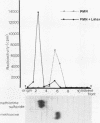
Abstract
Studies of the photosensitized oxidation have demonstrated that photodynamic oxidation of methionine is mediated by singlet oxygen (1O2). In this study, we demonstrated that phagocytosing human polymorphonuclear leukocytes (PMN), but not resting PMN, oxidized both intracellular and extracellular methionine to methionine sulfoxide. N-ethylmaleimide, which inhibits phagocytosis and cellular metabolism, inhibited the oxidation of methionine. Neutrophils from patients with chronic granulomatous disease did not oxidize methionine even in the presence of phagocytosis. The oxidation of methionine by phagocytosing normal PMN was inhibited by 1O2 quenchers, (1.4-diazabicyclo-[2,2,2]-octane, tryptophan, NaN3), myeloperoxidase (MPO) inhibitors (NaN3, KCN) and catalase. In contrast, superoxide dismutase, ethanol, and mannitol had no effect. Furthermore, 1O2 quenchers did not interfere with the production of superoxide (O2−) by phagocytosing PMN. The combination of catalase and SOD did not enhance the inhibition of methionine by phagocytosing PMN. On the other hand, deuterium oxide stimulated the oxidation of methionine by PMN almost 200%.
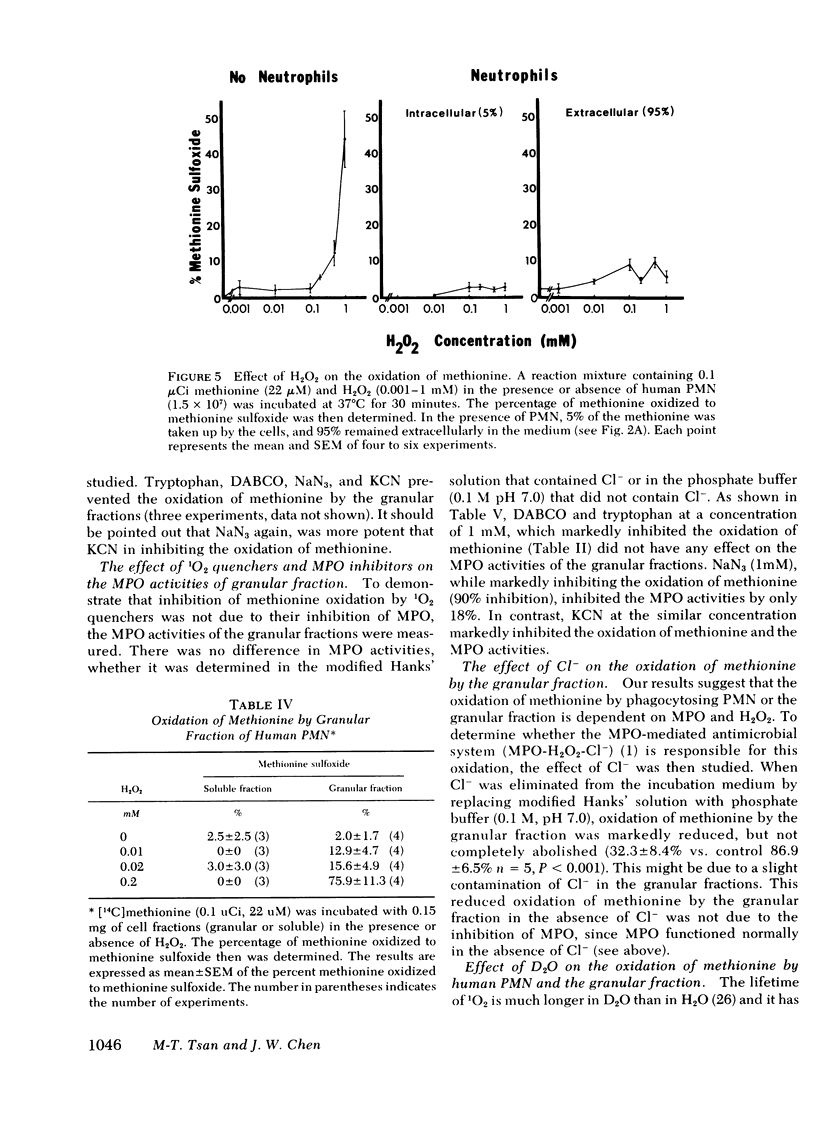
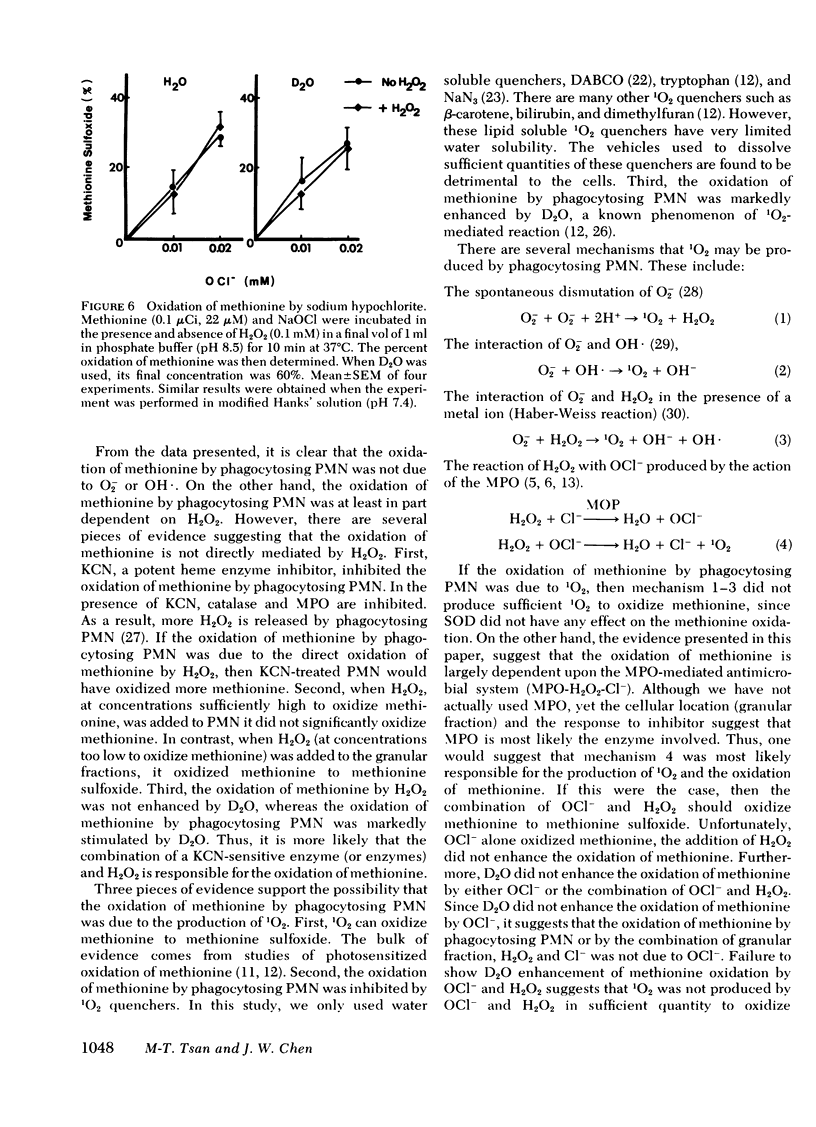
H2O2 at high concentrations oxidized methionine to methionine sulfoxide. However, when similar amounts of H2O2 were added to human PMN, they did not oxidize methionine. In contrast, when H2O2, at concentrations too low to oxidize methionine by itself, was added to the granular fraction, but not the soluble fraction, they oxidized methionine to methionine sulfoxide. The oxidation of methionine by the combination of H2O2 and granular fractions was inhibited by 1O2 quenchers and MPO inhibitors, but it was stimulated by deuterium oxide. Removal of chloride anion also prevented the oxidation of methionine by the granular fractions.
