Abstract
Methadone has been the most commonly used pharmacotherapy for the treatment of opioid dependence in U.S. public sector treatment, but availability of buprenorphine as an alternative medication continues to increase. Drawing data from two community-based clinical trials that were conducted nearly contemporaneously, this study examined retention in methadone vs. buprenorphine treatment over 6 months among urban African Americans receiving treatment in one of four publicly-funded programs (N= 478; 178 methadone; 300 buprenorphine). Adjusting for confounds related to medication selection, survival analysis revealed that buprenorphine patients are at substantially higher risk of dropout compared to methadone patients (HR= 2.43; p< .001). Buprenorphine’s retention disadvantage appears to be concentrated in the earlier phases of treatment (approximately the first 50 days), after which risk of subsequent dropout becomes similar for the two medications. These findings confirm a retention disparity between methadone and buprenorphine in this population, and suggest potential avenues for future research to enhance retention in buprenorphine treatment.
Keywords: methadone, buprenorphine, retention, dropout, African Americans
1. Introduction
Opioid dependence is a significant public health threat and social problem throughout the world. In 2009, there were an estimated 12–14 million heroin users globally, and in the US and Australia, prescription opioid abuse is a growing problem (United Nations Office on Drugs and Crime [UNODC], 2011). The age- and gender-adjusted mortality rate of individuals with opioid dependence is about 13 times that of the general population (Hulse, English, Milne, & Holman, 1999). Treatment with opioid agonists (primarily methadone and buprenorphine) has been shown in studies throughout the world to be effective in reducing illicit opioid use (Connock et al., 2007; Lawrinson et al., 2008; Mattick, Kimber, Breen, & Davoli, 2008). Both methadone and buprenorphine are considered “essential medicines” by the World Health Organization (WHO, 2010).
Enrollment in opioid agonist treatment is associated with reduced drug use (Hubbard, Craddock, & Anderson, 2003; Mattick et al., 2008; Zhang, Friedmann, & Gerstein, 2003), reduced HIV risk behaviors and seroconversion (Metzger et al., 1993; Nathan & Karan, 1989; Sullivan & Fiellin, 2005; Sullivan et al., 2008), and lower risk of death (Caplehorn, Dalton, Cluff, & Petrenas, 1994; Clausen, Anchersen, & Waal, 2008; Clausen, Waal, Thoresen, & Gossop, 2009; Degenhardt et al., 2011; Gibson et al., 2008; Gronbladh, Ohlund, & Gunne, 1990; Zanis & Woody, 1998). Premature discontinuation of opioid agonist treatment is associated with a range of adverse outcomes, including resumption of opioid use and mortality (Clausen et al., 2008; 2009; Magura & Rosenblum, 2001).
Given the consequences of premature treatment discontinuation, physicians, patients, and policy makers should have information on the relative ability of buprenorphine and methadone treatment to retain patients. A Cochrane Collaboration meta-analysis examined randomized clinical trials conducted through October 2006 that compared methadone and buprenorphine in terms of treatment retention as a dichotomous variable through the end of each study (Mattick et al., 2008). The analysis of the eight flexible dose trials, which varied from 6 to 52 weeks in duration, found that patients taking methadone were more likely to be retained than patients taking buprenorphine (RR= 0.85; 95% CI= 0.73–0.98). Six of eight fixed-dose comparison studies showed that patients taking a medium methadone dose (50–80 mgs) were more likely to be retained than patients taking a medium buprenorphine dose (7–15 mgs), while two studies showed no statistically significant differences between the medications.
The studies examined in the Cochrane meta-analysis were rigorous, however many were conducted in clinical research settings rather than typical community-based treatment settings. Also, some studies used the alcohol-based liquid preparation of buprenorphine which has been found to have higher bioavailability than the buprenorphine/naloxone (Suboxone®) formulation currently in widespread clinical use (Compton, Ling, Moody, & Chiang, 2006). Finally, many studies employed a slow buprenorphine dose induction schedule that may have contributed to early drop-out.
Since the Cochrane meta-analysis, additional studies have compared patient retention in methadone and buprenorphine treatment in randomized trials as well as community programs. For example, in a prospective patient preference study conducted in clinical settings in the UK, patients choosing methadone were more than twice as likely as patients choosing buprenorphine to be retained in treatment over a 6 month period (Pinto et al., 2010). A retrospective cohort study of 42,690 patients prescribed methadone or buprenorphine treatment in Australia found that, controlling for age and gender, buprenorphine patients were nearly twice as likely to drop out of treatment as methadone patients (Burns et al., 2009). A data linkage study conducted in Australia also found significantly longer median retention in methadone as compared to buprenorphine treatment (Bell, Trinh, Butler, Randall, & Rubin, 2009).
