Abstract
Abundant evidence supports a role of the sympathetic nervous system in the pathogenesis of obesity-related hypertension. However, the nature and temporal progression of mechanisms underlying this sympathetically mediated hypertension are incompletely understood. Recent technological advances allowing direct recordings of renal sympathetic nerve activity (RSNA) in conscious animals, together with direct suppression of RSNA by renal denervation and reflex-mediated global sympathetic inhibition in experimental animals and human subjects have been especially valuable in elucidating these mechanisms. These studies strongly support the concept that increased RSNA is the critical mechanism by which increased central sympathetic outflow initiates and maintains reductions in renal excretory function, causing obesity hypertension. Potential determinants of renal sympathoexcitation and the differential mechanisms mediating the effects of renal-specific versus reflex-mediated, global sympathetic inhibition on renal hemodynamics and cardiac autonomic function are discussed. These differential mechanisms may impact the efficacy of current device-based approaches for hypertension therapy.
Keywords: Obesity, Blood pressure, Hypertension, Sympathetic nervous system, Renin-angiotensin system, Renal nerves, Renal denervation, Baroreflex, Baroreflex sensitivity, Heart rate, Heart rate variability, Autonomic nervous system, Glomerular filtration rate, Renal function, Cardiac arrhythmogenesis, Device-based therapy
Introduction
Activation of the sympathetic nervous system plays an important role in the pathogenesis of hypertension, including hypertension associated with obesity [1, 2]. Although there is incomplete understanding of the role of the sympathetic nervous system in the pathogenesis of obesity hypertension, experimental and clinical studies conducted over the last few years have provided important insight into the mechanisms that account for sympathetic activation in obesity and the mechanisms that initiate and sustain the hypertension. This review will summarize these recent publications. Results from experimental and clinical studies using pharmacological strategies to block the sympathetic nervous system can be difficult to interpret from a mechanistic perspective because of incomplete blockade, off-target effects, and issues with patient compliance. These studies will not be presented. Rather, this review will focus on studies that have used nonpharmacological approaches to investigate the role of the sympathetic nervous system in the evolution of obesity hypertension. Particular attention will be given to experimental and clinical studies that have used novel device-based technology to suppress sympathetic activity and lower arterial pressure.
Device-Based Therapy for the Treatment of Resistant Hypertension
Recent technological advances have provided two nonpharmacological approaches for the treatment of resistant hypertension: electrical stimulation of the carotid sinus [3•, 4] and endovascular radiofrequency ablation of the renal nerves [5, 6•, 7]. In recent clinical trials these devices have substantially lowered arterial pressure in many patients with resistant hypertension [3•, 4, 5, 6•,7]. However, significant blood pressure lowering has not been uniform in this heterogeneous patient population and the specific pathophysiological context for maximum efficacy has not been established. Chronic electrical stimulation of the carotid sinus activates the carotid baroreflex and lowers arterial pressure by suppressing central sympathetic outflow [4, 8, 9]. In contrast, by selective denervation of the kidneys, catheter-based endovascular radiofrequency ablation of the renal nerves lowers arterial pressure by diminishing renal efferent sympathetic nerve activity [5]. It has been hypothesized that renal nerve ablation may also decrease central sympathetic outflow by reducing renal afferent nerve traffic [5, 10], but a recent report is inconsistent with this possibility [11]. Because obesity is highly prevalent in resistant hypertensive populations [3•, 6•, 7, 12], results from clinical studies using these devices are instructive for understanding the role of the sympathetic nervous system in mediating obesity hypertension. However, the mechanisms that account for resistant hypertension are different and more poorly understood than those mediating obesity hypertension, and mechanistic insight into the cardiovascular responses to suppression of sympathetic activity by device-based therapy in patients with resistant hypertension is confounded by the multiple antihypertensive drugs that are essential to their therapy.
