Abstract
Malassezia yeasts are commensal microorganisms which under insufficiently understood conditions can become pathogenic. We have previously shown that specific strains isolated from diseased human skin can preferentially produce agonists of the aryl hydrocarbon receptor (AhR), whose activation has been linked to certain skin diseases. Investigation of skin scale extracts from patients with Malassezia associated diseases demonstrated 10–1000 fold higher AhR activating capacity than control skin extracts. LC/MS/MS analysis of the patients’ extracts revealed the presence of indirubin, 6-formylindolo[3,2-b]carbazole (FICZ), indolo[3,2-b]carbazole (ICZ), malassezin, and pityriacitrin. The same compounds were also identified in 9/12 Malassezia species culture extracts tested, connecting their presence in skin scales with this yeast. Studying the activity of the Malassezia culture-extracts and pure metabolites in HaCaT cells by Reverse Transcriptase Real-Time PCR revealed significant alterations in mRNA levels of the endogenous AhR-responsive genes Cyp1A1, Cyp1B1 and AhRR. Indirubin and FICZ activated AhR in HaCaT and human HepG2 cells with significantly higher, yet transient, potency as compared to the prototypical AhR ligand, dioxin. In loco synthesis of these highly potent AhR inducers by Malassezia yeasts could have a significant impact on skin homeostatic mechanisms and disease development.
Introduction
Healthy human skin harbors a significant amount of commensal yeasts belonging to the genus Malassezia that can become pathogenic under currently inadequately understood conditions (Gaitanis et al, 2012; Ashbee 2007). These yeasts are implicated in the pathogenesis of skin diseases with diverse clinical presentation. Malassezia yeasts can cause pityriasis versicolor (PV), a minimally inflammatory condition despite a concomitant heavy fungal load, which however modifies the function of melanocytes, as evidenced by the formation of hyper- or hypopigmented plaques (Thoma et al, 2005). On the other hand, Malassezia yeasts are also implicated in exacerbations of certain inflammatory dermatoses, such asatopic dermatitis (AD) and seborrheic dermatitis (SD) (Gupta et al, 2004). All the aforementioned skin conditions are common and may have a significant impact on the quality of life of afflicted individuals (Sugita et al, 2010). Currently, the genus Malassezia comprises 14 species (Gaitanis et al, 2012). The most prevalent species in humans are M. globosa, M. restricta, M. sympodialis and M. furfur, with the first two species present on the skin of almost all individuals (Tajima et al, 2008). A major challenge in our effort to comprehend the role of Malassezia yeasts inskin diseases is to delineate pathogenic species or strains and to attribute virulence factors. During the last decade an array of indolic substances synthesized in vitro by M. furfur has been identified. These substances have been associated with the pathogenic potential of this species (Mayser & Gaitainis 2010). We have previously observed that M. furfur strains isolated from SD lesions (and from PV; unpublished observation) synthesize substances as are malassezin, indole[3,2-b]carbazole (ICZ) and pityriacitrin in significantly higher quantities in vitro compared to healthy skin isolates (Gaitanis et al, 2008). Malassezin has been shown to induce apoptosis in human melanocytes through Arylhydrocarbon Receptor (AhR) activation, while ICZ is a known potent ligand of this receptor. Furthermore, the existence of malassezin on human skin could be a chemical marker of the presence of M. furfur as the former has been described to be produced only by this species. To date the production of these bioactive indoles had been shown only in vitro and only for M. furfur species thus causing reservation in respect to their clinical relevance.
AhR is an orphan, ligand-dependent nuclear receptor with multifaceted biological functions. It has been shown to participate in the maintenance of skin homeostasis (Bock & Kohle 2009), the enhancement of wound healing (Barouti et al, 2009) and the partial mediation of ultraviolet radiation damage through the photochemical intracellular production of the potent AhR ligand, 6-formylindolo[3,2-b]carbazole (FICZ) (Fritsche et al, 2007). Regarding skin pathophysiology, it is well known that TCDD causes its detrimental effects through persistent activation of this receptor and the downstream signaling pathway. The appearance of chloracne, a characteristic acne-like eruption, is one of the heralding signs of TCDD intoxicationin humans (Panteleyev & Bickers 2006; Sorg et al, 2009). Furthermore, activation of the AhR plays an important role in mediating the biological/toxicological effects of a variety of environmental xenobiotics, including the Malassezia produced indirubin and ICZ, on the immune system (Esser et al, 2009; Vlachos et al, 2012).
