Abstract
Dietary fish oil, a source of polyunsaturated fatty acids (n-3 PUFA), has become increasingly popular for antidepressant therapy, in part because about half of patients treated with conventional antidepressants either fail to remit or discontinue therapy due to side effects. The inception of n-3 PUFA as a putative depression therapeutic may have stemmed from reports suggesting that dietary n-3 PUFA deficiency is linked to both altered membrane PUFA content as well as clinical depression. Several studies have examined n-3 PUFA treatment in depression, either singly or in combination with conventional antidepressant drugs. While results have been encouraging, fish oil treatment remains controversial. At least some of the reason for this is the lack of a defined site of action for n-3 PUFA that would be consistent with an antidepressant effect. This review will address this issue. While it is possible, even likely, that n-3 PUFA have multiple sites of action, this chapter will focus on sites at which n-3 PUFA modify G protein signaling and how those sites relate to both depression and antidepressant action. Much of the focus herein will be on specialized membrane domains (lipid rafts) and the effects that agents modifying those rafts have on elements of G protein signaling cascades. The relevance of specific alterations of G protein signaling for both depression and antidepressant action will be discussed, as will the ability for n-3 PUFA to act either as an antidepressant or in concert with conventional antidepressants.
Keywords: G-protein, lipid raft, depression, antidepressant, fatty acids, fish oil
INTRODUCTION
Docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and related n-3 PUFAs are major constituents of neural membranes and therefore potential mediators of neuronal signaling pathways [1]. A number of studies have addressed the possibility that dietary PUFA is relevant to psychiatric disease, particularly depression [1, 2]. Research has focused both on dietary deficiencies in these fatty acids contributing to pathology and the potential for supplementation to ameliorate malaise, either alone or acting synergistically with conventional therapies. The former is derived from longitudinal studies and the latter is very attractive because fish oil supplementation has a very low side effect profile and likely includes somatic benefits. This interest is perhaps most true for depression, where dozens of clinical trials have attempted to clarify whether fatty acids can affect disease outcome. To date, meta-analyses of these studies have proved inconclusive, largely because of inconsistencies between study design, particularly dosage and choice of fatty acid compound.
The lack of clear outcome of these clinical studies and the increasing therapeutic potential of fatty acids in other systems and disease states (e.g., cardiovascular) has lead to the investigation of putative mechanisms of n-3 PUFA action at the cellular level. From this, a diverse yet related collection of signaling pathways have been identified that are affected by n-3 PUFAs. Among these, we believe one of the most prominent and relevant to depression is the effect n-3 PUFAs have on G-protein coupled receptor (GPCR) signaling.
G-protein coupled receptors are a family of about 1, 000 transmembrane proteins that respond to a variety of hormones and neurotransmitters as well as odorant and tastant molecules. It is estimated that 50% of current pharmaceuticals target these receptors. Most intriguing for the possibility of modification by PUFA is the evidence that these receptors and their attendant signaling cascades are influenced by their distribution among membrane microdomains. These microdomains, referred to herein as lipid rafts (Fig. 1), selectively influence GPCR signaling, potentiating some signaling pathways and inhibiting others [3]. Fig. (2) illustrates this.
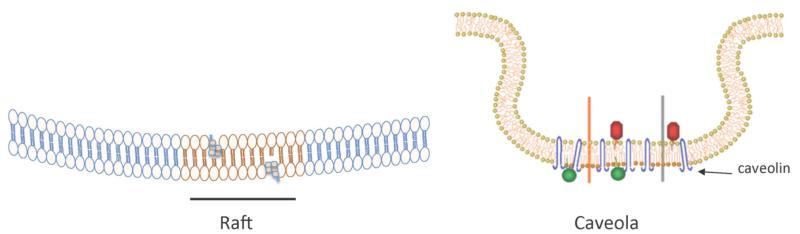
Fig. (1). Lipid rafts and caveolae.
Sphignolipid acyl chains are more tightly packed in rafts. Cholesterol also inserts here, creating a highly-ordered environment that “floats” in a less-ordered, loosely packed, phospholipid sea. Caveolae add the transmembrane protein caveolin to the specialized lipid domains and this helps create the depicted invaginations.