In contrast, two 6-month flexible-dose, random assignment studies in Sweden (Kakko et al., 2007) and Germany (Soyka, Zingg, Koller, & Kuefner, 2008), as well as a naturalistic study in Italy (Vigezzi et al., 2006), found no significant differences in treatment retention between patients treated with methadone or buprenorphine. An economic study using administrative records from the Veterans Health Administration in the U.S. found that patients initiating buprenorphine treatment stayed in treatment longer than their counterparts initiating methadone (Barnett, 2009). Thus, some questions remain regarding treatment retention in buprenorphine and methadone treatment as delivered in community-based treatment programs.
The clinical relevance of these questions is amplified by the fact that community-based treatment can come in multiple forms. In the U.S., for example, buprenorphine can be delivered in licensed opioid treatment programs with daily supervised dispensing, in office-based treatment with physician prescriptions as part of primary care, or in formerly drug-free community outpatient substance abuse treatment programs that have adopted buprenorphine. Research suggests that service delivery site for buprenorphine treatment can have an impact on patient retention (Miotto et al., 2012). Moreover, since buprenorphine became available in the U.S., individuals relying on the publicly-financed treatment sector often do not have equal access to this medication (Clark, Samnaliev, Baxter, & Leung, 2011; Ducharme & Abraham, 2008; Knudsen, Ducharme, & Roman, 2006; Koch, Arfken, & Schuster, 2006). African Americans in particular have faced disparities in accessing buprenorphine treatment (Mitchell et al., 2012; Stanton, 2006), and in various indicators of quality care in methadone treatment, such as timely admission (Gryczynski, Schwartz, Salkever, Mitchell & Jaffe, 2011) and adequate therapeutic dosing (D’Aunno & Pollack, 2002; Pollack, & D’Aunno, 2008).
In Baltimore, Maryland, a series of policy initiatives increased access to both methadone and buprenorphine treatment for low-income city residents (Agency for Healthcare Research Quality [AHRQ], 2011; Mitchell et al., 2012), thereby providing a unique opportunity to examine treatment retention for the two medications under conditions in which patients could select which of these treatments they wished to enter. The goal of this study was to compare treatment retention through 6 months for African American patients who selected methadone or buprenorphine treatment, as delivered in real-world clinical conditions within a large publicly-funded substance abuse treatment system.
2. Materials and Methods
2.1. Data Sources and Treatment Sites
The present study is a secondary data analysis drawn from two random-assignment studies conducted nearly contemporaneously in four community-based treatment programs in Baltimore, Maryland. The first study examined outcomes of patients receiving methadone with vs. without counseling during the first 4 months of treatment followed by methadone with standard counseling for 8 months (Schwartz, Kelly, O’Grady, Gandhi & Jaffe, 2012). This study was conducted between 2010 and 2011 in two methadone treatment programs (MTPs). The second study examined outcomes of buprenorphine patients receiving standard vs. intensive outpatient counseling (Mitchell et al., 2013). This study was conducted from 2010–2011 in two formerly drug-free outpatient community treatment programs that had adopted buprenorphine. Both parent studies found no significant differences in retention between study conditions. The studies had congruent and minimally-restrictive inclusion/exclusion criteria (opioid-dependent adults, newly admitted to the participating treatment clinic, not pregnant, and without an acute psychiatric or medical condition beyond the ability of the clinic physician to manage). Both studies were implemented in community treatment programs, and recruited participants immediately after formal admission to treatment. Neither study was advertised in the community. Thus, study participants reflect the natural pool of new admissions at each of the treatment programs.
The two programs in the methadone parent study were hospital-affiliated community clinics. Clinical services followed the standards for MTPs in the U.S. and the state of Maryland, with daily administration of methadone, and individual and group drug abuse counseling. Both clinics were open for medication 7 days a week. The two programs in the buprenorphine parent study were formerly drug-free substance abuse programs that had adopted buprenorphine as part of a large, city-wide initiative. One of the programs was co-located in a large community health center, while the other was a free-standing program affiliated with (and located adjacent to) a community outpatient mental health clinic. The programs both provided extensive counseling, predominantly group-level. Counseling groups were held 5 days a week, and new patients typically received a schedule of groups that they were expected to attend. On average, participants in standard outpatient treatment received 3. 7 hours of counseling per week, while those in intensive outpatient treatment received 5.2 hours of counseling per week (see Mitchell et al., 2013). New patients received the combination buprenorphine/naloxone (Suboxone ®) tablet or sublingual film on-site 5 to 6 days a week, with a take-home dose(s) for the weekend. After some time in treatment, participants would receive prescriptions that could be filled at local pharmacies, and could eventually receive a prescription for a month’s supply. The buprenorphine programs were established with the purpose of stabilizing and eventually transitioning patients to continued buprenorphine treatment in an office-based setting. At the time the two parent studies were in the field, Maryland had expanded Medicaid eligibility to indigent individuals regardless of whether they had a disability. The Medicaid program paid for the buprenorphine medication and its associated counseling as well as methadone treatment.