After documenting the preferential production of the AhR ligands ICZ and malassezin in M. furfur SD isolates we decided to validate their in vivo existence in Malassezia associated skin diseases (SD and PV) as compared to appropriate control samples. This was carried out by employing the very sensitive CALUX bioassay (Denison et al, 2004) that measures the ability and relative potency of a chemical or extract to activate the AhR and AhR-dependent gene expression. The extracts from diseased skin scales revealed significantly higher AhR activation potential in the CALUX bioassay as compared to controls. In parallel and in order to expand the biological significance of AhR ligands synthesis by Malassezia yeasts we: 1)analyzed Malassezia-culture and skin scales extracts for the existence of additional indolic AhR activators; 2)screened Malassezia species for the production of AhR ligands in order to establish the wider significance of this biochemical trait in this genus; 3)assessed the effect of Malassezia culture-extracts and indolic ingredients on HaCaT keratinocytes.
Results
AhR activation by human skin scale extracts
Organic solvent skin scale extracts were prepared from 10 patients with SD (N=6) or PV (N=4) and 6 healthy volunteers and evaluated for their ability to activate AhR-dependent gene expression in recombinant human hepatoma (HG2L7.5c1) cells containing a stably transfected AhR-responsive luciferase reporter gene. The extracts originating from the patients’ lesional skin scales were between 10–1000 times more potent in stimulating AhR-dependent luciferase activity than those from control healthy facial skin samples (N=3) or skin samples from anatomic areas that are not expected under physiological conditions to be colonized by Malassezia yeasts (N=3)(Table 1).
Table 1.
Quantitation (mol/mg of extract) of malassezin, indirubin, indolo[3,2-b]carbazole (ICZ), and 6-formylindolo[3,2-b]carbazole (FICZ) and pityriacitrin by HPLC/MS/MS in the extracts of skin originating patients with seborrheic dermatitis (SD) or pityriasis versicolor (PV), and healthy subjects (Healthy 1–3: elbows, palms and soles, Healthy 4–6: facial skin). Values are means of two independent measurements. The EC50 for stimulating AhR-dependent luciferase activity of each skin extract has been measured by the CALUX cell bioassay.
| Malassezin mol/mg skin extract | ICZ mol/mg skin extract | FICZ mol/mg skin extract | Indirubin mol/mg skin extract | Pityriacitrin mol/mg skin extract | EC50 μg/mL | |
|---|---|---|---|---|---|---|
| SD1 | ND1 | ND | ND | 8.1×10−11 | ND | 0.1 |
| SD2 | ND | ND | ND | 1.5×10−10 | ND | 0.08 |
| SD3 | 2×10−13 | ND | 1.1×10−11 | ND | 7.5×10−11 | 0.88 |
| SD4 | ND | ND | 5×10−13 | 4.2×10−12 | ND | 0.67 |
| SD5 | ND | ND | ND | ND | ND | >100 |
| SD6 | 2×10−13 | 1.0×10−11 | ND | ND | 4.1×10−12 | 7.9 |
| PV1 | ND | 2.1×10−11 | ND | ND | 1.5×10−11 | 4.9 |
| PV2 | 2.5×10−12 | ND | ND | 2.6×10−10 | ND | 0.07 |
| PV3 | 1.1×10−12 | ND | ND | 1.3×10−11 | ND | 0.09 |
| PV4 | ND | ND | ND | ND | ND | >100 |
| Healthy1 | ND | ND | ND | ND | ND | 53 |
| Healthy2 | ND | ND | ND | ND | ND | >100 |
| Healthy3 | ND | ND | ND | ND | ND | 100 |
| Healthy4 | ND | ND | ND | ND | ND | 83 |
| Healthy5 | ND | ND | ND | ND | ND | >100 |
| Healthy6 | ND | ND | ND | ND | ND | >100 |
ND: Not detected; Limits of detection (mol/mg): Malassezin: 4×10−14, ICZ: 8×10−12, FICZ: 2.9×10−13, Indirubin: 6×10−13, Pityriacitrin: 3×10−13
Identification, isolation and quantitation of indolic compounds from Malassezia furfur culture extracts
Using our previously described methodology (Gaitanis et al, 2008) we reevaluated the M. furfur culture extracts that had been included in the previous study. By chromatographic separations we isolated and identified by NMR and mass spectroscopy three additional indolic compounds known to be potent AhR activators, namely indirubin, FICZ and tryptanthrin. The quantitation of the more prevalent indolic AhR ligands in culture extracts of M. furfur strains was performed by HPLC/UV or LC/MS/MS using as analytical standards pure synthetic substances (Fig. S1). In order to rule out the likelihood of the photochemical production of these indoles all cultures were performed in the dark. As a negative control, extracts from agar slants without Malassezia inoculum were included. Comparison of M furfur strains isolated from healthy skin with SD isolates showed that the latter had elevated levels of FICZ (p=0.001), indirubin (p=0.001), and tryptanthrin (p=0.005) and pityriacitrin (p=0.001) (Table 2), in addition to the previously documented elevated levels of ICZ (p<0.001) and malassezin (p=0.001) (Gaitanis et al, 2008).