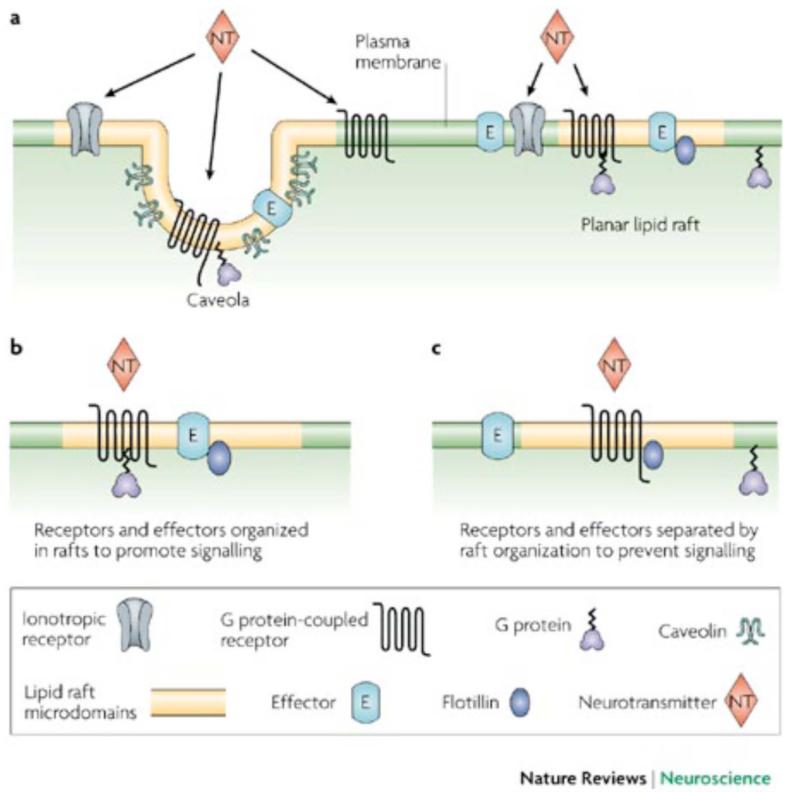
Fig. (2). Organization of signaling components into lipid rafts and caveolae.
Both caveolae and non-caveolae, planar, rafts recruit or segregate proteins to enhance or dampen signal transduction (From Allen et.al, 2007). On the left, all membrane components of a single signaling cascade are included within the same raft, augmenting signaling. On the right, the GPCR is shown within the raft while the G protein and effector enzyme are in non-raft domains. This would dampen signaling. Reprinted from [3] with permission of the publisher.
Dietary fish oil, a source of n-3 PUFA, has become increasingly popular for antidepressant therapy in part, because about half of patients treated with conventional antidepressants fail to remit or discontinue therapy due to side effects [4]. The inception of n-3 PUFA as a putative depression therapy may have stemmed from reports suggesting that dietary n-3 PUFA deficiency is linked to depression [5]. A study by Frasure-Smith and colleagues [6] showed that depressed patients had lower concentrations of total omega-3 and DHA, higher ratios of AA to DHA and higher n-6:n-3 ratios than controls; these findings have been confirmed by a recent meta-analysis [7]. In a small sample (n = 24), depressed subjects had lower red blood cell (RBC) membrane levels of omega-3 fatty acids than healthy controls and severity of depression correlated with both levels and dietary intake of omega-3 fatty acids [8]. The omega-3 fatty acid composition of RBC membrane phospholipids, and particularly DHA content, were significantly depleted among depressed compared with control subjects in a similar study [9]. Note, however, that there is significant controversy as to the effectiveness of fish oil in depression. In one study, patients with major depressive disorder (MDD) were found to have a reduction of total omega-3 fatty acids as well as alpha-linoleic acid (ALA) and EPA in serum cholesteryl esters compared with adults with minor depression or healthy controls (n = 74) [10]. These same authors did not observe increases in membrane n-3 PUFA following selective serotonin uptake inhibitor (SSRI) antidepressant treatment.
Studies with PUFA-deficient diets showed behavioral and cognitive deficits in mice that were reversed by addition of n-3 PUFA to their diets. Concentrations of DHA and EPA were highest in the frontal cortex [11]. Rats deprived of n-3 PUFA for a generation showed “depression-like” behaviors on several tests [12] and examination of the brains from these animals showed a deficiency in brain-derived neurtrophic factor (BDNF) as well as signs of a chronic decrease in cAMP, a product of GPCR signaling [13]. Transgenic mice with constitutively elevated n-3 PUFA showed many of the same behavioral characteristics seen in animals treated chronically with antidepressants [14]. Several mechanisms have been proposed for putative antidepressant effects of n-3 PUFA. The two major hypotheses for n-3 PUFA action include anti-inflammatory properties [15] and direct interactions with membranes (vide infra). Below we will discuss these membrane effects and their influence on G-protein signaling, suggesting a common mechanism between traditional antidepressants and n-3 PUFA.