2.2. Participants
Participants were 478 opioid-dependent African American adults entering publicly-funded agonist treatment with either methadone (n=178) or buprenorphine (n=300) in Baltimore who had enrolled in one of the parent studies. Since the buprenorphine study was explicitly focused on African Americans and had recruited a predominantly African American sample (94%), the analysis for the current study was restricted to African Americans. While the methadone study was open to all new admissions, the majority of the participants in this sample (77%) were African American. Characteristics of participants in the methadone and buprenorphine samples are shown in Table 1.
Table 1.
Participant baseline characteristics in methadone and buprenorphine treatment.
| Total (N = 478) | Methadone (n= 178) | Buprenorphine (n = 300) | p | |
|---|---|---|---|---|
|
|
||||
| Participant Characteristics | ||||
| Age, mean (SD) | 46.1 (6.4) | 45.2 (6.2) | 46.6 (6.4) | .020 |
| Female, % | 34.5 | 29.2 | 37.7 | .060 |
| Injection drug user, % | 28.0 | 38.2 | 22.0 | <.001 |
| Used cocaine within past 30d, % | 54.6 | 55.6 | 54.0 | .731 |
| Previous Treatment Experience | ||||
| Neither methadone nor buprenorphine | 36.2 | 45.5 | 30.7 | .001 |
| Methadone only | 27.2 | 42.1 | 18.3 | <.001 |
| Buprenorphine only | 20.9 | 7.3 | 29.0 | <.001 |
| Both methadone and buprenorphine | 15.7 | 5.1 | 22.0 | <.001 |
| Health | ||||
| Days of medical problems, past 30d, mean (SD) | 3.9 (8.8) | 1.2 (5.2) | 5.5 (10.1) | <.001 |
| Acute depression within past 30d, % | 20.5 | 3.9 | 30.3 | <.001 |
| Lifetime depression, % | 37.7 | 14.0 | 51.7 | <.001 |
Note: χ2 test used for categorical variables. Independent samples t-test used for continuous variables.
2.3. Measures
Retention
In both studies, treatment retention was determined by obtaining the number of days in treatment from clinic medication records and participant reports. Participants were interviewed at baseline and at 3- and 6-months post-baseline in the buprenorphine study and 4- and 12-months post-baseline in the methadone study. In the buprenorphine study, the goal of treatment was to eventually transition patients to ongoing office-based buprenorphine treatment. Thus, participants who left treatment at the original program but reported that they had successfully transitioned to ongoing buprenorphine treatment elsewhere by 6-month follow-up were classified as retained in treatment throughout the 6-month observation period. Similarly, those in the methadone study who transferred to another methadone treatment provider without an interruption in care and were still in that program were considered to have remained in treatment through 6 months.
Predictor variables and measures
The following predictor variables used in the current study were drawn from the 5th edition of the Addiction Severity Index (ASI; McLellan et al., 1992), an instrument used in both parent studies. Self-reported measures derived from the baseline ASI included participant gender, age, injection drug use status (yes/no), number of days of medical problems in the past 30 days, and lifetime and past 30 day depression (yes/no). Prior experience in methadone and buprenorphine treatment was obtained from the baseline supplemental self-report instruments used in each study. Prior medication treatment experience was operationalized as a series of categorical variables (methadone only, buprenorphine only, both methadone and buprenorphine, and the reference category of neither). Self-reported cocaine use (yes/no) in the 30 days prior to baseline was included as an additional predictor based on studies showing that cocaine users have lower retention rates in both methadone (Deck & Carlson, 2005; Grella, Wugalter, & Anglin, 1997; Joe, Simpson & Broome, 1999; Saxon, Wells, Fleming, Jackson, & Calsyn, 1996) and buprenorphine treatment (Pinto et al., 2010; Sullivan et al., 2010). The other predictor variables were selected based on our previous research, which found that African American patients entering methadone vs. buprenorphine treatment differ significantly on these characteristics (Mitchell et al., 2012). Thus, these variables are included in the present analysis to adjust for known differences between the methadone and buprenorphine samples. This approach allows a more accurate comparison of retention in methadone vs. buprenorphine treatment by controlling for potential confounds related to patients’ entry into one treatment or the other.
2.4. Statistical Analysis
Survival analysis modeling was used to examine the relationships between the independent variables and retention in treatment. The Kaplan-Meier estimate of the survival function was used to characterize the proportion of patients remaining in treatment over time. Time-to-dropout was analyzed using a parametric survival model with the Weibull distribution, with medication type (methadone vs. buprenorphine) as the primary effect of interest. In the Weibull model, the time ratio (TR) represents the multiplicative effect of a unit change in an independent variable on time to dropout, while the hazard ratio (HR) represents the multiplicative effect of a unit change on the risk of dropout. Interactions were tested between medication type and patient demographics, injection drug use status, and baseline cocaine use in order to determine whether response to the medications differed systematically based on these factors.
