstream effects of oxidative and nitrosative radicals produced during the immune response (Steiner et al., 2006). Under normal conditions the concentration of radical species produced is kept under control through antioxidant systems. However, during disease states an imbalance is formed by the overproduction of oxidative and nitrosative radicals and the underproduction of antioxidants or enzymes that are involved in their scavenging. This imbalanced state is called oxidative and nitrosative stress and can be detrimental to the survival of any cell type, including that of neurons. The radical species and their more stable secondary products can damage DNA (Cooke et al., 2003), alter the lipid composition of membranes (Stark, 2005) and post-translationally modify proteins.
Multiple studies have shown increased levels of oxidative and nitrosative stress after HIV infection in the CNS (Nakamura et al., 2002; Turchan et al., 2003; Boven et al., 1999). Some studies have even correlated increased levels of oxidative and nitrosative stress with the severity of HIV-D. Neurons are exposed to extensive amounts of oxidative species with the reduced concentration of endogenous antioxidant defenses such as glutathione (Castagna et al., 1995). This review will specifically focus on the effects of oxidative and nitrosative stress on the brain proteomics during HIV neuropathogenesis.
Oxidative and nitrosative stress in HIV infected brain
A redox imbalance is observed in patients infected with HIV. This state is characterized by the loss of reducing species and an accumulation of oxidizing ones. The major antioxidant in humans is the tripeptide, γ-glutamate-cysteine-glycine, or glutathione (GSH), which is found at millimolar concentrations. It provides neuroprotection by keeping protein sulfhydryls in the reduced state, reducing hydrogen peroxide with glutathione peroxidase and binding to secondary lipid peroxidation products (Pocernich et al., 2000; Pocernich et al., 2001). It has been shown that GSH concentration is reduced in plasma and cerebrospinal fluid after HIV infection (Choi et al., 2000; Castagna et al., 1995) and GSH deficiency is predictive of poor survival in clinical studies (Herzenberg et al., 1997). Thus, a decreased amount of GSH may leave cells prone to oxidative and nitrosative stress induced modifications.
Once HIV is in the brain, it can trigger oxidant production either through the infected cells or the cells that are activated by the viral proteins. The mechanisms of radical formation differ depending on the infection status of the cells; different cell types exhibit different responses to the interacting viral protein. We will next examine the production of key players in oxidative and nitrosative stress, superoxide and nitric oxide, in HIV-infected or activated brain cells.
Radical formation by HIV-infected cells
Microglia and macrophages, which express both CD4 and CCR5, are the main cell types in the CNS that are infected by HIV (Bagasra et al., 1996; Wiley et al., 1986). The factors upstream of microglia and macrophage activation are not well-characterized. One of the proposed mechanisms involves the HIV protein Nef that functions as a mediator of superoxide release by macrophages (Olivetta et al., 2005). NADPH oxidase is a transmembrane enzyme that produces superoxide during the oxidative burst of the immune system. The phosphorylation of NADPH oxidase cytosolic component, p47phox, is required for its translocation to the membrane and the assembly of the functional complex for the transfer of electrons from NADPH to oxygen (Vignais, 2002; DeLeo and Quinn, 1996). The stable transfection of human macrophage cell line with Nef alleles demonstrated that Nef induced the phosphorylation of p47phox and its translocation to the membrane. Furthermore, inhibition of Src kinase or phosphoinositide 3-kinase (PI3K) prevented both p47phox phosphorylation and superoxide release indicating that Nef regulates NADPH oxidase activity through the upstream activation of these kinases (Olivetta et al., 2009). HeLa cells stably transfected with Tat showed reduced manganese superoxide dismutase (Mn-SOD) mRNA level and protein expression (Westendorp et al., 1995). Mn-SOD is the main enzyme that reduces superoxide to hydrogen peroxide. A weakened antioxidant system in addition to the increased radical production may contribute to the disturbed redox state of the cells and lead to further expression of redox sensitive TNF-α and other cytokines that mediate inflammation (Tyor et al., 1992). This may lead to a domino effect involving the activation of more microglia and macrophages and the enhancement of the immune response.
Astrocytes comprise 70% of cells in the brain and can also be infected by HIV but in a CD4-independent mechanism (Brack-Werner, 1999; Sabri et al., 1999). Although HIV replication is restricted in astrocytes, their large number in the brain and their critical function in neuronal survival make astrocytes important players in HIV neuropathogenesis. Whether a similar mechanism of superoxide production exists in astrocytes or whether viral proteins other than Nef would lead to similar effects in macrophages has not been investigated.
Radical formation by uninfected cells
Uninfected macrophages can be activated either by cytokines released by infected/activated cells or by viral proteins, which results in the production of reactive oxygen species. Two major inflammatory mediators are TNF-α and IL-1. When HIV-infected macrophages are co-cultured with astrocytes, high levels of TNF-α are produced by macrophages (Genis et al., 1992) and this leads to an increase in intracellular calcium in astroglia (Koller et al., 1996). Intracellular calcium levels are important for normal mitochondrial function. The perturbation of intracellular calcium levels may lead to the production of more reactive oxygen species through multiple mechanisms (Starkov et al., 2004).
Nitric oxide (NO) is the major inducer of nitrosative stress in various disease states including HAND. NO is produced by neuronal (nNOS), endothelial (eNOS) and inducible (iNOS) nitric oxide synthases and is an important neurotransmitter in the brain. HIV infected macrophages release proinflammatory factors that induce iNOS in astrocytes (Hori et al., 1999; Hu et al., 1999). In a study using adult rat astroglia, iNOS expression was shown to be regulated by IL-1β/TNF-α (Marcus et al., 2003). Although, NO is required for regular neuronal function, its overproduction during neuroinflammation contributes to nitrosative stress.
In addition to cytokines, the viral envelope proteins and other viral proteins produced by infected astrocytes are capable of activating uninfected cells (Hegg et al., 2000) and promoting the production of reactive species. The most studied viral protein is Tat. The viral proteins gp120 and gp41 are also widely studied for their role in neurotoxicity. Tat can be secreted from infected cells (Chang et al., 1997) and activate neighboring cells. When macrophages and astrocytes are transiently exposed to Tat, the production of IL-1β and IL-6 is induced in an extracellular calcium-independent manner for a prolonged period of time, a hit-and-run phenomenon (Nath et al., 1999). The mechanism of IL-1β and IL-6 production may involve oxidative stress through the downregulation of Mn-SOD and the activation of NADPH oxidase. Tat treatment of Jurkat T cells and HeLa cells showed lower Mn-SOD transcription (Westendorp et al., 1995) indicating an impaired antioxidant system. In a recent study, the role of NADPH oxidase cytokine release in Tat treated macrophages and microglia was also investigated (Turchan-Cholewo et al., 2009). When the cells were exposed to Tat, an increase in superoxide levels was observed in a dose- and time-dependent manner. NADPH oxidase inhibitors and decoy peptides prevented superoxide production and release of IL-6 and TNF-α. In addition to its role in intracellular inflammatory signaling, increased superoxide levels contribute to the local concentration of radicals.