MEMBRANE FLUIDITY AND FATTY ACYLATION
Membranes are not homogeneous proteolipid sheets, but are quite structurally varied (Fig. 1). In addition to a heterogeneous distribution of protein, membrane lipids are divided into cholesterol-rich, cytoskeletal-associated domains, commonly referred to as lipid rafts and non-raft regions with a different lipid composition [16]. It is noteworthy that DHA, depending upon the cell type, can comprise as much as 50% of the plasma membrane lipid. Even in membranes where DHA is a lesser component, it appears to influence membrane function. Whereas cholesterol-rich lipid rafts represent a highly ordered structure, DHA is often considered the “anti-cholesterol”. Highly disordered DHA domains have been called the antithesis of lipid raft domains as well as the ultimate non-raft membrane domain [17, 18]. Regardless of diet, membrane DHA content varies tremendously throughout the body. The most inclusive membranes include synaptosomes, sperm, and the retinal rod outer segment, where DHA accounts for 50 mol% of the lipid species. Supplementation however can augment some membranes DHA content 2-10 fold [18].
Controversy exists over the site of DHA incorporation in membranes. Computational models suggest DHA and cholesterol do not interact, but analysis of actual raft and non-raft samples show DHA is present in both [18]. This discrepancy is likely the result of our limitation to resolve lipid raft domains. One group proposes that most of the DHA may be partitioning in discreet regions within rafts to avoid cholesterol interactions [19]. Computational analysis of DHA, stearic acid and cholesterol binding with rhodposin predicts interactions with all of these, but only DHA is suggested to associate in an arrangement that increase rhodpsin’s kinetics. This is consistent with in vitro experiments that show DHA increases phototransduction whereas cholesterol inhibits it [20].
The idea of DHA being anti-cholesterol extends into the clinic as well where it is suggested to benefit hypertriglyceridemia. The cardiovascular benefit of n-3 PUFAs has even lead to an unusual move for a dietary supplement: the U.S. FDA approval of a specific blend of omega-3-acid ethyl esters in a product called Lovaza. Given similar indications, n-3 PUFAs are often discussed alongside statins, even though they are chemically distinct compounds. Interestingly, however, statins are believed to worsen symptoms of depression and anxiety whereas n-3 PUFAs alleviate them [21]. This suggests that the effects of n-3 PUFAs on the brain may result from effects outside of cholesterol regulation and membrane fluidity.
Compared to n-3 PUFAs, the signaling disruption of statins is better understood. Statins do not simply prevent cholesterol synthesis, but inhibit the synthesis of many cholesterol precursors. The most significant is geranyl-geranyl pyrophosphate, a necessary lipid that through prenylation, covalently attaches to numerous proteins to mediate membrane association. G-proteins, especially G , are reliant on prenylation to anchor to the membrane and therefore signal through their receptors. n-3 PUFAs on the other hand may interfere with another lipid modification, fatty acylation. While this process may also be important in global membrane anchoring, it is generally considered to be more instrumental in targeting proteins to lipid raft membrane regions. Instead of a geranyl-geranyl pyrophosphate moiety, fatty acylation usually occurs with a medium chain, saturated fatty acid such as palmitic acid. It is unclear however, if sites of fatty acylation are completely faithful to palmitic acid, or whether similar length fatty acids (such as DHA or EPA) can take their place. At least one group suggests that palmitoylation of Fyn can be inhibited by DHA and EPA through acylation with these fatty acids instead. They also showed that this swap decreased Fyn localization to rafts, perhaps because n-3 PUFAs are less hydrophobic than palmitic acid [22].
If n-3 PUFAs are regulating fatty acylation in the brain, this effect may be responsible for many of the benefits of n-3 PUFA supplementation. Synaptic proteins are heavily palmitoylated, and changes in their acylation state are thought to be essential to proper trafficking and function [23]. Many neuropathologies such as Huntington’s disease [24, 25] and schizophrenia [26, 27] show alterations in protein acylation. In fact, almost all monoamine-activated GPCRs have putative or demonstrated palmitoylation sites [28, 29]. Serotonergic receptors are among the best studied [30].
Both increases and decreases in acylation may correlate with disease because as with phosphorylation, acylation levels may require fine-tuning. Unlike phosphorylation, palmitoylation cannot be easily examined because fatty acids are much less antigenic and difficult to radiolabel with strong emitters. Therefore the significance of palmitoylation in signaling is mostly derived from mutant proteins where acylation is blocked. As a result, acylation by other fatty acids such as n-3 PUFAs are also prevented. Fortunately advances in mass spectroscopy and the identification of acyl-transferase proteins may soon help offer insight into their role.