Our results suggest that the oxidation of methionine by phagocytosing PMN is dependent on the MPO-mediated antimicrobial system (MPO-H2O2-Cl−). They also suggest, but do not prove that the oxidation of methionine is mediated by 1O2. Oxidation of methionine may be one of the mechanisms that human PMN damage microorganisms.
Full text
PDF

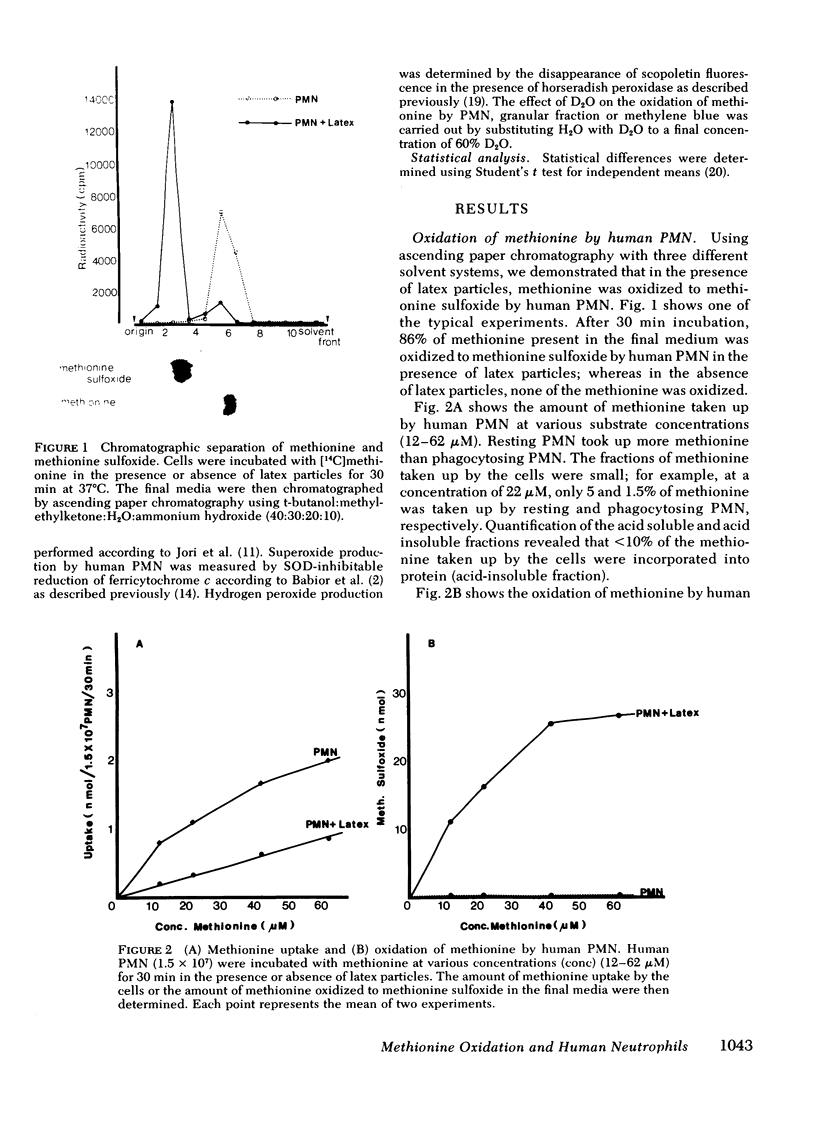
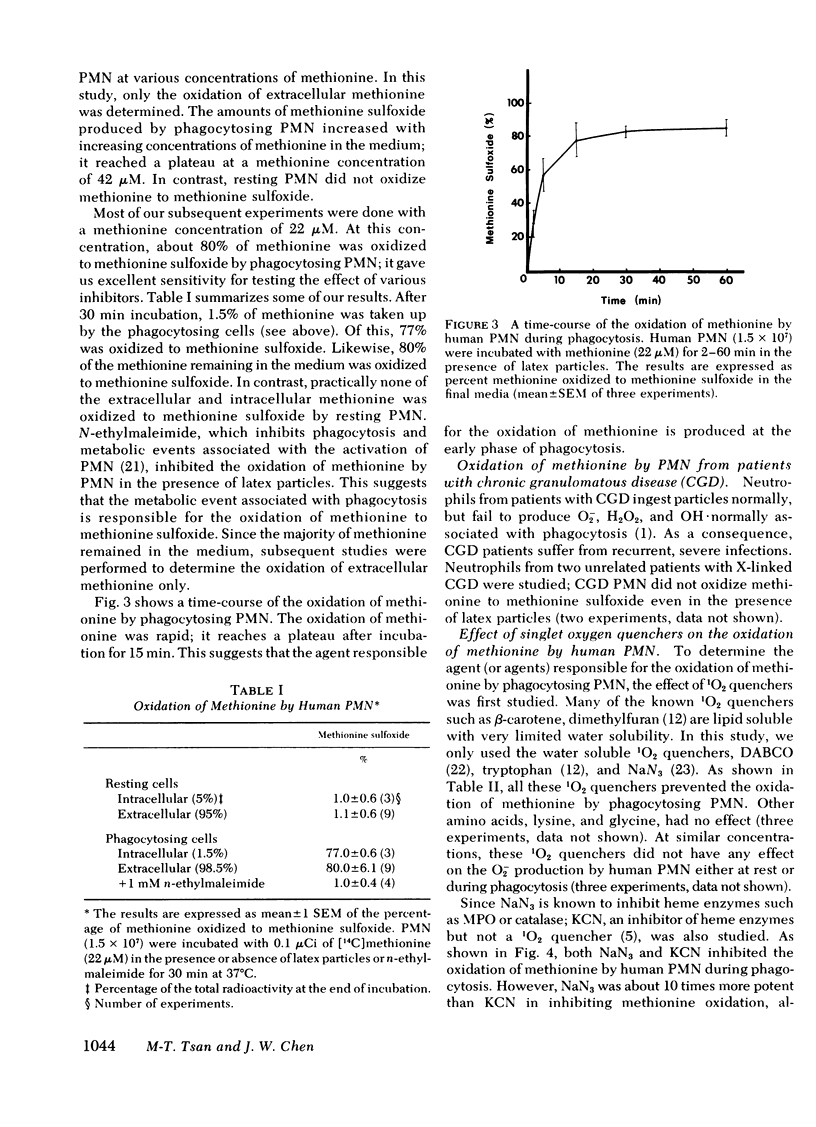
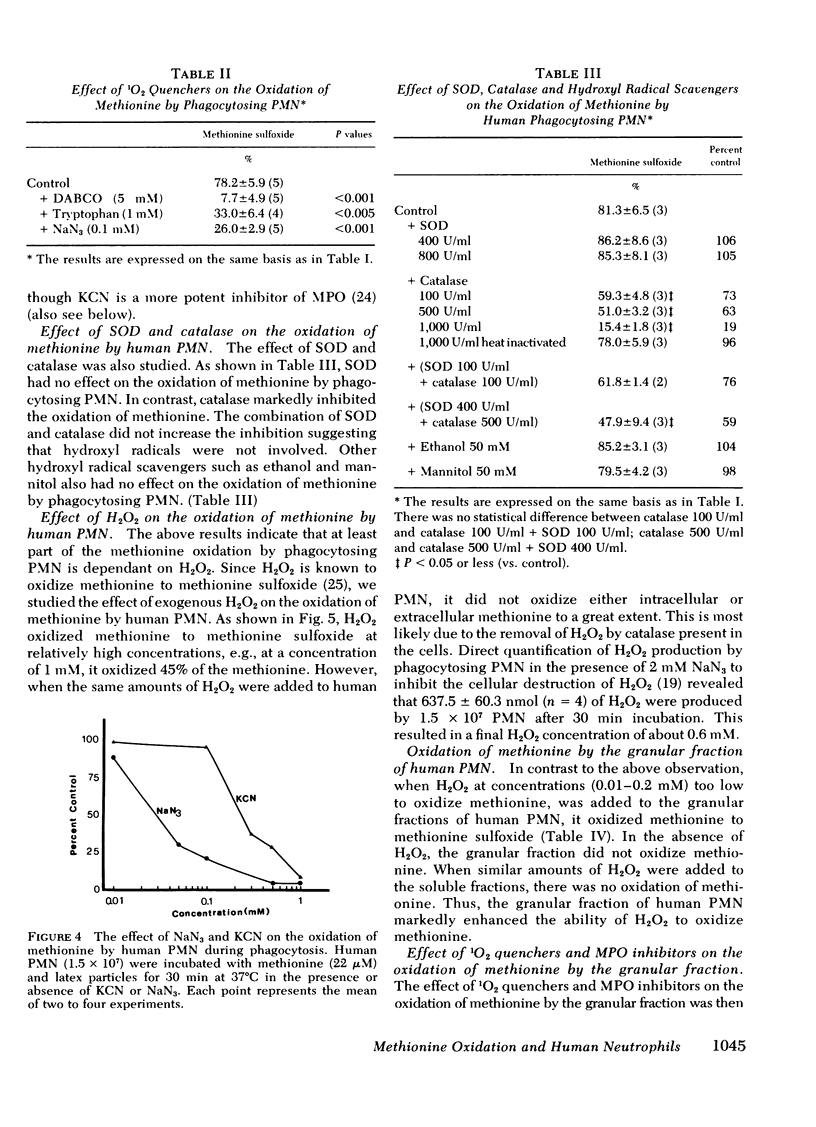



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Arneson R. M. Substrate-induced chemiluminescence of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1970 Feb;136(2):352–360. doi: 10.1016/0003-9861(70)90205-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Dye-sensitized selective photooxidation of methionine. Biochim Biophys Acta. 1968 Jan 22;154(1):1–9. doi: 10.1016/0005-2795(68)90252-3. [DOI] [PubMed] [Google Scholar]