Both Tat and gp120 can affect the concentration of excitatory amino acids and intracellular calcium levels in astrocytes and neurons. First, Na/H exchangers are activated on astrocytes, releasing glutamate and potassium (Holden et al., 1999; Perez et al., 2001). Then, NMDA receptors are activated, voltage gated calcium channels are opened on neurons and calcium is taken up. The increased calcium level disturbs the mitochondrial membrane integrity and reactive oxygen species are formed in neurons. Glutamate also inhibits the uptake of cysteine, one of the three amino acids in glutathione, and decreases glutathione levels, contributing further to oxidative stress (Gras et al., 2003).
Tat can also promote nitrosative stress in microglia and astrocytes. When microglia were treated with media containing Tat, a dose-dependent increase in iNOS expression and NO levels was observed (Polazzi et al., 1999). Tat can also induce the expression of iNOS in astrocytes (Liu et al., 2002) leading to the overproduction of NO, which can react with superoxide anion (O2•−) to form neurotoxic peroxynitrite (ONOO−). TNF-α also induces iNOS, leading to increased production of NO in HIV-infected macrophages (Bukrinsky et al., 1995). Another HIV protein, gp41, has been proposed to interact with a cell surface receptor in studies with mixed cortical cultures and has been shown to induce the expression of iNOS, leading to an increase in NO concentration in neurons and glial cells (Adamson et al., 1999) and may kill neurons through a nitric oxide (NO)-dependent mechanism (Adamson et al., 1996). The N-terminal region of gp41 induces iNOS protein activity (Adamson et al., 1999). Upon Tat or gp41 exposure local NO levels may increase; NO may irreversibly inhibit the electron transport chain in the mitochondria and superoxide may be produced. Alternatively, as a small diffusible molecule, NO may pass through the membrane and exert its effects in the extracellular space. In either case, NO may also react with other radical species or modify cellular molecules.
Post-translational modification (PTM) of proteins by reactive oxygen species (ROS) and reactive nitrogen species (RNS)
The function and lifetime of proteins can be regulated by their folding, localization, and altered interaction with other cellular macromolecules. The addition of PTMs on specific amino acid residues is one means for effecting such regulation. Unusual PTM patterns on specific proteins have been associated with various neurodegenerative diseases. As explained in detail in the previous section, upon HIV-infection there is an increase in the generation of reactive oxygen and nitrogen species as part of either the inflammatory signaling pathway or as a response to viral particles. Although the exact signaling pathways have not yet been delineated and the constructive and destructive effects of the reactive species are still debated, the modified proteins may serve as specific biomarkers (Table 1).
Table 1.
Major protein residues prone to modification by oxidative and nitrosative species and the major PTMs formed.
| Residue | Post-translational modification | |
|---|---|---|
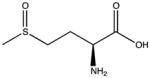
| Methionine | Methionine sulfoxide |
|
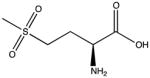
| Methionine sulfone |
|
|
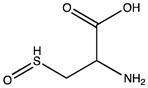
| Cysteine | Sulfenic acid |
|
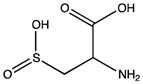
| Sulfinic acid |
|
|
| Sulfonic acid |
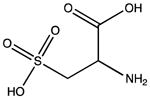
|
|
| Sulfenamide |
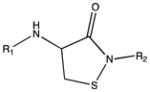
|
|
| S-nitrosylation |
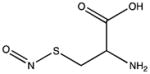
|
|
| Carbonylation | See Sayre et al. (2006) for 4-HNE, 4-ONE adduct derivatives | |
| Disulfide bridges |
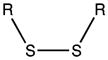
|
|
| Glutathionylation |
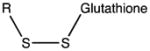
|
|
| Tyrosine | 3-nitrotyrosine |
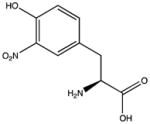
|
| 3,4-dihydroxyphenylalanine (DOPA) |
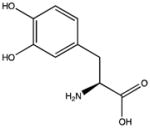
|
|
| Dopaquinone |
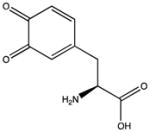
|
|
| Glutathionylation of tyrosine hydroperoxide |
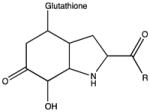
|
|
| Tryptophan | N-formylkynurenine |
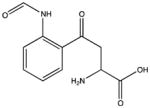
|
| Hydroxykynurenine |
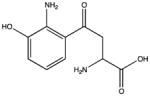
|
|
| Kynurenine |
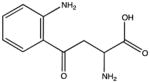
|
|
| Nitrohydroxytryptophan |
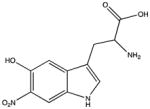
|
|
| 6-nitrotryptophan |
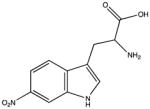
|
|
| Lysine, Proline, Arginine, Threonine Histidine | Carbonylation | See Madian and Regnier (2010b) |
The major ROS are superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). Superoxide is mainly generated by NADPH oxidase and is readily converted to H2O2 by superoxide dismutase (SOD). Alternatively, O2•− may react with NO to form peroxynitrite (ONOO−) (Figure 1). Unlike short-lived and negatively charged O2•−, H2O2 and the protonated form of ONOO− may diffuse through the membranes.
Figure 1.
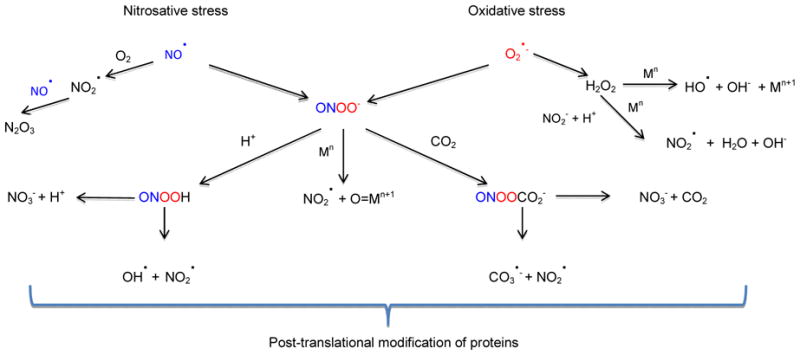
Reactive oxygen and nitrogen species and their products that are involved in the post-translational modification of proteins
Metal ions and metalloproteins play critical roles in radical formation and degradation. and For instance, H2O2 can react with the ferrous iron through the Fenton reaction to form OH• oxoferryl species (Yamazaki and Piette, 1990). On the other hand, myeloperoxidase (MPO), a hemeprotein, can oxidize NO2− to •NO2 (Figure 1). Another mechanism for •NO2 generation is the reaction of ONOO- with oxidized metal centers, such as those in MPO and SOD (Sampson et al., 1996). ONOO- and •NO2 are the main species involved in RNS-mediated PTMs of proteins. However, as explained in the reactions above, ROS are necessary for their production. Depending on the cellular localization of the protein, the exposure of redox sensitive residues, local antioxidant concentrations and the extent of free radicals, proteins may have multiple PTMs. For example, Apolipoprotein B-100 precursor from human plasma has 20 PTMs (Madian and Regnier, 2010a). Oxidative and nitrosative PTMs of proteins take place either directly by ROS/RNS or by their secondary products. Although peptide backbone cleavage and cross-linking may occur, they would not be suitable as biomarkers because these modifications may also take place endogenously by other mechanisms in vivo. Thus, we will focus on PTMs that occur on amino acid side chains.