GPCRs AND FATTY ACIDS
The enormous diversity of GPCRs throughout biology justifies the current deficiencies in our understanding of them, despite decades of research and many spectacular findings. The range of GPCR ligands was recently expanded to include fatty acids. Long-hydrophobic carbon chains were controversial as GPCR ligands because they were structurally very dissimilar from most described ligands and because they could associate with the membrane very directly.
Traditionally the focus of fatty acids and GPCRs has been on the ability of fatty acids to be metabolized into ligands for GPCRs. This is the basis for the dueling-roles of n-6 and n-3 fatty acids, as their various metabolites are implicated in opposing biologic roles. For example, both the n-6 archadonic acid and the n-3 eicosapentaenoic acid are metabolized into various eicosanoids, a well studied class of GPCR ligands in the immune and cardiovascular systems [31]. Many of the effects on inflammation are reviewed elsewhere [32]. Pariante and colleagues have suggested that anti-inflammatory properties of n-3 PUFA are the mediators of the observed antidepressant effects [33]. While this is possible, even likely, we suggest multiple roles for these compounds, and focus on membrane properties thereof.
Recently, research has also begun to focus the role of these fatty acids themselves, rather than their metabolites, on GPCR activation. In the past decade, several previously orphaned GPCRs have been identified as receptors for lipid messengers [34]. The first characterized, GPR40, possesses promiscuity for many lengths and saturation of fatty acids, whereas others, like GPR120, are more selective. Presently, it is believed that all of these receptors are coupled to the Gαq pathway, which is described below. It has also been hypothesized that particular fatty acids interact differently with the same receptor.
Still, the physiologic significance of these free fatty acids receptors is not well established. The leading clue to their function is their distribution pattern in the body. Most are expressed in at least one type of immune cell, whereas GPR41 and GPR43 are also highly expressed in adipose tissue, GPR40 is also found in brain and pancreas tissue, and GPR120 is also found in adipose, lung, gut and brain tissues [34].
Most physiologic characterization has been spent investigating the relationship of these receptors in metabolic disorders. Oh and colleagues hypothesize that GPR120 plays a significant role in both insulin resistance and inflammation. They demonstrated that n-3 PUFAs increase insulin sensitivity in obese mice only when GPR120 is present [35]. Other efforts to study free fatty acid receptors in metabolic disorders have been reviewed [36, 37].
As an extension of this, much of the work on these receptors in the nervous system relate to the gut-brain axis as these compounds, which are generated in the GI system, yet freely penetrate the blood-brain barrier, have signaling properties in both brain and gut. The ability of GPR40 and GPR120 to help mediate taste perception lead some researchers to ponder if these are valuable targets for fighting obesity [38]. It is even possible that these receptors are important in neurogenesis and neurodevelopment [39]. One preliminary line of evidence is that immunostaining of newborn neurons is especially high for GPR40 versus mature neurons [40]. Other ideas about free fatty acid receptor signaling in the brain are purely speculative. Below, we describe many second-messenger signaling pathways that are affected by fatty acids that may be acting in concert with these receptors.
REGULATION OF CAMP THROUGH LIPID RAFTS IN DEPRESSION
The localization of G proteins to specific membrane domains such as caveolae [41] and lipid rafts has generated interest in these cholesterol and sphingolipid-rich detergent-resistant membrane domains and how they affect G protein targeting and function [3]. More recent data suggest that lipid rafts represent areas where Gαq signaling is promoted [42] and where signaling through Gαs is inactivated [3, 43, 44]. There is a long experimental history of agents that increase “membrane fluidity” increasing agonist- and Gαs - mediated adenylyl cyclase. Levitski and his colleagues [45] examined this thoroughly in turkey erythrocytes and we observed this in synaptic membranes [46]. Gαs activates adenylyl cyclase more efficiently outside of lipid rafts and chronic treatment with antidepressants facilitates G protein exodus from those rafts [44, 47]. Treatment of lymphocytes with DHA displaced phospholipase D (PLD) from lipid rafts, increasing PLD activity by facilitating association with its non-raft small G protein activator [48].