3. Results
Medication doses were individually determined in both the methadone and buprenorphine programs. As previously reported (Schwartz et al., 2012), methadone was typically started between 20mg–30mg, then increased gradually to a maintenance dose. The mean methadone dose in the methadone sample was 84.2 (SD= 22.8). Buprenorphine was typically started between 4mg–8mg of the buprenorphine/naloxone combination (Suboxone®) tablet or sublingual film, then increased gradually to a maintenance dose. The modal buprenorphine maintenance dose was 16 mg. Dose induction with buprenorphine was typically rapid, with nearly 80% of the patients reaching a maintenance dose (defined as 7 consecutive days at the same dose) within 4 days of starting treatment.
Descriptive statistics and bivariate tests of group differences for the methadone and buprenorphine samples can be found in Table 1. Buprenorphine patients compared to methadone patients were significantly older (p=.020), less likely to inject drugs (p<.001), had more days of recent medical problems (p<.001) and were more likely to have a history of recent and lifetime depression (both ps<.001). Participants in each type of treatment were more likely to have prior experience with the same medication they were currently taking (p< .001).
Table 2 presents the results of the survival analysis, in both time ratio and hazard ratio metrics. The model shows that during the first 6 months, buprenorphine patients stayed about one third as long as methadone patients (TR=.31, p=<.001). Over this period, buprenorphine patients were over twice as likely to drop out of treatment as were methadone patients (HR= 2.43). Overall 6-month retention rates were 57.7% for buprenorphine and 78.1% for methadone.
Table 2.
Weibull survival model predicting days until dropout: buprenorphine vs. methadone.
| Time Ratio (95% CI) | Hazard Ratio (95% CI) | p | |
|---|---|---|---|
| Treatment | |||
| Buprenorphine (ref=methadone) | 0.31 (.17 .54) | 2.43 (1.60–3.69) | <.001 |
|
| |||
| Participant Characteristics | |||
| Age | 1.01 (.98–1.04) | 0.99 (.97–1.02) | .613 |
| Female Gender | 0.88 (.57–1.35) | 1.10 (.80–1.52) | .559 |
| Injection drug user | 0.77 (.48–1.25) | 1.21 (.84–1.74) | .296 |
| Cocaine Use, past 30 days | 0.52 (.34 .81) | 1.63 (1.18–2.25) | .004 |
| Previous Treatments (ref=none) | |||
| Prior Methadone only | 1.83 (1.02–3.29) | 0.64 (.41–.99) | .044 |
| Prior Buprenorphine only | 1.44 (.81–2.56) | 0.76 (.50–1.17) | .218 |
| Both methadone and buprenorphine | 1.01 (.56–1.82) | 0.99 (.64–1.54) | .976 |
| Health | |||
| Acute depression, past 30 days | 1.17 (.66–2,08) | 0.89 (.58–1.37) | .592 |
| Lifetime depression | 0.88 (.53–1.45) | 1.10 (.76–1.61) | .607 |
| Medical problems, # of days | 1.00 (.98–1.03) | 1.00 (.98–1.02) | .878 |
Note: CI = Confidence Interval.
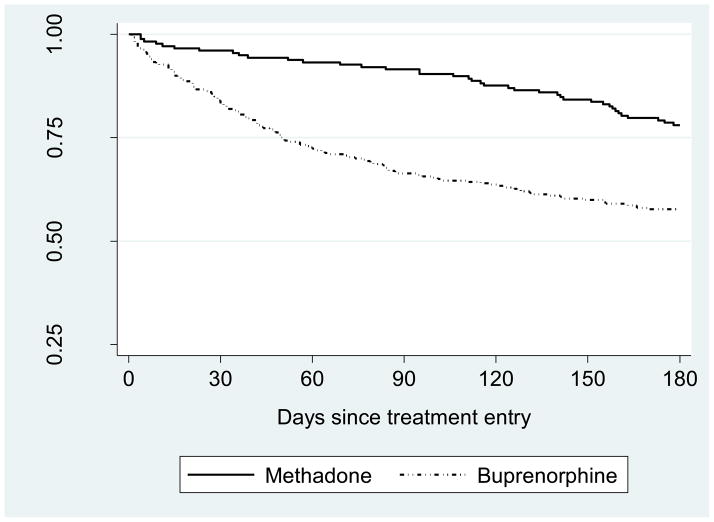
Figure 1 shows the unadjusted Kaplan-Meier survival curves for each medication. All remaining cases are censored at 180 days. The methadone patients show a higher probability of staying in treatment than the buprenorphine patients for the 6-month period. However, the advantage of methadone over buprenorphine appears to be concentrated in the early part of the observation period. The buprenorphine curve shows a steep drop during approximately the first 50 days, after which the rate of dropout decreases somewhat but remains higher than that of methadone through approximately 90 days. The methadone retention curve drops more gradually over the time period than the buprenorphine curve. During the second half of the observation period from ~90 days to 180 days, the survival curves of methadone and buprenorphine appear approximately parallel.