Table 2.
Quantitation (μg/mg of extract) of malassezin, indirubin, indolo[3,2-b]carbazole (ICZ), tryptanthrin and 6-formylindolo[3,2-b]carbazole (FICZ) by HPLC/UV in the extracts of clinical M. furfur strains originating from seborrheic dermatitis, pityriasis versicolor, folliculitis and healthy subjects. Values are means and standard deviations of three independent measurements.
| Strain | Skin of origin of the sample | Malassezin (μg/mg) | Indirubin (μg/mg) | ICZ (μg/mg) | Tryptanthrin (μg/mg) | FICZ (μg/mg) | Pityriacitrin (μg/mg) |
|---|---|---|---|---|---|---|---|
| M. furfur Bul19 | SD1 | 4.08±0.35 | 0.084±0.010 | 0.26±0.01 | 0.59±0.03 | 1.33±0.15 | 4.82±0.33 |
| M. furfur Bul22 | SD | 3.33±0.38 | 3.90±0.33 | 2.50±0.11 | 1.52±0.09 | 1.14±0.10 | 3.62±0.30 |
| M. furfur Bul23 | SD | 3.52±0.29 | 0.25±0.02 | 0.36±0.02 | 0.38±0.03 | 0.45±0.04 | 3.63±0.28 |
| M. furfur Bul412 | SD | 2.02±0.18 | 3.36±0.34 | 0.95±0.07 | 0.53±0.03 | 0.72±0.05 | 2.76±0.27 |
| M. furfur CBS9585 | SD | 2.02±0.20 | 1.40±0.13 | 0.40±0.02 | 0.24±0.02 | 0.73±0.06 | 4.93±0.35 |
| M. furfur CBS9596 | SD | 4.12±0.25 | 2.66±0.21 | 1.60±0.12 | 0.58±0.03 | 0.68±0.06 | 2.31±0.23 |
| M. furfur GS19A | SD | 2.16±0.15 | 0.022±0.01 | 0.38±0.02 | 0.05±0.01 | ND | 2.49±0.25 |
| M. furfur WCH114 | SD | 4.21±0.40 | 1.37±0.15 | 1.47±0.11 | 0.66±0.05 | 0.58±0.03 | 0.75±0.35 |
| M. furfur WCH100 | PV2 | 3.17±0.37 | 1.58±0.14 | 0.82±0.05 | 1.65±0.11 | 0.67±0.04 | 3.07±0.28 |
| M. furfur WCH106 | Folliculitis | 5.99±0.51 | 1.48±0.14 | 3.27±0.29 | 4.50±0.38 | 0.57±0.05 | 8.27±0.45 |
| M. furfur GS1B | HS3 | 0.38±0.03 | 0.006±0.001 | ND4 | 0.029±0.005 | ND | 0.41±0.04 |
| M. furfur GS2A | HS | 0.40±0.03 | 0.006±0.001 | ND | 0.031±0.005 | ND | 0.57±0.03 |
| M. furfur GS2B | HS | 0.39±0.04 | 0.009±0.002 | ND | 0.094±0.007 | ND | 0.55±0.03 |
| M. furfur GS4A | HS | 0.39±0.04 | 0.009±0.002 | ND | 0.16±0.01 | ND | 1.18±0.09 |
| M. furfur GS46A | HS | 0.45±0.02 | 0.010±0.001 | ND | 0.19±0.01 | ND | 0.77±0.06 |
| M. furfur GS9A | HS | 0.56±0.04 | 0.026±0.002 | ND | 0.37±0.02 | ND | 0.35±0.05 |
| M. furfur GA9B | HS | 0.43±0.04 | 0.008±0.002 | ND | 0.088±0.07 | ND | 0.68±0.05 |
SD: Seborrheic dermatitis;
PV: Pityriasis versicolor;
HS: healthy subject;
ND: Not detected
Identification and quantitation of selected indolic products in cultures of different Malassezia species
To expand the significance of indole synthesis by Malassezia species we screened Type strains of species that are mainly isolated from human skin. At least one of these AhR ligands was detected in 9/12 Malassezia species strains studied (Table 3). Overall, the M. furfur pathogenic strains were the most efficient producers of these indoles (Tables 2,3). However, with the exception of M.sympodialis and M.restricta, all other isolates from humans (M.furfur, M.obtusa, M.globosa, M.slooffiae, M.japonica, M.yamatoensis) in addition to the animal isolates M.pachydermatis and M.nana, also synthesize at least some AhR ligands.
Table 3.