A number of studies suggest that chronic antidepressant treatment increased physical coupling between Gαs and adenylyl cyclase. This was investigated using immunoprecipitation of Gαs adenylyl cyclase complexes with anti- Gαs antibodies [49]. This study also provided independent verification that there was no increase in Gαs content after antidepressant treatment. The total amount of adenylyl cyclase immunoprecipitated by anti- Gαs increased after antidepressant treatment, consistent with the idea that antidepressant treatment increases coupling between Gαs and adenylyl cyclase [49]. This is consistent with the observation that chronic treatment with antidepressants results in long-term increases in cellular cAMP [50] as illustrated in Fig. (3).
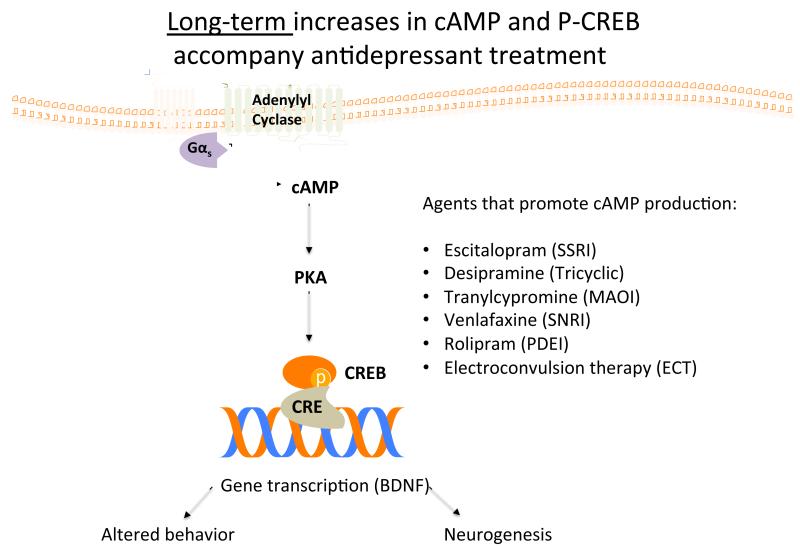
Fig. (3). Long-term_increases in cAMP and P-CREB accompany antidepressant treatment.
Chronic, but not acute, antidepressant treatment enhances cAMP signaling by increasing Gαs-adenylyl cyclase coupling. Likewise, n-3 PUFAs also increase BDNF, a neurotropic factor increased with CREB phosphorylation, perhaps through enhancement of G-protein signaling. Modified from [50].
More recent data demonstrated that Gαs (and not other G proteins) became more detergent soluble after antidepressant treatment and antidepressants have been shown to decrease Gαs localization in lipid rafts [47, 51-53]. This has been observed after chronic treatment with fluoxetine, desipramine, escitalopram and phenelzine. It has also been shown that some antidepressant and some antipsychotic drugs (no antipsychotic tested affects Gαs raft localization of Gαs -adenylyl cyclase coupling) concentrated in lipid rafts during the course of chronic treatment [54]. Taken together, these data suggested that chronic antidepressant treatment moved Gαs to a region of the plasma membrane where it was less complexed with cytoskeletal elements and more available to activate adenylyl cyclase. This hypothesis is consistent with the increased “cAMP tone” that investigators, looking at products of genes activated by cAMP response elements, have observed subsequent to antidepressant treatment [50].
Note, however, that while both lipid raft disruption and chronic antidepressant treatment increase Gαs-adenylyl cyclase coupling, antidepressant treatment is selective both in the signaling proteins that it effects (only Gαs) and the cell types in which it works [47, 55]. HEK 293 cells show concentration of antidepressants in lipid rafts but do not show increased Gαs-adenylyl cyclase coupling unless those HEK-293 cells are expressing type VI adenylyl cyclase. It is noteworthy that the acute effects of cytoskeletal-disrupting drugs at increasing the coupling between Gαs and adenylyl cyclase are also not additive with chronic antidepressant treatment [47]. There may be an intimate relationship between Gαs, adenylyl cyclase and microtubules in lipid rafts [56] (see Fig. 4).