Increased Renal Sympathetic Nerve Activity in Obesity Hypertension
There is considerable evidence that the kidneys dominate in the long-term control of arterial pressure by altering body fluid volume through pressure natriuresis and that long-term increases in arterial pressure can only be achieved by mechanisms that decrease renal excretory function [13]. Because the sympathetic nervous system is activated in obesity hypertension, one way in which pressure natriuresis could be shifted to a higher pressure and therefore cause hypertension during weight gain is by increasing sympathetic outflow to the kidneys [14-17]. This possibility is supported by the demonstration of increased renal norepinephrine (NE) spillover in both the early prehypertensive and advanced stages of hypertension in obese human subjects [1, 18, 19]. These indirect measures of renal sympathetic nerve activity (RSNA) are consistent with the report that bilateral renal denervation before weight gain prevented the development of obesity-induced hypertension in dogs fed a high-fat diet [20]. Two recent longitudinal studies in rabbits and dogs fed a high-fat diet provide further insight into the importance of increased RSNA in mediating obesity hypertension [21, 22]. Rabbits and dogs fed a high-fat diet exhibit many of the same hemodynamic, neurohormonal, renal, and metabolic abnormalities associated with obesity in humans [2, 21, 22].
Rabbits instrumented for telemetric recordings of arterial pressure and RSNA were studied over a 3-week period of high-fat feeding [22]. Throughout this period, there was progressive weight gain along with increases in plasma levels of glucose, insulin, and leptin. RSNA, arterial pressure, and heart rate were all increased 1 week after initiation of the high-fat diet. These responses persisted throughout the 3 weeks of fat feeding. These observations from direct recording of RSNA support the concept that increased sympathetic outflow to the kidney both initiates and sustains obesity-related hypertension. The observation that baroreflex control of RSNA was depressed throughout the 3 weeks of the high-fat diet raises the possibility that baroreflex dysfunction may have contributed to chronic increases in RSNA. However, this interpretation is not easily validated by experimentation and the controversy surrounding it is discussed below.
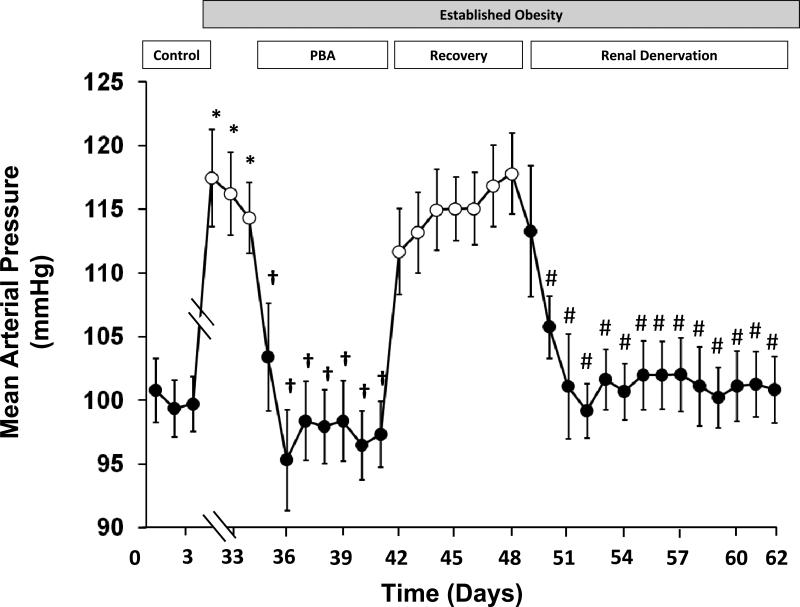
To investigate the precise mechanisms whereby the sympathetic nervous system mediates obesity induced hypertension, the effects of global and renal specific suppression of sympathetic activity were compared in dogs with a 50 % increase in body weight after 4 weeks of feeding a high-fat diet [21]. At this time they were insulin resistant, hyperinsulinemic, and hypertensive. Prolonged baroreflex activation (PBA) by chronic electrical stimulation of the carotid sinus during a week of established hypertension (Fig. 1) reduced arterial pressure to control levels in parallel with sustained suppression of central sympathetic outflow, reflected by reductions in circulating plasma NE concentration. After terminating baroreflex activation, plasma NE concentration and arterial pressure returned to their previously elevated levels. Arterial pressure fell once again from hypertensive to normotensive control levels after bilateral renal denervation (Fig. 1) but, in contrast to PBA, without reductions in plasma NE concentration. Because renal denervation abolished the hypertension and because activation of the baroreflex in hypertension suppresses RSNA [14, 15], the authors attributed the antihypertensive effects of global reduction in sympathetic activity by PBA to suppression of RSNA. Because renal denervation did not reduce the high circulating levels of NE associated with weight gain, the authors surmised that sensory afferent signals from the kidneys do not contribute importantly to chronic sympathetic overactivity in obesity hypertension. Thus, despite obesity being a common feature in patients with resistant hypertension, this observation does not support the hypothesis that renal afferents add to systemic sympathetic activation in these subjects, as long as there is no overt renal disease. Most importantly, these responses demonstrate that the renal nerves play a critical role in maintaining obesity hypertension. Despite the concept of baroreceptor resetting and the uncertainty as to whether the baroreflex completely resets in response to long-term changes in arterial pressure [14, 15], it is clear from this and a number of earlier studies during PBA that direct electrical stimulation of baroreceptor afferents has pronounced and sustained effects to suppress sympathetic activity and lower arterial pressure [3•, 4, 9, 16].