Modifications on Met and Cys
The oxidation of methionine to methionine sulfoxide (MetO) is the most common oxidative modification (Madian and Regnier, 2010a). In strong oxidizing environments MetO can be further oxidized to form methionine sulfone (MetO2) (Levine et al., 2000). This residue is very sensitive to the redox environment and can be oxidized by almost all ROS/RNS. Methionine sulfoxide reductases are able to reduce MetO to Met with a reaction catalyzed by NADPH. The non-complex nature of oxidation and its reversibility make this PTM a potential antioxidant defense to protect other residues from critical damage (Levine et al., 2000). Studies with methionine sulfoxide reductase knockout mice support this hypothesis; the mice have increased oxidation on residues other than methionine and have a decreased life span (Moskovitz et al., 2001). However, a recent report identified residual metal ions in buffers and columns that can catalyze radical formation and rapid MetO generation during sample preparation (Zang et al., 2012). To use Met oxidized proteins as biomarkers, precautions against artificial Met oxidation need to be taken.
Cysteine residues are also very redox sensitive and have been implicated as one of the main players in redox signaling. Oxidation of cysteines may lead to the formation of sulfenic acid (Cys-SOH), sulfinic acid (Cys-SO2H), sulfonic acid (Cys-SO3H), sulfenamide and disulfide bridges within the protein or with other proteins/peptides. Cysteine oxidation has been reviewed in detail (Paulsen and Carroll, 2010). Additionally, cysteines can be S-nitrosylated by RNS or carbonylated by fatty acid peroxidation products, such as 4-hydroxynenal (4-HNE), 4-oxo-2-nonenal (4-ONE), and nitroalkenes (Sayre et al., 2006; Batthyany et al., 2006). Although Cys-SOH, disulfide bridges, S-nitrosylation and carbonylation on cysteines have long been known to be reversible through reactions of antioxidant systems, the reversibility of Cys-SO2H has only recently been observed. The crystal structure of sulfiredoxin enzyme complexed with peroxiredoxin showed that unfolding of the oxidized peroxiredoxin is necessary to expose the Cys-SO2H for sulfiredoxin activity (Jonsson et al., 2008).
Intermolecular disulfide bond formation and Cys-SOH have been implicated in activity changes in phosphatases, kinases, and transcription factors (Paulsen and Carroll, 2010), whereas S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been shown to be important for its binding to Siah1, an E3 ubiquitin ligase, and translocation to the nucleus (Hara et al., 2006). The Tat protein of HIV has a polycysteine region, which binds two cadmium, and two zinc ions that are critical for its activity (Frankel et al., 1988). S-nitrosylation of HIV-Tat protein leads to loss of its activity (Li et al., 2008b). Additionally, S- nitrosylation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) has been demonstrated in the brains of patients with HAND and in the HIV-gp120 transgenic mouse model, leading to decreased Akt activity (Banerjee et al., 2012).
The diverse PTMs that can take place on cysteines and their reversibility make cysteine a key regulatory position in an oxidizing environment, such as in HIV neuropathogenesis. The oxidized proteins can serve as biomarkers with appropriate preservation and handling of the samples (Tsikas and Frolich, 2004).
Modifications of aromatic residues
All aromatic residues are prone to oxidative and nitrosative modifications to some extent and prolonged ROS/RNS exposure may lead to multiple modification forms on a single residue as well as multiple modifications on the same molecule. The discovery of novel PTMs on tyrosine and tryptophan and their functional implications are emerging research fields. Recent studies report endogenous 3,4-dihydroxyphenylalanine and dopaquinone modifications on protein tyrosine (Zhang et al., 2010b) and the conjugation of GSH to oxidized tyrosine (Nagy et al., 2012) in addition to the long-studied and highly debated 3-nitrotyrosine. Furthermore, the oxidized forms of tryptophan to N-formylkynurenine (Helland et al., 2008; Hunzinger et al., 2006), hydroxykynurenine and kynurenine, 6-nitro tryptophan (Yamakura et al., 2003; Yamakura and Ikeda, 2006; Uda et al., 2012) and nitrohydroxytryptophan (Rebrin et al., 2007; Bregere et al., 2010) have been detected. Tryptophan oxidation and nitration can affect protein function. The oxidation of tryptophan enhances the copper binding ability of the protein MopE in the methanotropic bacteria Methylococcus capsulatus (Helland et al., 2008). The nitration of a tryptophan on the substrate binding cavity of succinyl-CoA:3-ketoacid CoA transferase increases the specific activity of the enzyme (Bregere et al., 2010). Abnormalities in the tryptophan pathway have been studied by several groups in individuals with neurological or psychiatric complications of HIV infection (Davies et al., 2010). This includes increased levels of 3-hydroxykyenurenine in the brain (Sardar et al., 1995) and L-kynurenine, kynurenic acid and quniolinic acid in CSF (Heyes et al., 1992a; Heyes et al., 1992b). These changes are due to activation of indoleamine-2,3-dioxygenase (Sardar and Reynolds, 1995).
Although oxidative modifications are mostly associated with ROS, peroxynitrite, which is formed by the reaction of superoxide and nitric oxide, is capable of producing both oxidative and nitrosative modifications on proteins (Figure 1). In addition to nitrating tyrosine and tryptophan and S-nitrosylating cysteine, peroxynitrite can oxidize cysteine, methionine, tryptophan, tyrosine, phenylalanine and histidine residues. A detailed review of peroxynitrite reactivity with amino acids and proteins has been published (Alvarez and Radi, 2003). Thus, peroxynitrite can be classified as an ROS and an RNS, both in its formation and its effect on proteins. Noteworthy factors that affect how the residues will be modified are carbon dioxide levels and the proximity of metal centers and cysteines to aromatic residues (Alvarez and Radi, 2003). Treatment of human serum albumin (HSA) with peroxynitrite in phosphate buffer decreased the level of protein thiols and lead to the nitration of 1 tyrosine per 10 albumin molecules. The addition of carbon dioxide increased tyrosine nitration 4-fold and decreased cysteine oxidation by half. Furthermore, blocking the thiols on HSA led to increased tyrosine nitration both in the presence and absence of carbon dioxide (Alvarez et al., 1999). Therefore, special attention is required in the choice of protein (amino acid sequence) and reaction components for producing protein controls and antigens for oxidative and nitrosative PTM detection.