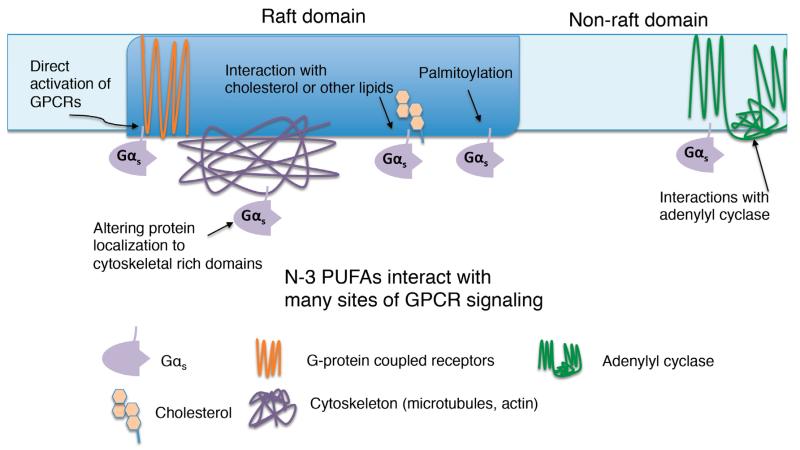
Fig. (4). GPCR signaling is altered by n-3 PUFAs: possible sites of action.
Fatty acids, including n-3 PUFAs, influence GPCR signaling by activating GPCRs, interfering with G-protein localization, due either to altered raft properties or altered acylation. N-3 PUFA may also alter membrane fluidity.
Postmortem studies reveal increased lipid raft localization of Gαs in suicide victims compared to age-matched control subjects [57], a finding consistent with an attenuation of Gαs - adenylyl cyclase coupling. It appears that human peripheral tissue may show similar effects. A study of about 1, 500 subjects shows that that AlF4- (Gαs) stimulated adenylyl cyclase activity is significantly lower in platelets from depressed than from non-depressed subjects [58]. Similarly, decreased activation of platelet PGE2-activated adenylyl cyclase resolved, as early as one week, in those subjects showing an antidepressant response at 6 weeks [59] (Fig. 4).
As mentioned above, clinical trials of n-3 PUFA, alone or in combination with conventional antidepressants, have had mixed results. A recent meta-analysis by Mischoulon et. al suggests a slight advantage of n-3 PUFA over placebo, something that many conventional antidepressants often struggle to achieve [60]. Further, recent data by Rapaport and colleagues demonstrate a clear advantage to n-3 PUFA supplementation of SSRI therapy [61]. This suggests the possibility that n-3 PUFA may have multiple sites and mechanisms of action. Two possible sites or mechanisms include direct changes in lipid composition and combination between n-3 PUFAs and palmitic acid for the acylation of lipid raft associated proteins.
A number of authors have suggested the involvement of BDNF in both depression and antidepressant action and Pandey and colleagues have measured both BDNF and trkB in depression and antidepressant response [62]. trkB, the receptor for BDNF, is lipid raft associated. N-3 PUFA treatment has been suggested to increase BDNF and the structural changes associated with synaptic plasticity in rats [3, 63].
CONCLUSIONS
While the role of n-3 PUFA as an antidepressants continues to remain unsettled, this review has suggested a mechanism where their actions are consistent with those of antidepressants. By extension, a mechanism for lowered n-3 PUFA playing a role in depression is also insinuated. Curiously, the observations that n-3 PUFA might potentiate SSRI action have also been lent credence to a potential role for n-3 PUFAs in depression sincee modification of G protein acylation would provide an independent site of action for fish oil, both in modifying antidepressants and in modifying G protein signaling (hence neurotransmitter responsiveness). The information provided in this review, both data and speculation, confirm the importance of continued investigation into the role of fish oil, both as a modulator of G protein signaling and as a therapeutic partner for depression.