Fig. 1.
Changes in mean arterial pressure during prolonged baroreflex activation and following bilateral surgical renal denervation in dogs with obesity-induced hypertension. Values are mean ±SEM (n=6). *P<0.05 versus control, days 2–3; †P<0.05 versus established obesity, days 33–34; # P<0.05 versus recovery from prolonged baroreflex activation (PBA), days 46–47. During days 4–31, obesity hypertension was induced by feeding dogs a high-fat diet. During this time, body weight increased ~ 50 %. After day 31, dietary fat intake was minimal, and there was no further change in body weight throughout the remainder of the study.
The Renin-Angiotensin System in Obesity Hypertension
In obesity hypertension, increased RSNA may shift pressure natriuresis to a higher arterial pressure by increasing sodium reabsorption directly and also indirectly through the antinatriuretic effects of angiotensin II (ANG II) as a result of stimulating renin secretion. While increases in PRA have been reported in experimental animals and in human subjects during the development of obesity hypertension, sustained increases in PRA have not been a consistent finding [1, 2, 20, 21]. Under conditions of constant sodium intake, increases in PRA occurred initially in dogs fed a high-fat diet, but those increases did not persist as weight gain progressed over the 4 weeks of the high-fat diet [21]. Because progressive sodium retention leading to accumulation of extracellular fluid volume and higher arterial pressure developed concomitantly with weight gain, it is likely that these responses counteracted the neural drive for renin secretion, resulting in only a subtle final increase in renin secretion not easily discerned by intermittent measurements of PRA even in this well controlled longitudinal study. This observation does not discount the importance of even small increases in ANG II in mediating the hypertension since for the prevailing level of volume expansion and hypertension, even normal levels of ANG II would be considered inappropriately elevated. When measured 7 days after PBA and 14 days after renal denervation in the study illustrated in Fig. 1, the antihypertensive responses to global and renal-specific inhibition of sympathetic activity were associated with substantial reductions in PRA. Similarly, in a case study, Schlaich et al. reported a 50 % decrease in PRA immediately following renal nerve ablation in a subject with resistant hypertension [23]. These observations point to the contribution of sympathetic drive in stimulating renin secretion in obesity hypertension and are in keeping with the efficacy of ANG II antagonists and angiotensin-converting enzyme inhibitors in reducing the severity of hypertension in obese animals and humans [2].
Sympathetic Activity and Renal Function in Obesity Hypertension
In obese humans, impaired pressure natriuresis is initially due to neurally-mediated sodium reabsorption because GFR and renal blood flow are increased [2]. Tomaszewski et al. reported increases in estimated GFR in a large cohort of apparently healthy young men (mean age=18 years) with elevated BMI and an unfavorable metabolic profile [24]. In a more recent study, creatinine clearance and MSNA were measured in 18 lean and 25 overweight or obese college students [25•]. The overweight/obese subjects were not hypertensive, but their systolic blood pressure and heart rate were slightly greater than in the lean controls. Additionally, overweight/obese subjects had impaired glucose tolerance and elevated fasting levels of insulin. Creatinine clearance was higher in overweight/obese subjects when compared to their lean counterparts, and increases in creatinine clearance were directly correlated with increases in MSNA. Increases in GFR early in the pathogenesis of obesity-related hypertension provide further evidence that neurally mediated increases in tubular sodium reabsorption play a causal role in the initiation of obesity hypertension [2, 21•].