Nitration of tyrosine has been the focus of many mechanistic studies since the late 1990s. Enzymes such as myeloperoxidase produce nitrogen dioxide radical either from nitrite in the presence of ROS or from peroxynitrite (Sampson et al., 1996; Burner et al., 2000). Additionally, nitrogen dioxide radical can be formed from the homolytic cleavage of peroxynitrous acid (ONOOH) or nitrosoperoxocarbonate (ONOOCO2−), the former being very slow (Figure 1). Nitrogen dioxide radical alone is not sufficient to nitrate tyrosines in the free radical pathway because the tyrosyl radical also needs to be formed. This mainly takes place either by the carbonate radical or oxo-metal complexes. The CSF of individuals with HAND has 3-nitrotyrosine modified L-prostaglandin synthase, which leads to loss of its activity (Li et al., 2008a). Similar mechanisms are found for the nitration of tryptophan residues with the possible formation of N-nitrosylation (Sala et al., 2004). Because tryptophan is critical for the synthesis of serotonin in the brain and is part of the tryptophan oxidation pathway in inflammation (Murray, 2010), any alteration of free and protein bound tryptophan by ROS and RNS could be important in HIV neuropathogenesis.
Other residues
Upon oxidative stress, many carbonylation reactions take place on various residues. The carbonyl is either formed by the direct oxidation of the amino acid and protein backbone or by the addition of oxidized lipids and advanced glycation end products. A detailed list of carbonylated forms of threonine, arginine, histidine, aspartic acid, lysine, leucine, and asparagine residues has been described (Madian and Regnier, 2010b). However, the biological samples that are used to analyze carbonylation need to be fresh to prevent artificial carbonylations. Protein carbonyl levels in CSF of individuals with HAND are significantly elevated (Turchan et al., 2003). In experimental models where the brain is exposed to HIV-Tat proteins, prominent increases in protein carbonyl have also been found (Aksenov et al., 2003). During oxidative stress, lipid peroxidation yields short chain aldehydes that are prone to the nucleophilic attack of lysine and histidine in addition to cysteine. Some of these species are 4-HNE, 4-ONE and acrolein (Sayre et al., 2006). Elevated levels of 4-HNE have been demonstrated in the brains and CSF of individuals with HAND. These modifications are most prominent in the frontal lobe and basal ganglia (Haughey et al., 2004). The CSF levels are particularly increased in individuals who show neurological progression (Sacktor et al., 2004).
Furthermore, nitric oxide derived reactive species can nitrate unsaturated fatty acids and lead to the formation of nitroalkene adducts. One such example is the nitroalkene adduct found on the histidines of GAPDH in red blood cells obtained from healthy humans (Sayre et al., 2006; Batthyany et al., 2006). Finally, upon exposure to ROS, Amadori and glyoxal adducts of lysine may form. Peroxynitrite can further modify the Amadori adduct to generate N-ε (carboxymethyl) lysine (Nagai et al., 2002).
Methods for detecting post-translationally modified proteins
One of the tasks of proteomics studies is to compare changes in the individual proteome that occur as the result of disease or condition. This includes not only the up- or down-regulation of specific proteins, but also changes to their post-translational modifications, which would include those modifications described here that result from oxidative or nitrosative stress. Mass spectrometry plays a key role in such studies, as PTMs provide a change in mass within the protein’s sequence that can be located by tandem mass spectrometry techniques that compare the sequence spectra with those predicted from the human genome. Proteomics methods for PTM analysis can be global, intended primarily to identify or discover all of the protein isoforms carrying a specific modification, or quantitative, generally targeting specific PTMs that are key in understanding the biochemical pathways associated with the disease or condition. Global analyses may be quantitative or semiquantitive as well, such as the iTRAQ and ICAT methods described below, while more targeted analyses provide the opportunity for absolute quantitation of targeted isoforms using stable isotope analogs.
The goal of many such studies is the development of suitable biomarkers for the disease, specific proteins or PTMs whose up- or down-regulation may be used for diagnosis, for elucidating the response to specific drugs or therapies, or monitoring the disease outcome. In this case, these two analytical approaches are generally referred to as the discovery and validation phases of biomarker development. In the discovery phase a preliminary list of potential biomarkers is generated from a limited number of samples from healthy and diseased individuals. The aim is only to monitor differences between the sample groups. In addition to this, a validation phase is necessary for testing the specificity of the potential biomarkers for the disease of interest, reproducibility of the method with biological and technical replicates and the presence of the potential biomarker in large sample sets.
In the discovery phase, ROS/RNS-induced protein PTM analysis involves mainly immunological detection, chemical derivatization and chromatography (generally reverse phase high-performance liquid chromatography) coupled to mass spectrometry. During the validation, high throughput enzyme-linked immunosorbent assays (ELISA) and mass spectrometric methods targeted to specific analytes are utilized. Because different methods have their own advantages and disadvantages, using multiple methods may provide further confirmation before the establishment of a biomarker for clinical use.
Discovery phase
The location of the PTM on the protein and the nature of the modified chemical group on the residue are very important for immunological and chemical derivatization techniques. While location of the PTM on the protein may limit its accessibility by the antibody, the modified chemical group directly determines the specificity of the chemical method.
Diverse classes of protein carbonyls are isolated mainly after chemical derivatization with dinitrophenylhydrazine, biotin hydrazide, and oxidation-dependent element coded affinity tags followed by either 2-D gel separation and Western analysis with an antibody targeting the derivatizing agent or separation on an immunosorbent column and LC-MS/MS. Girard’s P reagent also reacts with carbonyls resulting in the formation of quaternary amines. A detailed review is presented for the identification of carbonylated proteins and their oxidation sites (Madian and Regnier, 2010b). Upon trypsinization, the quaternary amine containing peptides can be selected by strong cation exchange chromatography. It is essential to use fresh samples for carbonylation studies because the carbonyl group is not stable during storage even at low temperatures.
Biotin switch (Jaffrey and Snyder, 2001), reductive ligation (Zhang et al., 2010a), SNO-resin assisted capture (SNO-RAC) (Forrester et al., 2009), and d-switch (Sinha et al., 2010) methodologies have been developed for the identification of S-nitrosylated proteins. While the former two methods rely on either Western analysis or avidin pull-down followed by LC-MS/MS, the latter two involve isobaric tags for S-nitrosylated protein and peptide quantitation (such as ITRAQ, see Figure 2), respectively. SNO-RAC is designed for the quantitation of various S-nitrosylated peptides in up to eight samples and the non-nitrosylated proteins are not analyzed. On the other hand, d-switch focuses on certain proteins and sites with the aim of quantitating the S-nitrosylation level. The appropriate method should be selected depending on the purpose of the study and the degree of protein and site identity knowledge from previous studies.
Figure 2.