ABBREVIATIONS
- AA
Arachidonic acid
- ALA
Alpha-linoleic acid
- BDNF
Brain-derived neurtrophic factor
- cAMP
Cyclic adenosine monophosphate
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- GPCR
G-protein coupled receptor
- MDD
Major depressive disorder
- PLD
Phospholipase D
- n-3 PUFA
n-3 Polyunsaturated fatty acids
- RBC
Red blood cells
- SSRI
Selective serotonin uptake inhibitor
REFERENCES
- [1].Levant B. N-3(omega-3) polyunsaturated fatty acids in the pathophysiology and treatment of depression: pre-clinical evidence. In J.M. Witkin and X. Li (eds), Therapeutic potential of some natural products in the treatment of major depressive disorder. CNS Neurol. Disord. Drug Targets. 2013;12(3):XXX–XXX. doi: 10.2174/1871527311312040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kang JX, Gleason ED. Omega-3 fatty acids and hippocampal neurogenesis in depression. in j.m. witkin and x. li (eds), therapeutic potential of some natural products in the treatment of major depressive disorder. CNS Neurol. Disord. Drug Targets. 2013;12(3):XXX–XXX. doi: 10.2174/1871527311312040004. [DOI] [PubMed] [Google Scholar]
- [3].Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007;8(2):128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- [4].Rush A, Trivedi M, Wisniewski S. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a star* d report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- [5].Hibbeln JR, Umhau JC, George DT, Salem N., Jr. Do plasma polyunsaturates predict hostility and depression? World Rev. Nutr. Diet. 1997;82:175–186. doi: 10.1159/000059633. [DOI] [PubMed] [Google Scholar]
- [6].Frasure-Smith N, Lespérance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol. Psychiatry. 2004;55(9):891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- [7].Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry. 2010;68(2):140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [8].Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J. Affect. Disord. 1998;48(2-3):149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- [9].Peet M, Murphy B, Shay J. Depletion of Omega-3 Fatty Acid Levels in Red Blood Cell Membranes of Depressive Patients. Biol. Psychiatry. 1998;43(5):315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- [10].Maes M, Smith R, Christophe A, Cosyns P. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J. Affect. Discord. 1996;38(1):35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- [11].Franc s, H., Dra P, Smirnova M, Carrié I. Nutritional (N-3) polyunsaturated fatty acids influence the behavioral responses to positive events in mice. Neurosci. Lett. 2000;285(3):223–227. doi: 10.1016/s0304-3940(00)01065-x. [DOI] [PubMed] [Google Scholar]
- [12].DeMar JC, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of N-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006;47(1):172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- [13].Rao JS, Ertley RN, Lee HJ, DeMar JC. N-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex bdnf via a p38 mapk-dependent mechanism. Mol. Psychiatry. 2007;12(1):36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- [14].He C, Qu X, Cui L, Wang J, Kang JX. improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA. 2009;106(27):11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wall R, Ross RP, Fitzgerald GF. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- [16].Lingwood and Simons Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- [17].Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem. Phys. Lipids. 2008;153(1):57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- [18].Wassall SR, Stillwell S. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta. 2009;1788(1):24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- [19].Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 2009;139(9):1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- [20].Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. USA. 2006;103(13):4888–4893. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Farooqui AA, Ong WY, Horrocks LA, Chen P. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res. Rev. 2007;56(2):443–471. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [22].Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-Bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000;275(1):261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- [23].Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 2010;11(3):161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- [24].Singaraja RR, Huang K, Sanders SS, Milnerwood AJ, Hines R, Lerch JP, Franciosi S. Altered Palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet. 2011;20(20):3899–3909. doi: 10.1093/hmg/ddr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ehrnhoefer DE, Sutton L, Hayden MR. Small changes, big impact: posttranslational modifications and function of huntingtin in huntington disease. Neuroscientist. 2011;17(5):475–492. doi: 10.1177/1073858410390378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mukai J, Dhilla A, Drew LJ, Stark KL, Cao LX, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat. Neurosci. 2008;11(11):1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mukai J, Liu H, Burt RA, Swor DE, Lai WS. Evidence that the gene encoding zdhhc8 contributes to the risk of schizophrenia. Nat. Genet. 2004;36(7):725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- [28].Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J. Mol. Endocri. 2004;32(2):325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- [29].Escribá PV, Wedegaertner PB, Goñi FM, Vögler O. Lipid--protein interactions in gpcr-associated signaling. Biochim. Biophys. Acta. 2007;1768(4):836–852. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [30].Renner U, Glebov K, Lang T, Papusheva E. Localization of the mouse 5- hydroxytryptamine1A receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol. Pharmacol. 2007;72(3):502–513. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- [31].Schmitz J, Ecker D. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008;47(2):147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [32].Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81(2-3):187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Su KP, Huang SY, Peng CY, Lai HC, Huang CL. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-[alpha]-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67(6):550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C. The Orphan G protein-coupled receptor gpr40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003;278(13):11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- [35].Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan WQ, Li PP, Lu WD, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing gpcrs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 2011;32(9):543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hara T, Hirasawa A, Ichimura A. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J. Pharm. Sci. 2011;100(9):3594–3601. doi: 10.1002/jps.22639. [DOI] [PubMed] [Google Scholar]