One possible explanation of the increased GFR in obesity is that increased RSNA increases sodium reabsorption prior to the macula densa. Increased sodium reabsorption in the proximal tubule or loop of Henle, sites of action of the renal nerves and ANG II, would increase fractional sodium reabsorption and decrease sodium delivery to the macula densa. This, in turn, would elicit a tubuloglomerular feedback (TGF) signal to dilate the afferent arteriole and increase GFR. Further, in overperfused kidneys, the vasoconstrictor effect of ANG II on efferent arterioles would exacerbate the hyperfiltration. Glomerular hyperfiltration may lead to progressive glomerular injury and add to the impairment of pressure natriuresis and hypertension attributed to enhanced tubular reabsorption.
The study in obese dogs illustrated in the figure provides novel insight into the differential renal actions of global and renal specific suppression of sympathetic activity by baroreflex activation and by renal denervation, respectively [21•]. PBA not only suppressed renin secretion and abolished hypertension as discussed above, but also chronically diminished the 35 % increase in GFR associated with obesity. Because suppression of sympathetic activity by baroreflex activation reduced the elevated rate of tubular sodium reabsorption, it was concluded that by increasing sodium delivery to the macula densa, PBA may reduce GFR by constriction of the afferent arteriole through the TGF mechanism indicated above and by diminishing the vasoconstrictor effects of ANG II on the efferent arterioles as a result of suppressing renin secretion. Support for this contention was provided by a follow up study in normotensive dogs [26]. In this study, the chronic blood pressure lowering effects of PBA were associated with sustained reductions in renal blood flow and GFR, and these responses to baroreflex activation were abolished during chronic administration of the calcium channel blocker amlodipine, which dilates the preglomerular vasculature.
As illustrated in Fig. 1, PBA and renal denervation produced comparable reductions in arterial pressure and abolished obesity hypertension. However, in contrast to the reduction in glomerular hyperfiltration during baroreflex activation, GFR was increased further (by 10 %) at 2 weeks after surgical denervation of the kidneys. Because GFR increased in the absence of a change in fractional sodium reabsorption, the authors speculated that complete surgical renal denervation may decrease preglomerular resistance as a result of achieving reductions in RSNA that exceed those seen with suppression of sympathetic activity during baroreflex activation. The decrease in preglomerular resistance in this study is consistent with a reported 56 % increase in renal plasma flow immediately following renal nerve ablation in a patient with resistant hypertension [23]. Thus, by decreasing preglomerular resistance and increasing glomerular pressure, renal denervation may predispose obese patients to glomerular injury due to glomerular hypertension, particularly if the systemic antihypertensive response to renal nerve ablation is modest, as seen in some patients with resistant hypertension.
Despite the importance of the kidneys in long-term control of arterial pressure, there has been little focus on changes in renal function during device-based therapy in patients with resistant hypertension. Most reports to date indicate that estimated GFR is unchanged several months after endovascular renal nerve ablation in subjects with resistant hypertension [6, 7, 27]. However, with the exception of one pilot study [28], ablation of the renal nerves with this technology has been restricted to selected patients with little or no baseline impairment in estimated GFR, and follow-up has typically been 1 year or less. In a recent study, Alnima et al. reported a decrease in estimated GFR of ~8 % after 6 months of baroreflex activation therapy in a large cohort of patients with resistant hypertension [29]. Thus, this modest reduction in GFR is consistent with the response to PBA reported in obese dogs, as discussed above [21•]. In the study reported by Alnima et al., the reduction in GFR did not intensify after 12 months of therapy, and reductions in GFR did not occur in subjects in the low GFR category (estimated GFR<60 mL/min.). Therefore, whether the differential renal effects of global and renal-specific inhibition of sympathetic activity indicated in the experimental studies discussed above truly enhance or limit the long-term clinical benefit of lowering arterial pressure in subjects with resistant hypertension during treatment with these novel device-based therapies is unknown. The answer to this question will require careful long-term, temporal determination of renal function in subjects with different levels of renal impairment.