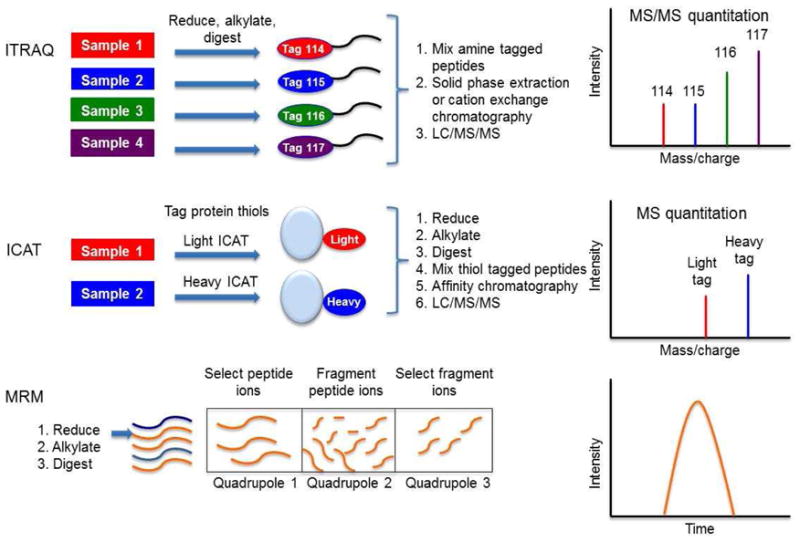
Generic schemes for the mass spectrometric quantitation methods used in proteomic discovery and validation phases. iTRAQ: isobaric tag for absolute and relative quantitation; ICAT: isotope coded affinity tags; MRM: multiple reaction monitoring
The detection of cysteine oxoforms has been advanced in the past few years with the development of small-molecule probes. The traditional indirect methods were based on the lack of reactivity of oxidized cysteines with thiol active reagents and the restoration of reactivity upon treatment with reducing agents. Another means of cysteine oxoform detection is the labeling of free thiols by biotinylated iodoacetamide followed by either immunologic detection or enrichment on an immunosorbent column and LC-MS/MS. Isotope-coded affinity tags (ICAT) have been incorporated for the quantitation of thiol reactive and non-reactive cysteines (Sethuraman et al., 2004) (Figure 2). A modified form of ICAT, called OxICAT, can also detect the reversibility of the oxidation on the cysteines (Leichert et al., 2008). However, neither method can distinguish which cysteine oxoform is present. Recently, sulfenylation-specific alternative methods have been developed. One such method is the modified biotin switch assay in which arsenite is used for selective reduction (Saurin et al., 2004). Additionally, the specific reaction of dimadone derivatives conjugated to biotin is utilized for sulfenation detection (Poole et al., 2005; Charles et al., 2007; Nelson et al., 2010). With the availability of new detection methods, protein sulfenation research may become more prevalent in the following years.
Although oxidized tyrosine has not attracted much attention, there is increased interest in N-formylkynurenine modified tryptophan. An antibody has been developed against this PTM and may accelerate the discovery of this modification in various ROS exposed tissues and biological fluids (Ehrenshaft et al., 2009). Similarly, 4-HNE modified protein detection is mostly based on immunological methods.
The nitration of tyrosine is widely studied and has been associated with various diseases such as diabetes (Pacher et al., 2005), neurodegenerative and cardiovascular diseases (Butterfield et al., 2011; Eleuteri et al., 2009), and cancer (Chazotte-Aubert et al., 2000). The detection methods are based on either immunological or chemical enrichment followed by western analysis or LC-MS/MS. All chemical derivatization methods include the reduction of nitro to amino group in the early steps. Over the past decade, although various nitrated proteins have been identified, the sites of modification are rarely detected by mass spectrometric methods. The challenge may be due to the presence of proposed denitratase activity in the samples (Kamisaki et al., 1998; Kuo et al., 1999; Irie et al., 2003; Smallwood et al., 2007), potential multiple modifications on the same peptide, or the overall low abundance of nitrotyrosine-containing proteins. Conversely, unexpected PTMs may exist in the complex human samples that the antibodies have not been tested against using in vitro-treated standard proteins. One such modification is nitrohydroxytryptophan, which was discovered due to its ability to bind to the clone 1A6 3-nitrotyrosine antibody (Rebrin et al., 2007). Additionally, as mentioned in the previous section, peroxynitrite treatment causes multiple PTMs in addition to nitrotyrosine, and peroxynitrite-treated antigens need to be fully characterized before antibody production to ensure specificity and analytical reliability (Tsikas, 2010; Tsikas, 2012).
Tryptophan is found less frequently than tyrosine on proteins. However, the surface exposed residues are important for protein-protein interactions. If the tryptophan is at the active site cavity it may alter the enzyme activity. For example, nitrohydroxytryptophan containing succinyl-CoA:3-ketoacid coenzyme A transferase was shown to have a 30% increase in activity. A specific antibody for 6-nitrotryptophan was generated using 6-nitrotryptophane peptide conjugated to keyhole limpet hemocyanin as the antigen. This antibody was used for western analysis of PC12 cells treated with ONOO− or NGF. The immunostained bands were further analyzed by mass spectrometry and 5 modified proteins along with their modification sites were identified (Ikeda et al., 2007). Another group is developing chemical methods for nitrated tryptophan containing proteins (Nuriel et al., 2011). Thus, nitrated tryptophan studies may become more widespread in the next few years with the availability of new detection methods.
Validation phase
After the discovery of various modified proteins that exhibit differences between the control and diseased sample groups, it is essential to validate the results in large sample sets. The two most commonly used methods in proteomics biomarker validation studies are multiple reaction monitoring (MRM) assays and ELISA. During the validation phase, the specificity, reproducibility, expense and the time needed for assay development become key factors prior to assessing clinical applicability. For MRM assays, tryptic peptides are ionized by electrospray on a triple quadrupole mass spectrometer. The peptide ion of interest and the fragment ions it generates are selected in the first and third quadrupole, respectively. With the use of synthetic standard peptides, MRM assays provide high specificity whereas the specificity of ELISAs depends mainly on the specificity of the antibody used. While MRM assays can be performed reproducibly (Prakash et al., 2010; Prakash et al., 2012), conflicting ELISA results have been reported among different immunoassays in some cases (Safinowski et al., 2009; Sturgeon and Seth, 1996; Sturgeon et al., 2011). The synthetic peptides used in MRM assays and the antibodies used in ELISAs contribute to the cost of these methods. Finally, the development of ELISAs depends on the availability of specific antibodies and may require years. However, it is possible to perform MRM assays as soon as the target peptides are determined for most peptides.
Conclusions
The prevalence of mild forms of HAND and the lack of the diagnostic capability of pre-HAART markers have prompted the search for novel HAND markers that are specific for the early stages of this neurologic disease. Studies that have been conducted over the past two decades have clearly shown an increase in oxidative and nitrosative stress upon HIV infection and throughout the development of HAND. ROS/RNS modified proteins may serve as biomarkers for the clinical assessment of HAND patients throughout disease progression and following the treatment.
A differential protein expression study of autopsy brain tissues of HAND and HIV non-dementia patients has indicated an overlap between HAND and non-viral neurodegenerative diseases (Zhou et al., 2010). The majority of the differentially expressed proteins were involved in energy metabolism (mitochondria) and signal transduction pathways and were previously shown to be associated with non-viral neurodegenerative diseases. In contrast to the low specificity of differentially expressed proteins in HAND, ROS/RNS-modified proteins may provide specificity to this neurological disease. In the immunohistochemistry analysis of autopsy brain tissue, an increase in nitration was detected (Boven et al., 1999) but the modified proteins were not identified. In a more recent study, S-nitrosylated Akt was identified in the post-mortem brain tissues from HAND patients and in the HIV-gp120 transgenic mouse brains (Banerjee et al., 2012).