Figure 1.
Kaplan-Meier survival curves for methadone and buprenorphine.
Only two other independent variables in the equation were associated with time-to-dropout. Patients who reported using cocaine in the 30 days prior to treatment admission stayed in treatment only about half as long as patients who did not use cocaine (TR=.52, p=.004), and were 63% more likely to discontinue treatment earlier than non-cocaine users (HR=1.63). While patients with prior experience in methadone treatment only tended to stay in treatment almost twice as long as those without prior opioid pharmacotherapy (TR=1.83; p=.04), the prior treatment experience variables were not jointly significant (p=.15). None of the other independent variables in the equation were significantly associated with time-to-dropout. These variables are nevertheless retained in the equation to help isolate the effect of medication type because they are associated with treatment selection (Mitchell et al., 2012).
Interactions were tested between treatment type and participant demographics, injection drug use, and cocaine use. Each interaction was non-significant, indicating that there was no evidence of a differential treatment retention response in methadone vs. buprenorphine based on these variables.
4. Discussion
This multi-clinic study compared retention in methadone and buprenorphine treatment among 478 African American patients receiving opioid pharmacotherapy in a large urban publicly-funded treatment system. Consistent with previous evidence from clinical trials (Mattick et al., 2008) and real-world observational studies (Burns et al., 2009; Pinto et al., 2010), the current study found methadone to have superior retention compared with buprenorphine. Although not all studies have found a retention disadvantage for buprenorphine relative to methadone (e.g., Barnett, 2009; Kakko et al., 2007; Soyka et al., 2008; Vigezzi et al., 2006), the balance of the literature suggests that a retention disparity (favoring methadone) exists between these two medications (Mattick et al., 2008).
In a Cochrane Collaboration review of the efficacy of buprenorphine treatment, Mattick and colleagues (2008) discuss several possible factors that may have contributed to that review’s finding of lower retention rates in buprenorphine treatment as compared to methadone. First, as a partial agonist, buprenorphine may be less reinforcing than methadone. Moreover, buprenorphine has a milder withdrawal syndrome and may thus be easier for patients to discontinue than methadone. Another issue that may be particularly critical in the first few days of treatment is buprenorphine’s potential to precipitate opioid withdrawal in patients who begin buprenorphine treatment very soon after using a full opioid agonist, such as heroin or methadone (Johnson, Strain, & Amass, 2003). Conversely, lengthy dose induction periods may lead to insufficient management of withdrawal and craving, prolonging the length of time until subjective patient stabilization. Finally, clinical trials may not always examine buprenorphine treatment as optimally delivered since doses in such trials are often dispensed according to a protocol (Maremmani & Gerra, 2010). Others have pointed out that outcome differences between methadone and buprenorphine are actually clinically minor, and that there is comparatively greater variation in effectiveness between different methadone programs (Barnett, Rodgers, & Bloch, 2001).
Medication doses in the present report were individually determined in both the methadone and buprenorphine programs. The most common buprenorphine maintenance dose was 16 mg, which is higher than used in most of the clinical trials literature (Mattick et al., 2008). However, early studies used the alcohol solution of buprenorphine, which has higher bioavailability than the tablet formulation (Compton et al., 2006). With chronic administration, bioavailability is similar for buprenorphine solution and buprenorphine/naloxone combination tablets as used in the current study (Strain, Moody, Stoller, Walsh & Bigelow, 2004). Moreover, in the present study, the dose induction periods were individually determined and nearly 80% of the buprenorphine patients reached their maintenance dose within 4 days of treatment entry (operationally defined as 7 days at the same dose). Therefore, slow induction in buprenorphine dose is not a likely explanation for the higher early drop-out from buprenorphine treatment.
Another possibility is that the retention disparity between methadone and buprenorphine has less to do with the differing pharmacological properties of the medications than with differences in the way that the respective treatments are structured. Methadone has been a mainstay of public sector treatment for opioid dependence for many years, whereas buprenorphine is relatively newer. Opioid-dependent individuals considering treatment may thus have a better sense of what methadone has to offer, and what is expected of patients (e.g., daily dosing, some counseling attendance). There may have been less awareness of the expectations in buprenorphine treatment as implemented in Baltimore’s public sector system, where buprenorphine was made available in a number of formerly drug-free substance abuse programs. In some ways, these programs developed procedures that paralleled those of methadone clinics in their handling of the medication (e.g., daily on-site administration of buprenorphine). Yet, group counseling was considered a central aspect of treatment, and patients were expected to attend. One plausible explanation for why patients were more likely to discontinue buprenorphine than methadone treatment is a greater mismatch between patient and program expectations.