Autonomic Activity and Cardiac Arrhythmogenesis in Obesity
Obesity has a profound effect on cardiac automaticity, and obese subjects have an increased risk of arrhythmias and sudden death [30-33]. While alterations in autonomic drive to the heart likely contribute to impaired cardiac automaticity in obesity, the causal mechanisms that lead to cardiac autonomic imbalance and the functional significance of sympathetic-parasympathetic interactions in mediating cardiac arrhythmias is unclear. The role of heightened cardiac sympathetic activity in arrhythmogenesis in the prehypertensive stages of obesity has been discounted by early clinical studies indicating that, in contrast to increased sympathetic outflow to the kidneys and skeletal muscle vasculature, cardiac NE spillover is depressed in overweight or obese subjects in early disease progression [1, 18, 19]. In contrast, increases in both heart rate and cardiac NE spillover to levels exceeding those present in lean healthy normotensive individuals are present when hypertension is manifested [19]. A recent clinical study showed that overweight and obese children have higher resting heart rate and blood pressure than their leaner counterparts [34]. In another study, overweight or obese young adults had higher levels of MSNA and heart rates than their lean counterparts [25•]. Taken together, these data suggest that increased cardiac sympathetic activation may occur concomitantly with increases in arterial pressure in obese humans.
In contrast, experimental and clinical studies have consistently identified reduced cardiac vagal activity in obese experimental animals and in human subjects [35-37], providing evidence for attenuated parasympathetic control of heart rate and suggesting a possible role in arrhythmogenesis. However, the studies mentioned above have investigated only steady state levels of activity of both cardiac autonomic limbs under resting conditions, disregarding their complementary and dynamic influence on cardiac automaticity during daily activity. Low heart rate variability is a risk factor for cardiac arrhythmias and mortality [38-42], and is present in obese individuals in association with impaired baroreflex control of heart rate [43-46•]. Heart rate variability reflects the balance of sympathetic and parasympathetic modulation of cardiac rhythmicity and their control by the arterial baroreflex. Thus, consideration should be given to reactive autonomic influences on cardiac function such as those occurring during obstructive sleep apnea (OSA), which is prevalent in obesity. OSA is associated with sympathetic activation, large increases in arterial pressure, and cardiac arrhythmias. The depressed baroreflex sensitivity and autonomic imbalance associated with obesity may allow unbuffered surges in sympathetic activity induced by acute apneic episodes to increase the risk of arrhythmias.
The potential for PBA to confer cardiac protection in obesity was recently investigated in the dog model of obesity hypertension. Based on continuous 24-hour recordings of arterial pressure and heart rate, dogs fed a high-fat diet showed tachycardia and marked reductions in heart rate variability and baroreflex control of heart rate even before increases in arterial pressure and substantial weight gain developed [47]. These responses intensified with weight gain and the development of hypertension. Although the central and peripheral mechanisms that account for decreased baroreflex sensitivity during weight gain and obesity have not been completely elucidated, chronic electrical activation of the baroreflex in obese dogs not only abolished the tachycardia but also restored baroreflex control of heart rate dynamics, presumably by improving cardiac sympathovagal balance [46]. In sharp contrast, selective elimination of renal sympathetic drive by surgical renal denervation had no effect on either steady state or dynamic measures of heart rate [47].
Therefore, the role of altered sympathetic-parasympathetic interactions in obesity-related autonomic cardiac modulation is more complex than is evident from single time-point measurements of autonomic influences on the heart or mean values of heart rate. The mechanisms by which electrical baroreflex activation improves cardiac sympathovagal balance need further exploration. It is likely that these mechanisms improve heart rate variability through modulation of the sympathetic and parasympathetic limbs of the baroreflex. Therapies targeted at restoration of baroreflex control of heart rate have the potential to reduce the risk of life-threatening arrhythmias, frequently associated with obesity hypertension.
Causes of Sympathetic Activation in Obesity
Sustained activation of the sympathetic nervous system in obesity hypertension has been attributed to hyperleptinemia, OSA, hyperinsulinemia, increased circulating lipids, increased circulating ANG II, and impaired baroreflexes, although there is often little supporting evidence for cause and effect relationships. New insight into potential mechanisms for sympathetic activation follows.