Most HAND biomarker studies conducted to date have utilized cerebrospinal fluid (CSF) from HAND patients (Angel et al., 2012; Laspiur et al., 2007; Rozek et al., 2007) or from macaques with simian immunodeficiency virus encephalitis (Pendyala et al., 2009). However, there are only a few studies that analyze ROS/RNS-modified proteins (Turchan et al., 2003; Li et al., 2008a) or antioxidant enzyme levels in CSF (Velazquez et al., 2009). Although an increase in carbonylated and nitrated proteins has been detected via immunologic methods, the identity of these proteins and their modification sites are mostly unknown. L-Prostaglandin D synthase (LPDGS) is one of the nitrated proteins that was identified in CSF from HAND patients and was further studied (Li et al., 2008a). The site of modification was determined by liquid chromatography coupled to high-resolution tandem mass spectrometry (Beasley et al., 2010). We are currently using multiple mass spectrometric methods for both the discovery and validation of novel oxidative and nitrosative stress-induced PTMs using brain tissue and CSF from HAND patients. The identification of the protein modification sites may provide new insights on altered molecular function and make the development of MRM assays possible for potential clinical applications.
Biomarker discovery and validation has come a long way in the past decade with the development of new detection methods and the availability of more sensitive instrumentation. Novel PTMs have broadened the potential targets for oxidative and nitrosative stress induced biomarker investigation. The challenge in the discovery phase is constructing a reasonable target list, while in the validation phase the main challenge is the development of reproducible assays and proving the specificity of the biomarker for the disease. Despite these challenges, an increased interest in clinical proteomics is expected in the near future.
Acknowledgments
RJC and LU were supported by grants R01NS039253 (Cotter R, PI) from the National Institute of Neurological Disorders and Stroke and P30MH075673 (McArthur J, PI) from the National Institute of Mental Health. AN is supported by intramural NIH funds.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adamson DC, Kopnisky KL, Dawson TM, Dawson VL. Mechanisms and structural determinants of HIV-1 coat protein, gp41-induced neurotoxicity. J Neurosci. 1999;19:64–71. doi: 10.1523/JNEUROSCI.19-01-00064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- Angel TE, Jacobs JM, Spudich SS, Gritsenko MA, Fuchs D, Liegler T, Zetterberg H, Camp DG, 2nd, Price RW, Smith RD. The cerebrospinal fluid proteome in HIV infection: Change associated with disease severity. Clin Proteomics. 2012;9:3. doi: 10.1186/1559-0275-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: Identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Liao L, Russo R, Nakamura T, McKercher SR, Okamoto S, Haun F, Nikzad R, Zaidi R, Holland E, Eroshkin A, Yates JR, 3rd, Lipton SA. Isobaric tagging-based quantification by mass spectrometry of differentially regulated proteins in synaptosomes of HIV/gp120 transgenic mice: Implications for HIV-associated neurodegeneration. Exp Neurol. 2012;236:298–306. doi: 10.1016/j.expneurol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley Ashley, Anderson Caroline, McArthur Justin, Sacktor Ned, Nath Avindra, Cotter Robert. Characterization of nitrotyrosine-modified proteins in cerebrospinal fluid. Clinical Proteomics. 2010:29–41. [Google Scholar]
- Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004;45:549–559. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS. Increased peroxynitrite activity in AIDS dementia complex: Implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bregere C, Rebrin I, Gallaher TK, Sohal RS. Effects of age and calorie restriction on tryptophan nitration, protein content, and activity of succinyl-CoA:3-ketoacid CoA transferase in rat kidney mitochondria. Free Radic Biol Med. 2010;48:609–618. doi: 10.1016/j.freeradbiomed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Nottet HS, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: Implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burner U, Furtmuller PG, Kettle AJ, Koppenol WH, Obinger C. Mechanism of reaction of myeloperoxidase with nitrite. J Biol Chem. 2000;275:20597–20601. doi: 10.1074/jbc.M000181200. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of alzheimer’s disease. Free Radic Res. 2011;45:59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, Bottiglieri T, Lazzarin A. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: Effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–1683. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Charles RL, Schroder E, May G, Free P, Gaffney PR, Wait R, Begum S, Heads RJ, Eaton P. Protein sulfenation as a redox sensor: Proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- Chazotte-Aubert L, Hainaut P, Ohshima H. Nitric oxide nitrates tyrosine residues of tumor-suppressor p53 protein in MCF-7 cells. Biochem Biophys Res Commun. 2000;267:609–613. doi: 10.1006/bbrc.1999.2003. [DOI] [PubMed] [Google Scholar]
- Cherner M, Cysique L, Heaton RK, Marcotte TD, Ellis RJ, Masliah E, Grant I HNRC Group . Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007;13:23–28. doi: 10.1080/13550280601089175. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 tat-transgenic mice. J Biol Chem. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Davies NW, Guillemin G, Brew BJ. Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int J Tryptophan Res. 2010;3:121–140. doi: 10.4137/ijtr.s4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: Molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- Ehrenshaft M, Silva SO, Perdivara I, Bilski P, Sik RH, Chignell CF, Tomer KB, Mason RP. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic Biol Med. 2009;46:1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleuteri E, Magno F, Gnemmi I, Carbone M, Colombo M, La Rocca G, Anzalone R, Tarro Genta F, Zummo G, Di Stefano A, Giannuzzi P. Role of oxidative and nitrosative stress biomarkers in chronic heart failure. Front Biosci. 2009;14:2230–2237. doi: 10.2741/3375. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol. 2009;27:557–559. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Chen L, Cotter RJ, Pabo CO. Dimerization of the tat protein from human immunodeficiency virus: A cysteine-rich peptide mimics the normal metal-linked dimer interface. Proc Natl Acad Sci U S A. 1988;85:6297–6300. doi: 10.1073/pnas.85.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras G, Chretien F, Vallat-Decouvelaere AV, Le Pavec G, Porcheray F, Bossuet C, Leone C, Mialocq P, Dereuddre-Bosquet N, Clayette P, Le Grand R, Creminon C, Dormont D, Rimaniol AC, Gray F. Regulated expression of sodium-dependent glutamate transporters and synthetase: A neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol. 2003;13:211–222. doi: 10.1111/j.1750-3639.2003.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006;1762:502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Hegg CC, Hu S, Peterson PK, Thayer SA. Beta-chemokines and human immunodeficiency virus type-1 proteins evoke intracellular calcium increases in human microglia. Neuroscience. 2000;98:191–199. doi: 10.1016/s0306-4522(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Helland R, Fjellbirkeland A, Karlsen OA, Ve T, Lillehaug JR, Jensen HB. An oxidized tryptophan facilitates copper binding in methylococcus capsulatus-secreted protein MopE. J Biol Chem. 2008;283:13897–13904. doi: 10.1074/jbc.M800340200. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci U S A. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Jordan EK, Lee K, Saito K, Frank JA, Snoy PJ, Markey SP, Gravell M. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Res. 1992a;570:237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, Bhalla RB, Der M, Markey SP. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992b;40:71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Hori K, Burd PR, Furuke K, Kutza J, Weih KA, Clouse KA. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: Astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood. 1999;93:1843–1850. [PubMed] [Google Scholar]