Although the overall risk of treatment dropout was higher for buprenorphine patients than for methadone patients, the survival curves show a more complex picture. While methadone treatment is characterized by a pattern of gradual attrition, buprenorphine patients are at highest risk of dropout in the early phases of treatment. The retention disparity between methadone and buprenorphine emerges early, but the retention curves for the respective medications eventually stabilize and become parallel.
This finding is consistent with several other studies that have compared retention in methadone and buprenorphine (for example, Fischer et al., 1999; Kakko et al., 2007; Mattick et al., 2003, 2008; Pinto et al., 2010). One of the few studies that did not find differences in retention between methadone and buprenorphine was conducted by Maremmani and collaborators (2007). However, that study only included patients who had been retained in treatment through 90 days, thus missing the impact of early drop out. The pattern of elevated risk of dropout in the earlier segments of the treatment episode was also found in studies of buprenorphine treatment delivered in primary care (Fiellin et al., 2008; Stein, Cioe, & Friedmann, 2005).
The current study’s findings are especially consistent with those of the SUMMIT trial, which examined outcomes in methadone and buprenorphine treatment as delivered in real-world clinical settings in the UK (Pinto et al., 2010). That study found that retention disparities between methadone and buprenorphine were considerably more pronounced than those found in the controlled trials literature. The treatment retention (survival) curves for these two medications in the current research are almost indistinguishable from those identified in the SUMMIT trial. The similarities in retention patterns are remarkable considering these studies were conducted in different countries, within different public healthcare systems, and with a different population (primarily White Europeans vs. African Americans).
The shape of the retention disparity between methadone and buprenorphine could have important clinical implications. It is possible that interventions to reduce early dropout from buprenorphine treatment could potentially enhance the effectiveness of buprenorphine to be on par with methadone. Future research on approaches to decrease early discontinuation of buprenorphine treatment is needed. Identification of effective strategies to decrease early dropout could help to maximize the individual and public health benefits of buprenorphine treatment.
Recent cocaine use prior to treatment entry predicted drop-out, a finding consistent with research in methadone (Deck & Carlson, 2005; Grella et al., 1997; Joe et al., 1999; Saxon et al., 1996) as well as buprenorphine treatment (Pinto et al., 2010; Sullivan et al., 2010). Interestingly, some research has not found a relationship between baseline cocaine use and retention in buprenorphine treatment (Sullivan et al., 2011), and there is some evidence that buprenorphine may actually reduce cocaine use as well as opioid use (Montoya et al., 2004). The elevated risk of treatment drop-out for cocaine users could be attributable to a number of factors. For example, programs may have referred patients to a higher level of care to manage their cocaine use. However, this explanation is limited because, in most cases, such patients would be referred to a provider where they could continue their medication for opioid dependence, and this study conceptualized retention as continued enrollment in medication treatment either at the original program or elsewhere. Another possibility is that patients with co-occurring cocaine use left treatment at higher rates because of problems secondary to their cocaine use. Patients continuing to use cocaine may have also been at higher risk for involuntary discharge from the treatment programs (e.g., for rule infractions or perceived lack of progress in treatment).
While this study made use of data from two separate clinic trials that were being conducted nearly contemporaneously, the use of secondary data carries certain limitations. For example, we were limited in considering only those measures that were common to both parent studies. Although we attempted to account for differing patient characteristics among those seeking treatment with methadone and buprenorphine (Mitchell et al., 2012), we cannot discount the possibility that other unmeasured factors may impact upon patients’ treatment selection and therefore lead to biased inferences for the medication effect. This limitation is balanced by the open inclusion criteria and the use of multiple program sites, which allows for an appraisal of retention differences between methadone and buprenorphine treatment in real-world clinical settings.
It is important to note that the focus on the low-income African American population in the present study may limit generalizability to other groups and other treatment systems. However, low-income urban African American heroin users are an important population that stands to benefit from opioid agonist treatment. This group relies on the publicly-funded treatment sector, where buprenorphine has had a relatively slow rollout (Ducharme & Abraham, 2008; Knudsen, Ducharme, & Roman, 2006; Koch, Arfken, & Schuster, 2006) and has been characterized by other access barriers (Clark et al., 2011). The current study sheds light on retention disparities between methadone and buprenorphine treatment for this underserved population.
Acknowledgments
Funding for this study was provided by RC1DA028407 (PI, Mitchell) and R0113636 (PI, Schwartz) from the National Institute on Drug Abuse, which did not play a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. We thank the National Institute on Drug Abuse for funding the parent studies, as well as the participating treatment programs for their clinical assistance: Total Health Care, Partners in Recovery, Sinai Hospital Addiction Recovery Program, and the University of Maryland Drug Treatment Center. Finally, we thank Melissa Irwin and Kyra Walls for their assistance with manuscript preparation.