Leptin
Recent experimental and clinical studies support a strong link between circulating leptin, secreted primarily from adipocytes, and the initiation and maintenance of obesity hypertension. Based on 9102 semiannual arterial pressure and height/weight assessments in children (mean age=10.2 years) with follow up for 4.5 years, Tu et al. reported significantly higher arterial pressure and a greater proportion of arterial pressure measurements in the prehypertensive/hypertensive range when body mass index (adjusted for age and sex) was in the overweight/obesity category [34]. In addition, plasma leptin concentration was 3–4 times higher in overweight/obese children than in normal weight children. In a large cohort of adults (n=5599), Shankar and Xiao reported that higher plasma leptin levels are positively associated with hypertension in both men and women [48]. These findings suggest that leptin is a critical link between obesity and hypertension, consistent with seminal studies showing that leptin- or leptin receptor–deficient, obese mice have little or no increase in arterial pressure compared to their lean controls [2, 49].
As discussed above, in an experimental model of obesity-induced hypertension, plasma leptin concentration increased progressively with weight gain in rabbits fed a high-fat diet [22]. Moreover, nerve recordings showed that intracerebroventricular injection of a leptin antagonist decreased the elevated levels of RSNA in these conscious hypertensive rabbits, providing direct evidence for a causal relationship with central leptin signaling accounting for increased renal sympathetic outflow [50•]. In contrast, despite hyperinsulinemia, an insulin antagonist did not affect RSNA, adding to the evidence that insulin does not promote obesity hypertension by chronically stimulating the sympathetic nervous system [2]. Suppression of RSNA by the leptin antagonist is consistent with a much earlier report that renal NE spillover is directly correlated with plasma leptin concentration in humans with essential hypertension [51]. Taken together, these studies provide strong evidence that associations between leptin and arterial pressure are causal and that leptin plays a critical role in both the initiation and maintenance of obesity hypertension by increasing RSNA through its actions on the central nervous system.
Obstructive Sleep Apnea
OSA is commonly associated with obesity, and OSA and hypertension are believed to be causally related, possibly through sustained activation of the sympathetic nervous system [12, 52]. However, the precise mechanism whereby OSA leads to persistent sympathetic activation is unclear. In an interesting twist, Witkowski et al. showed that OSA was a consequence as well as a cause of increased sympathetic activity in patients with OSA who underwent renal nerve ablation therapy for the treatment of resistant hypertension [53]. They reported that the apnea–hypopnea index improved in 8 of 10 patients 6 months after renal denervation therapy. A possible mechanistic link between the improved incidence of OSA and renal denervation was provided by an earlier study showing that displacement of fluid from the legs to the neck overnight strongly relates to the severity of OSA in patients with both controlled and resistant hypertension [54]. Thus, by reducing rostral fluid shifts as a result of increasing renal excretory function and reducing body fluid volume, renal denervation may decrease the prevalence of OSA by diminishing peripharyngeal fluid accumulation, which may predispose to upper airway obstruction. If this hypothesis is correct, the finding would suggest a positive feedback between increased RSNA and OSA, i.e., increases in RSNA mediated by mechanisms independent of airway obstruction would promote fluid retention, and the resultant fluid retention would exacerbate OSA. In turn, increased OSA would increase RSNA further, leading to more fluid retention and more severe hypertension. Consistent with this possibility, measurements of resting muscle sympathetic nerve activity in hypertensive subjects showed that the sympathetic activation in metabolic syndrome occurs independently of OSA, but that OSA markedly potentiates the sympathoexcitation [46•].
Baroreflex Dysfunction
Several studies have demonstrated that baroreflex control of sympathetic activity is impaired in animals and humans with established obesity, but the time course and physiological significance of this dysfunction is unclear [43, 46•, 55, 56]. Armitage et al. recently reported that impaired baroreflex control of RSNA was an early as well as a chronic response in the development of obesity hypertension in rabbits fed a high-fat diet [22]. However, this dysfunction occurred in parallel with increases in plasma leptin concentration, and it is unclear whether impaired baroreflex suppression of central sympathetic outflow added to the early renal sympathoexcitatory effects of leptin. Another obstacle to accepting this possibility is that baroreceptors reset to the level of arterial pressure to which they are exposed and, therefore, if resetting is complete, the baroreflex could not produce sustained suppression of sympathetic activity in hypertension. However, because of technical limitations, few studies have assessed the magnitude to which resetting of the baroreflex occurs [14, 15]. In this regard, studies in chronically instrumented animals have shown that baroreflex resetting is incomplete and that baroreflex-mediated suppression of renal sympathetic outflow is sustained in experimental models of hypertension [14, 15]. In particular, use of Fos-like immunohistochemistry showed chronic activation of the neurons in the central baroreflex pathway in dogs with obesity hypertension of ~ 6 weeks in duration [57]. This finding was interpreted to indicate that chronic activation of the baroreflex diminishes the increase in central sympathetic outflow in obesity-induced hypertension. Based on these observations, it follows that baroreflex dysfunction may contribute to sustained increases in RSNA in obesity hypertension. The authors believe that the importance of baroreflex dysfunction as an accessory determinant of chronic increases in RSNA in obesity hypertension is an open question and should not be casually dismissed or accepted.