- Hu S, Ali H, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Gp-41-mediated astrocyte inducible nitric oxide synthase mRNA expression: Involvement of interleukin-1beta production by microglia. J Neurosci. 1999;19:6468–6474. doi: 10.1523/JNEUROSCI.19-15-06468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzinger C, Wozny W, Schwall GP, Poznanovic S, Stegmann W, Zengerling H, Schoepf R, Groebe K, Cahill MA, Osiewacz HD, Jagemann N, Bloch M, Dencher NA, Krause F, Schrattenholz A. Comparative profiling of the mammalian mitochondrial proteome: Multiple aconitase-2 isoforms including N-formylkynurenine modifications as part of a protein biomarker signature for reactive oxidative species. J Proteome Res. 2006;5:625–633. doi: 10.1021/pr050377+. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Yukihiro Hiraoka B, Iwai H, Matsumoto T, Mineki R, Taka H, Takamori K, Ogawa H, Yamakura F. Detection of 6-nitrotryptophan in proteins by western blot analysis and its application for peroxynitrite-treated PC12 cells. Nitric Oxide. 2007;16:18–28. doi: 10.1016/j.niox.2006.04.263. [DOI] [PubMed] [Google Scholar]
- Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci U S A. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001:pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- Jonsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee YC, Murad F. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci U S A. 1998;95:11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller H, Thiem K, Siebler M. Tumour necrosis factor-alpha increases intracellular Ca2+ and induces a depolarization in cultured astroglial cells. Brain. 1996;119 ( Pt 6):2021–2027. doi: 10.1093/brain/119.6.2021. [DOI] [PubMed] [Google Scholar]
- Kuo WN, Kanadia RN, Shanbhag VP, Toro R. Denitration of peroxynitrite-treated proteins by ‘protein nitratases’ from rat brain and heart. Mol Cell Biochem. 1999;201:11–16. doi: 10.1023/a:1007024126947. [DOI] [PubMed] [Google Scholar]
- Laspiur JP, Anderson ER, Ciborowski P, Wojna V, Rozek W, Duan F, Mayo R, Rodriguez E, Plaud-Valentin M, Rodriguez-Orengo J, Gendelman HE, Melendez LM. CSF proteomic fingerprints for HIV-associated cognitive impairment. J Neuroimmunol. 2007;192:157–170. doi: 10.1016/j.jneuroim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: Roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50:301–307. doi: 10.1080/713803735. [DOI] [PubMed] [Google Scholar]
- Li W, Malpica-Llanos TM, Gundry R, Cotter RJ, Sacktor N, McArthur J, Nath A. Nitrosative stress with HIV dementia causes decreased L-prostaglandin D synthase activity. Neurology. 2008a;70:1753–1762. doi: 10.1212/01.wnl.0000282761.19578.35. [DOI] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-tat protein is clade dependent. J Neurosci. 2008b;28:12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madian AG, Regnier FE. Profiling carbonylated proteins in human plasma. J Proteome Res. 2010a;9:1330–1343. doi: 10.1021/pr900890k. [DOI] [PubMed] [Google Scholar]
- Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010b;9:3766–3780. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: An evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: An evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, Albert SM, Kieburtz K, deMarcaida JA, Cohen B, Epstein LG. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MF. Insights into therapy: Tryptophan oxidation and HIV infection. Sci Transl Med. 2010;2:32ps23. doi: 10.1126/scitranslmed.3001082. [DOI] [PubMed] [Google Scholar]
- Nagai R, Unno Y, Hayashi MC, Masuda S, Hayase F, Kinae N, Horiuchi S. Peroxynitrite induces formation of N( epsilon )-(carboxymethyl) lysine by the cleavage of amadori product and generation of glucosone and glyoxal from glucose: Novel pathways for protein modification by peroxynitrite. Diabetes. 2002;51:2833–2839. doi: 10.2337/diabetes.51.9.2833. [DOI] [PubMed] [Google Scholar]
- Nagy P, Lechte TP, Das AB, Winterbourn CC. Conjugation of glutathione to oxidized tyrosine residues in peptides and proteins. J Biol Chem. 2012;287:26068–26076. doi: 10.1074/jbc.M112.371690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Masutani H, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid Redox Signal. 2002;4:455–464. doi: 10.1089/15230860260196245. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nelson KJ, Klomsiri C, Codreanu SG, Soito L, Liebler DC, Rogers LC, Daniel LW, Poole LB. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 2010;473:95–115. doi: 10.1016/S0076-6879(10)73004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel T, Hansler A, Gross SS. Protein nitrotryptophan: Formation, significance and identification. J Proteomics. 2011;74:2300–2312. doi: 10.1016/j.jprot.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetta E, Mallozzi C, Ruggieri V, Pietraforte D, Federico M, Sanchez M. HIV-1 nef induces p47(phox) phosphorylation leading to a rapid superoxide anion release from the U937 human monoblastic cell line. J Cell Biochem. 2009;106:812–822. doi: 10.1002/jcb.22041. [DOI] [PubMed] [Google Scholar]
- Olivetta E, Pietraforte D, Schiavoni I, Minetti M, Federico M, Sanchez M. HIV-1 nef regulates the release of superoxide anions from human macrophages. Biochem J. 2005;390:591–602. doi: 10.1042/BJ20042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications, emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Trauger SA, Kalisiak E, Ellis RJ, Siuzdak G, Fox HS. Cerebrospinal fluid proteomics reveals potential pathogenic changes in the brains of SIV-infected monkeys. J Proteome Res. 2009;8:2253–2260. doi: 10.1021/pr800854t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem Int. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Levi G, Minghetti L. Human immunodeficiency virus type 1 tat protein stimulates inducible nitric oxide synthase expression and nitric oxide production in microglial cultures. J Neuropathol Exp Neurol. 1999;58:825–831. doi: 10.1097/00005072-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Poole LB, Zeng BB, Knaggs SA, Yakubu M, King SB. Synthesis of chemical probes to map sulfenic acid modifications on proteins. Bioconjug Chem. 2005;16:1624–1628. doi: 10.1021/bc050257s. [DOI] [PubMed] [Google Scholar]