- Khan A. U. Singlet molecular oxygen from superoxide anion and sensitized fluorescence of organic molecules. Science. 1970 Apr 24;168(3930):476–477. doi: 10.1126/science.168.3930.476. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J., Jr, Karnovsky M. L. Role of peroxide in the stimulation of the hexose monophosphate shunt during phagocytosis by polymorphonuclear leukocytes. Enzyme. 1972;13(1):110–131. doi: 10.1159/000459652. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Membrane transport in the rabbit alveolar macrophage. The specificity and characteristics of amino acid transport systems. Biochim Biophys Acta. 1971 Jul 6;241(1):155–169. doi: 10.1016/0005-2736(71)90313-0. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Douglass K. H., McIntyre P. A. Hydrogen peroxide production and killing of Staphylococcus aureus by human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):437–444. [PubMed] [Google Scholar]
- Tsan M. F., McIntyre P. A. Stimulation by propylthiouracil of the hexose monophosphate shunt in human polymorphonuclear leucocytes during phagocytosis. Br J Haematol. 1975 Oct;31(2):193–208. doi: 10.1111/j.1365-2141.1975.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Newman B., McIntyre P. A. Surface sulphydryl groups and phagocytosis-associated oxidative metabolic changes in human polymorphonuclear leucocytes. Br J Haematol. 1976 Jun;33(2):189–204. doi: 10.1111/j.1365-2141.1976.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Tsan M., McIntyre P. A. The requirement for membrane sialic acid in the stimulation of superoxide production during phagocytosis by human polymorphonuclear leukocytes. J Exp Med. 1976 Jun 1;143(6):1308–1316. doi: 10.1084/jem.143.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]