Conclusions
There is abundant evidence that obesity is characterized by activation of the sympathetic nervous system and that sympathetic activation contributes to obesity-related hypertension. However, the time course of this activation and the specific mechanisms that account for this form of hypertension are incompletely understood. Findings from recent experimental and clinical studies have provided further insight into these issues and strongly support the concept that increased RSNA is the critical link between increased central sympathetic outflow and reductions in renal excretory function that initiate and sustain the hypertension. Device-based therapy that suppresses RSNA is efficacious in reducing arterial pressure in many patients with resistant hypertension, who are commonly obese. However, mechanistic insight from experimental studies using these technologies and/or their approaches for suppressing sympathetic activity indicate that appreciable differences may exist between global and renal-specific sympathoinhibition on renal hemodynamics and autonomic control of cardiac function. Whether the differences observed in experimental studies translate into either untoward outcomes or additional clinical benefit with device-based therapy will depend on the findings from appropriately designed prospective clinical trials.
Acknowledgement
The authors’ studies cited in this report were funded by National Heart, Lung, and Blood Institute Grant HL-51971.
Footnotes
Conflict of Interest Thomas E. Lohmeier has received consulting fees from CVRx and research support from National Institutes of Health.
Radu Iliescu declares that he has no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
•Of Importance
- 1.Esler M, Straznicky N, Eikelis N, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, da Dilva AA, Brandon E, et al. Pathophysiology of obesity-induced hypertension and target organ damage. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. Elsevier; New York: 2007. pp. 447–468. [Google Scholar]
- 3•.Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal trial. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008. [The first randomized controlled trial investigating safety and efficacy of baroreflex activation therapy as treatment for resistant hypertension.] [DOI] [PubMed] [Google Scholar]
- 4.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation. Mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlaich MP, Sobotka PA, Krum H, et al. Renal denervation as a therapeutic approach for hypertension. Novel implications for an old concept. Hypertension. 2009;54:1195–1201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 6•.Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet. 2011;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [The first randomized controlled trial investigating safety and efficacy of catheter-based renal denervation as treatment for resistant hypertension.] [DOI] [PubMed] [Google Scholar]
- 7.Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension. One-year results from Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 8.Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 9.Lohmeier TE, Iliescu R, Dwyer TM, et al. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann J, Heusser K, Schmidt BM, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients. Prospective case studies. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 13.Guyton AC. Arterial Pressure and Hypertension. Saunders; Philadelphia, PA: 1980. [Google Scholar]
- 14.Lohmeier TE, Hildebrandt DA, Warren S, et al. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288:R828–R836. doi: 10.1152/ajpregu.00591.2004. [DOI] [PubMed] [Google Scholar]
- 15.Lohmeier TE, Drummond HA. The baroreflex in the pathogenesis of hypertension. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. Elsevier; New York: 2007. pp. 265–279. [Google Scholar]
- 16.Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation. J Appl Physiol. 2012;113:1652–1658. doi: 10.1152/japplphysiol.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 18.Vaz M, Jennings G, Turner A. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 19.Rumantir MS, Vaz M, Jennings GL, et al. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 20.Kassab S, Kato T, Wilkins C, et al. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 21•.Lohmeier TE, Iliescu R, Liu B, et al. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [Demonstrates both similar and differential effects of chronic baroreflex activation and renal denervation on arterial pressure, neurohormonal responses, renal function, and heart rate in dogs with obesity-induced hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage JA, Burke SL, Prior LJ, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 23.Schlaich MP, Sobotka PA, Krum, et al. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 24.Tomaszewski M, Charchar, Maric C. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007;71:816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 25•.Lambert E, Sari CI, Daywood T, et al. Sympathetic Nervous System Activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [This study provides early evidence for glomerular hyperfiltration, a precursor of kidney disease, in parallel with increases in systolic blood pressure and muscle sympathetic nerve activity in overweight/obese college students when compared to their lean counterparts.] [DOI] [PubMed] [Google Scholar]