- Prakash A, Rezai T, Krastins B, Sarracino D, Athanas M, Russo P, Ross MM, Zhang H, Tian Y, Kulasingam V, Drabovich AP, Smith C, Batruch I, Liotta L, Petricoin E, Diamandis EP, Chan DW, Lopez MF. Platform for establishing interlaboratory reproducibility of selected reaction monitoring-based mass spectrometry peptide assays. J Proteome Res. 2010;9:6678–6688. doi: 10.1021/pr100821m. [DOI] [PubMed] [Google Scholar]
- Prakash A, et al. Interlaboratory reproducibility of selective reaction monitoring assays using multiple upfront analyte enrichment strategies. J Proteome Res. 2012;11:3986–3995. doi: 10.1021/pr300014s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Bregere C, Kamzalov S, Gallaher TK, Sohal RS. Nitration of tryptophan 372 in succinyl-CoA:3-ketoacid CoA transferase during aging in rat heart mitochondria. Biochemistry. 2007;46:10130–10144. doi: 10.1021/bi7001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek W, Ricardo-Dukelow M, Holloway S, Gendelman HE, Wojna V, Melendez LM, Ciborowski P. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. J Proteome Res. 2007;6:4189–4199. doi: 10.1021/pr070220c. [DOI] [PubMed] [Google Scholar]
- Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: Independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Safinowski M, Wilhelm B, Reimer T, Weise A, Thome N, Hanel H, Forst T, Pfutzner A. Determination of nitrotyrosine concentrations in plasma samples of diabetes mellitus patients by four different immunoassays leads to contradictive results and disqualifies the majority of the tests. Clin Chem Lab Med. 2009;47:483–488. doi: 10.1515/CCLM.2009.095. [DOI] [PubMed] [Google Scholar]
- Sala A, Nicolis S, Roncone R, Casella L, Monzani E. Peroxidase catalyzed nitration of tryptophan derivatives. mechanism, products and comparison with chemical nitrating agents. Eur J Biochem. 2004;271:2841–2852. doi: 10.1111/j.1432-1033.2004.04219.x. [DOI] [PubMed] [Google Scholar]
- Sampson JB, Rosen H, Beckman JS. Peroxynitrite-dependent tyrosine nitration catalyzed by superoxide dismutase, myeloperoxidase, and horseradish peroxidase. Methods Enzymol. 1996;269:210–218. doi: 10.1016/s0076-6879(96)69023-5. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Reynolds GP. Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995;187:9–12. doi: 10.1016/0304-3940(95)11324-p. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Bell JE, Reynolds GP. Increased concentrations of the neurotoxin 3-hydroxykynurenine in the frontal cortex of HIV-1-positive patients. J Neurochem. 1995;64:932–935. doi: 10.1046/j.1471-4159.1995.64020932.x. [DOI] [PubMed] [Google Scholar]
- Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: Focus on HNE and one. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Sethuraman M, McComb ME, Huang H, Huang S, Heibeck T, Costello CE, Cohen RA. Isotope-coded affinity tag (ICAT) approach to redox proteomics: Identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J Proteome Res. 2004;3:1228–1233. doi: 10.1021/pr049887e. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite longstanding suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Sinha V, Wijewickrama GT, Chandrasena RE, Xu H, Edirisinghe PD, Schiefer IT, Thatcher GR. Proteomic and mass spectroscopic quantitation of protein S-nitrosation differentiates NO-donors. ACS Chem Biol. 2010;5:667–680. doi: 10.1021/cb100054m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood HS, Lourette NM, Boschek CB, Bigelow DJ, Smith RD, Pasa-Tolic L, Squier TC. Identification of a denitrase activity against calmodulin in activated macrophages using high-field liquid chromatography--FTICR mass spectrometry. Biochemistry. 2007;46:10498–10505. doi: 10.1021/bi7009713. [DOI] [PubMed] [Google Scholar]
- Stark G. Functional consequences of oxidative membrane damage. J Membr Biol. 2005;205:1–16. doi: 10.1007/s00232-005-0753-8. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, Malpica T, Pocernich C, Butterfield DA, Nath A. Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal. 2006;8:2089–2100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- Sturgeon CM, Seth J. Why do immunoassays for tumour markers give differing results?--a view from the UK national external quality assessment schemes. Eur J Clin Chem Clin Biochem. 1996;34:755–759. [PubMed] [Google Scholar]
- Sturgeon CM, Sprague SM, Metcalfe W. Variation in parathyroid hormone immunoassay results--a critical governance issue in the management of chronic kidney disease. Nephrol Dial Transplant. 2011;26:3440–3445. doi: 10.1093/ndt/gfr614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Munoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol. 2001;60:885–892. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- Tsikas D. Analytical methods for 3-nitrotyrosine quantification in biological samples: The unique role of tandem mass spectrometry. Amino Acids. 2012;42:45–63. doi: 10.1007/s00726-010-0604-5. [DOI] [PubMed] [Google Scholar]
- Tsikas D. Measurement of nitrotyrosine in plasma by immunoassays is fraught with danger: Commercial availability is no guarantee of analytical reliability. Clin Chem Lab Med. 2010;48:141–3. doi: 10.1515/CCLM.2010.015. author reply 145–6. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Frolich JC. Trouble with the analysis of nitrite, nitrate, S-nitrosothiols and 3-nitrotyrosine: Freezing-induced artifacts? Nitric Oxide. 2004;11:209–13. doi: 10.1016/j.niox.2004.09.001. author reply 214–5. [DOI] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-tat. Antioxid Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Velazquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Melendez LM. Antioxidant enzyme dysfunction in monocytes and CSF of hispanic women with HIV-associated cognitive impairment. J Neuroimmunol. 2009;206:106–111. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais PV. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki I, Piette LH. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990;265:13589–13594. [PubMed] [Google Scholar]
- Zang L, Carlage T, Murphy D, Frenkel R, Bryngelson P, Madsen M, Lyubarskaya Y. Residual metals cause variability in methionine oxidation measurements in protein pharmaceuticals using LC-UV/MS peptide mapping. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;895–896:71–76. doi: 10.1016/j.jchromb.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Zhang D, Wang H, Whorton AR, Xian M. Reductive ligation mediated one-step disulfide formation of S-nitrosothiols. Org Lett. 2010a;12:4208–4211. doi: 10.1021/ol101863s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Monroe ME, Chen B, Chin MH, Heibeck TH, Schepmoes AA, Yang F, Petritis BO, Camp DG, 2nd, Pounds JG, Jacobs JM, Smith DJ, Bigelow DJ, Smith RD, Qian WJ. Endogenous 3,4-dihydroxyphenylalanine and dopaquinone modifications on protein tyrosine: Links to mitochondrially derived oxidative stress via hydroxyl radical. Mol Cell Proteomics. 2010b;9:1199–1208. doi: 10.1074/mcp.M900321-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Diefenbach E, Crossett B, Tran SL, Ng T, Rizos H, Rua R, Wang B, Kapur A, Gandhi K, Brew BJ, Saksena NK. First evidence of overlaps between HIV-associated dementia (HAD) and non-viral neurodegenerative diseases: Proteomic analysis of the frontal cortex from HIV+ patients with and without dementia. Mol Neurodegener. 2010;5:27-1326-5-27. doi: 10.1186/1750-1326-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