- [38].Cartoni C, Yasumatsu K, Ohkuri T. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010;30(25):8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boneva NB, Kikuchi M, Minabe Y, Yamashima T. Neuroprotective and ameliorative actions of polyunsaturated fatty acids against neuronal diseases:implication of fatty acid-binding proteins (FABP) and G protein- coupled receptor 40(GPR40) in adult neurogenesis. J. Pharmacol. Sci. 2011;116(2):163–172. doi: 10.1254/jphs.10r34fm. [DOI] [PubMed] [Google Scholar]
- [40].Yamashima T. A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory. Prog. Neurobiol. 2008;84(2):105–115. doi: 10.1016/j.pneurobio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [41].Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J. Biol. Chem. 1995;270(26):15693. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- [42].Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth BA, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J. Biol. Chem. 2004;279(33):34614. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- [43].Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes galpha s internalization through lipid rafts: a study in living cells. Mol. Pharmacol. 2005;67(5):1493. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- [44].Allen JA, Jiang ZY, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol. Pharmacol. 2009;76(5):1082–1093. doi: 10.1124/mol.109.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rimon G, Hanski E, Braun S. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature. 1978;276(5686):394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- [46].Rasenick MM, Stein PJ, Bitensky MW. The Regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981;294(5841):560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- [47].Donati RG, Rasenick MM. Chronic antidepressant treatment prevents accumulation of gs alpha in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (Lipid Rafts) Neuropsychopharmacology. 2005;30(7):1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- [48].Diaz O, Berquand A, Dubois M, Agostino SD, Sette C, Bourgoin S, Lagarde M, Nemoz G, Prigent AF. The mechanism of docosahexaenoic acid-induced phospholipase D activation in human lymphocytes involves exclusion of the nzyme from lipid rafts. J. Biol. Chem. 2002;277(42):39368–39378. doi: 10.1074/jbc.M202376200. [DOI] [PubMed] [Google Scholar]
- [49].Chen J, Rasenick MM. Chronic Treatment of C6 Glioma Cells with Antidepressant drugs increases functional coupling between a G protein (Gs) and adenylyl cyclase. J. Neurochem. 1995;64(2):724–732. doi: 10.1046/j.1471-4159.1995.64020724.x. [DOI] [PubMed] [Google Scholar]
- [50].Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element- binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J. Neurosci. 2007;27(29):7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Toki S, Donati RJ, Rasenick MM. Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of Gs [alpha] from plasma membrane. J. Neurochem. 1999;73(3):1114. doi: 10.1046/j.1471-4159.1999.0731114.x. [DOI] [PubMed] [Google Scholar]
- [52].Donati RJ, Thukral C, Rasenick MM. Chronic treatment of C6 glioma cells with antidepressant drugs results in a redistribution of Gs alpha. Mol. Pharmacol. 2001;59(6):1426–1432. doi: 10.1124/mol.59.6.1426. [DOI] [PubMed] [Google Scholar]
- [53].Zhang L, Rasenick MM. Chronic treatment with escitalopram but not r- citalopram translocates G alpha S from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound. J. Pharmacol. Exp. Ther. 2010;332(3):977. doi: 10.1124/jpet.109.162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, Lambert JJ, Zieglgänsberger W, Holsboer F, Rupprecht R. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J. Neurosci. 2005;25(44):10198–10206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Menkes DB, Aghajanian GK, Gallager DW. Chronic antidepressant treatment enhances agonist affinity of brain α 1-adrenoceptors. Eur. J. Pharmacol. 1983;87(1):35–41. doi: 10.1016/0014-2999(83)90047-x. [DOI] [PubMed] [Google Scholar]
- [56].Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 2006;281(36):26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- [57].Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM. Postmortem brain tissue of depressed suicides reveals increased Gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J. Neurosci. 2008;28(12):3042. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hines LM, Tabakoff B. Platelet adenylyl cyclase activity: a biological marker for major depression and recent drug use. Biol. Psychiatry. 2005;58(12):955–962. doi: 10.1016/j.biopsych.2005.05.040. [DOI] [PubMed] [Google Scholar]
- [59].Mooney JJ, Cole JO, Schatzberg AF, Gerson B, Schildkraut JJ. Pretreatment urinary MHPG levels as predictors of antidepressant responses to alprazolam. Am. J. Psychiatry. 1985;142(3):366–367. doi: 10.1176/ajp.142.3.366. [DOI] [PubMed] [Google Scholar]
- [60].Freeman MP, Fava M, Lake J, Trivedi MH, Wisner KL, Mischoulon D. Complementary and alternative medicine in major depressive disorder: the american psychiatric association task force report. J. Clin. Psychiatry. 2010;71(6):669–681. doi: 10.4088/JCP.10cs05959blu. [DOI] [PubMed] [Google Scholar]
- [61].Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 2012;32(1):61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pandey GN, Dwivedi Y, Rizavi HS, Ren XG, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(4):645–651. doi: 10.1016/j.pnpbp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [63].Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34(2):199–211. doi: 10.1016/j.psyneuen.2008.08.025. [DOI] [PubMed] [Google Scholar]