- 26.Iliescu R, Irwin ED, Georgakopoulos D, Lohmeier TE. Renal responses to chronic suppression of central sympathetic outflow. Hypertension. 2012;60:749–756. doi: 10.1161/HYPERTENSIONAHA.112.193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahfoud F, Cremers B, Link B, et al. Renal hemodynamics and renal function after catheter-based sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 28.Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alnima T, de Leeuw PW, Tan ES, Kroon AA. Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01159. In press. [DOI] [PubMed] [Google Scholar]
- 30.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. J Am Med Assoc. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 32.Pietrasik G, Goldenberg I, McNitt S, et al. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:181–184. doi: 10.1111/j.1540-8167.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 33.Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity—results of meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Tu W, Eckert GJ, DiMeglio LA, et al. Intensified effect of adiposity on blood pressure on overweight and obese children. Hypertension. 2011;58:818–824. doi: 10.1161/HYPERTENSIONAHA.111.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Vliet BN, Hall JE, Mizelle HL, et al. Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol. 1995;269:H629–H637. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 36.Aronne LJ, Mackintosh R, Rosenbaum M, et al. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regulatory Integrative Comp Physiol. 1995;269:R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 37.Truett AA, Borne AT, Poincot MA, West DB. Autonomic control of blood pressure and heart rate in obese hypertensive dogs. Am J Physiol Regulatory Integrative Comp Physiol. 1996;270:R541–R549. doi: 10.1152/ajpregu.1996.270.3.R541. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 40.Reed MJ, Robertson CE, Addison PS. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM. 2005;98:87–95. doi: 10.1093/qjmed/hci018. [DOI] [PubMed] [Google Scholar]
- 41.Billman GE. Heart rate variability – a historical perspective. Front Physiol. 2011;2:1–13. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–107. doi: 10.1007/s10741-010-9179-1. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Dell'Oro R, et al. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 44.Beske S, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–H635. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 45.Skrapari I, Tentolouris T, Perrea D, et al. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity. 2007;15:1685–1693. doi: 10.1038/oby.2007.201. [DOI] [PubMed] [Google Scholar]
- 46•.Grassi G, Seravalle G, Quarti-Trevano F, et al. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens. 2010;28:1313–1320. doi: 10.1097/HJH.0b013e328337a9fd. [Measurements of muscle sympathetic nerve activity show that the sympathetic activation of metabolic syndrome occurs independently of obstructive sleep apnea (OSA) but that OSA has sustained effects to potentiate the sympathoexcitation.] [DOI] [PubMed] [Google Scholar]
- 47.Iliescu R, Tudorancea E, Irwin E, Lohmeier TE. Chronic baroreflex activation improves baroreflex control of heart rate in obesity. Hypertension. 2012;60:A477. doi: 10.1152/ajpheart.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56:623–628. doi: 10.1161/HYPERTENSIONAHA.109.148213. [DOI] [PubMed] [Google Scholar]
- 49.Mark AL, Shaffer RA, Correia MLG, et al. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 50•.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [Demonstrates that the central actions of endogenous leptin stimulate renal sympathetic nerve activity in conscious, obese hypertensive rabbits.] [DOI] [PubMed] [Google Scholar]
- 51.Eikelis N, Schlaich M, Aggarwal A, et al. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 52.Wolk R, Abu SM. Shamsuzzaman, Somers VK. Obesity, sleep apnea, and Hypertension. Hypertension. 2003;42:1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 53.Witkowski A, Prejbisz A, Florczak E. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 54.Friedman O, Bradley TD, Chan CT, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 55.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol. 2010;588.9:1515–1525. doi: 10.1113/jphysiol.2009.186387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severly impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol. 2012;302:H2083–H2091. doi: 10.1152/ajpheart.01200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohmeier TE, Warren S, Cunningham JT. Sustained activation of the central baroreceptor pathway in obesity hypertension. Hypertension. 2003;42:96–102. doi: 10.1161/01.HYP.0000076092.10923.FD. [DOI] [PubMed] [Google Scholar]