Footnotes
All subjects provided informed consent and study procedures were in accord with the standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accord with the Helsinki Declaration of 1975.
Disclosures
Until 2009, Dr. Schwartz was Program Director and Fellow at the Open Society Institute-Baltimore, which made grants to support drug abuse treatment in Baltimore. Drs. Schwartz and Jaffe also serve as members of the Board of Baltimore’s Substance Abuse Treatment Authority (Baltimore Substance Abuse Treatment Systems). A NIDA-funded study in which Dr. Schwartz serves as a Co-Investigator receives medication in-kind from Reckitt Benckiser Pharmaceuticals. Dr. O’Grady has been reimbursed for his time by Reckitt Benckiser Pharmaceuticals. Dr. Olsen was the BSAS Medical Director from 2009 to 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Healthcare Research and Quality. AHRQ Healthcare Innovations Exchange. Rockville, MD: U.S. Department of Health and Human Services; 2011. Dec, Community partnerships and provider training increase service capacity and access to long-term treatment for individuals with heroin addiction. Retrieved May 14, 2012, from http://www.innovations.ahrq.gov/content.aspx?id=1827. [Google Scholar]
- Barnett PG, Rodgers JH, Bloch DA. A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction. 2001;96:683–690. doi: 10.1046/j.1360-0443.2001.9656834.x. [DOI] [PubMed] [Google Scholar]
- Barnett PG. Comparison of costs and utilization among buprenorphine and methadone patients. Addiction. 2009;104:982–992. doi: 10.1111/j.1360-0443.2009.02539.x. [DOI] [PubMed] [Google Scholar]
- Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104:1193–1200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- Burns L, Randall D, Hall WD, Law M, Butler T, Bell J, Degenhardt L. Opioid agonist pharmacotherapy in New South Wales from 1985 to 2006: Patient characteristics and patterns and predictors of treatment retention. Addiction. 2009;104:1363–1372. doi: 10.1111/j.1360-0443.2009.02633.x. [DOI] [PubMed] [Google Scholar]
- Caplehorn JR, Dalton MS, Cluff MC, Petrenas AM. Retention in methadone maintenance and heroin addicts’ risk of death. Addiction. 1994;89:203–209. doi: 10.1111/j.1360-0443.1994.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, Baxter JD, Leung GY. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30(8):1425–1433. doi: 10.1377/hlthaff.2010.0532. [DOI] [PubMed] [Google Scholar]
- Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): A national prospective cross-registry study. Drug and Alcohol Dependence. 2008;94:151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opiate users: Opioid maintenance therapy, age and causes of death. Addiction. 2009;104(8):1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Compton P, Ling W, Moody D, Chiang N. Pharmacokinetics, bioavailability and opioid effects of liquid versus tablet buprenorphine. Drug and Alcohol Depend. 2006;82:25–31. doi: 10.1016/j.drugalcdep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. [Review] Health Technology Assessment. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- D’Aunno T, Pollack HA. Changes in methadone treatment practices: Results from a national panel study, 1988–2000. Journal of the American Medical Association. 2002;288:850–856. doi: 10.1001/jama.288.7.850. [DOI] [PubMed] [Google Scholar]
- Deck D, Carlson MJ. Retention in publicly funded methadone maintenance treatment in two Western States. Journal of Behavioral Health Services and Research. 2005;32:43–60. doi: 10.1007/BF02287327. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment, Prevention, and Policy. 2008;3:17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, Schottenfeld RS. Long-term treatment with buprenorphine/naloxone in primary care: Results at 2–5 years. The American Journal on Addictions. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stuhlinger G, Kasper S. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462–468. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- Grella CE, Wugalter SE, Anglin MD. Predictors of treatment retention in enhanced and standard methadone maintenance treatment for HIV risk reduction. Journal of Drug Issues. 1997;27:203–224. [Google Scholar]
- Gronbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: Impact of methadone treatment. Acta Psychiatrica Scandinavica. 1990;82:223–227. doi: 10.1111/j.1600-0447.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Schwartz RP, Salkever DS, Mitchell SG, Jaffe JH. Patterns in admission delays to outpatient methadone treatment in the United States. Journal of Substance Abuse Treatment. 2011;41:431–439. doi: 10.1016/j.jsat.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS) Journal of Substance Abuse Treatment. 2003;25:125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Hulse GK, English DR, Milne E, Holman CD. The quantification of mortality resulting from the regular use of illicit opiates. Addiction. 1999;94:221–229. doi: 10.1046/j.1360-0443.1999.9422216.x. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Broome KM. Retention and patient engagement models for different treatment modalities in DATOS. Drug and Alcohol Dependence. 1999;57:113–125. doi: 10.1016/s0376-8716(99)00088-5. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug and Alcohol Dependence. 2003;70:S59–77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, Rawlings B, Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: A randomized controlled trial. American Journal of Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. Early adoption of buprenorphine in substance abuse treatment centers: Data from the private and public sectors. Journal of Substance Abuse Treatment. 2006;30:363–373. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Koch AL, Arfken CL, Schuster CR. Characteristics of U.S. substance abuse treatment facilities adopting buprenorphine in its initial stage of availability. Drug and Alcohol Dependence. 2006;83:274–278. doi: 10.1016/j.drugalcdep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, Zhao C. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103:1484–1492. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- Magura S, Rosenblum A. Leaving methadone treatment: Lessons learned, lessons forgotten, lessons ignored. Mount Sinai Journal of Medicine. 2001;68(1):62–74. [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. Journal of Substance Abuse Treatment. 2007;33:91–98. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Gerra G. Buprenorphine-based regimens and methadone for the medical management of opioid dependence: Selecting the appropriate drug for treatment. American Journal on Addictions. 2010;19:557–568. doi: 10.1111/j.1521-0391.2010.00086.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: A randomized double-blind trial with 405 opioid dependent patients. Addiction. 2003;98:441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008;16(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: An 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndrome. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Miotto K, Hillhouse M, Donovick R, Cunningham-Rathner J, Charuvastra C, Torrington M, Ling W. Comparison of buprenorphine treatment for opioid dependence in 3 settings. Journal of Addiction Medicine. 2012;6:68–76. doi: 10.1097/ADM.0b013e318233d621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Kelly SM, Gryczynski J, Myers CP, Jaffe JH, O’Grady KE, Schwartz RP. African American patients seeking treatment in the public sector: Characteristics of buprenorphine vs. methadone patients. Drug and Alcohol Dependence. 2012;122:55–60. doi: 10.1016/j.drugalcdep.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, Jaffe JH. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug and Alcohol Dependence. 2013;128(3):222–229. doi: 10.1016/j.drugalcdep.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Fudala PJ. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clinical Pharmacology and Therapeutics. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan JA, Karan LD. Substance abuse treatment modalities in the age of HIV spectrum disease. Journal of Psychoactive Drugs. 1989;21:423–429. doi: 10.1080/02791072.1989.10472188. [DOI] [PubMed] [Google Scholar]
- Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: A field comparison of buprenorphine versus methadone maintenance treatment. Journal of Substance Abuse Treatment. 2010;39:340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Pollack HA, D’Aunno T. Dosage patterns in methadone treatment: Results from a national survey, 1988–2005. Health Services Research. 2008;43:2143–2163. doi: 10.1111/j.1475-6773.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91:1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction. 2012;107:943–952. doi: 10.1111/j.1360-0443.2011.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. The International Journal of Neuropsychopharmacology. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- Stanton A. Summary Report. Rockville, MD: WESTAT; 2006. The SAMHSA Evaluation of the Impact of the DATA Waiver Program. [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Brief report: Buprenorphine retention in primary care. Journal of General Internal Medicine. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug and Alcohol Dependence. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA. Buprenorphine: Its role in preventing HIV transmission and improving the care of HIV-infected patients with opioid dependence. Clinical Infectious Diseases. 2005;41:891–896. doi: 10.1086/432888. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O’Connor PG, Fiellin DA. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of Substance Abuse Treatment. 2008;35:87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, O’Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, Fiellin DA. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. The American Journal on Addictions. 2010;19:53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Botsko M, Cunningham CO, O’Connor PG, Hersh D, Mitty J, BHIVES Collaborative. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. Journal of Acquired Immune Deficiency Syndrome. 2011;56:S54–61. doi: 10.1097/QAI.0b013e3182097576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report. United Nations Publication; 2011. Sales No. E.11.XI.10. Retrieved on May 11, 2012, from http://www.unodc.org/documents/data-and-analysis/WDR2011/World_Drug_Report_2011_ebook.pdf. [Google Scholar]
- World Health Organization. WHO model list of essential medicines. Geneva, Switzerland: WHO; 2010. Mar, Retrieved on May 14, 2012, from http://apps.who.int/medicinedocs/documents/s17833en/s17833en.pdf. [Google Scholar]
- Vigezzi P, Guglielmino L, Marzorati P, Silenzio R, De Chiara M, Corrado F, Cozzolino E. Multimodal drug addiction treatment: A field comparison of methadone and buprenorphine among heroin- and cocaine-dependent patients. Journal of Substance Abuse Treatment. 2006;31:3–7. doi: 10.1016/j.jsat.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug and Alcohol Dependence. 1998;52:257–260. doi: 10.1016/s0376-8716(98)00097-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Friedmann PD, Gerstein DR. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98:673–684. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]