Quantitation (μg/mg of extract) of malassezin, indirubin, indolo[3,2-b]carbazole (ICZ), tryptanthrin and 6-formylindolo[3,2-b]carbazole (FICZ) by HPLC/UV in the extracts of 13 Type and reference Malassezia species strains. Values are means and standard deviations of three independent measurements.
| Malassezia species | Malassezin (μg/mg) | Indirubin (μg/mg) | ICZ (μg/mg) | Tryptanthrin (μg/mg) | FICZ (μg/mg) | Pityriacitrin (μg/mg) |
|---|---|---|---|---|---|---|
| M. furfur CBS1878 | 2.02±0.18 | 0.062±0.005 | 0.42±0.03 | 0.49±0.04 | 0.84±0.02 | 0.75±0.05 |
| M. furfur CBS6001 | 3.52±0.25 | ND | ND | ND | ND | ND |
| M. pachydermatis CBS6534 | 0.28±0.02 | 0.002 | ND | Traces | ND | ND |
| M. sympodialis CBS7222 | ND1 | ND | ND | ND | ND | ND |
| M. obtusa CBS7876 | Traces2 | 0.006±0.001 | ND | Traces | ND | ND |
| M. globosa CBS7966 | Traces | 0.006±0.001 | ND | 0.074±0.04 | ND | ND |
| M. slooffiae CBS7971 | Traces | ND | ND | ND | ND | ND |
| M. restricta CBS7991 | ND | ND | ND | ND | ND | ND |
| M. dermatis CBS9170 | Traces | ND | ND | ND | ND | ND |
| M. japonica CBS9432 | 0.30±0.02 | 0.005±0.001 | ND | 0.017±0.02 | ND | 0.33±0.05 |
| M. nana CBS9557 | 0.34±0.03 | ND | ND | ND | ND | ND |
| M. yamatoensis CBS9726 | 0.29±0.02 | 0.002±0.001 | Traces | Traces | ND | 0.24±0.04 |
| M. caprae CBS10434 | ND | ND | ND | ND | ND | ND |
ND: Not detected;
Trace: detectable but not quantifiable
Identification of indolic Malassezia metabolites in human skin scale samples
The identification of indirubin and FICZ, along with the previously known Malassezia metabolites malassezin, pityriacitrin and ICZ, in EtOAc extracts of human skin scales was performed by LC/MS/MS. FICZ and ICZ were identified in 2 out of 7 patients’ samples, pityriacitrin in 3 out of 10, malassezin in 4 out of 10, while indirubin was found in 5 out of 10. The concentration of the identified metabolites ranged between 0.2 to 260 pmol/mg of lesional skin scales extract (Table 1). None of these compounds was found in the control skin samples.
Relative AhR activation potency of chemically pure indolic Malassezia metabolites
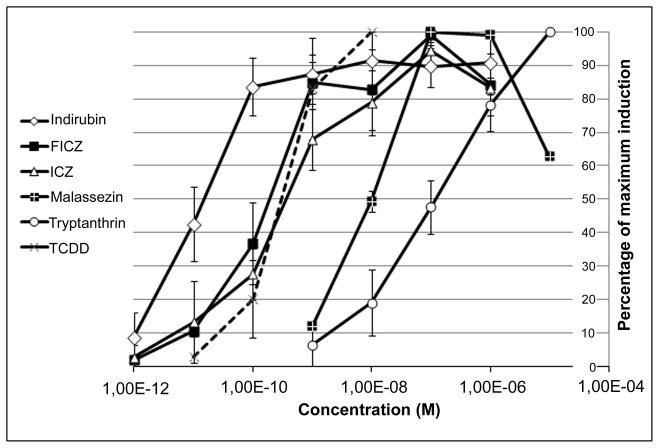
Using the human hepatoma CALUX cell bioassay, the Malassezia metabolites were evaluated for their ability to stimulate AhR-dependent reporter gene expression at 6 hours of incubation. All tested compounds were potent AhR activators, with ICZ and FICZ equipotent to the prototypical AhR agonist TCDD; malassezin and tryptanthrin were 20- and 200-fold less potent than TCDD, respectively. Interestingly, indirubin was 20-fold more potent than TCDD as an activator of the AhR in the human cell line (compare EC50s of 26 pM to 523 pM, respectively)(EC50s: FICZ at 348 pM, ICZ at 600 pM, malassezin at 11 nM and tryptanthrin at 107 nM) (Fig. 1).
FIGURE 1.
AhR induction in Recombinant human hepatoma (HG2L7.5c1) cells after incubation for 6 h measured with the CALUX cell bioassay. The results are normalized to the maximal activity of each compound.
Activation of AhR pathway by M. furfur culture extracts in HaCaT cells
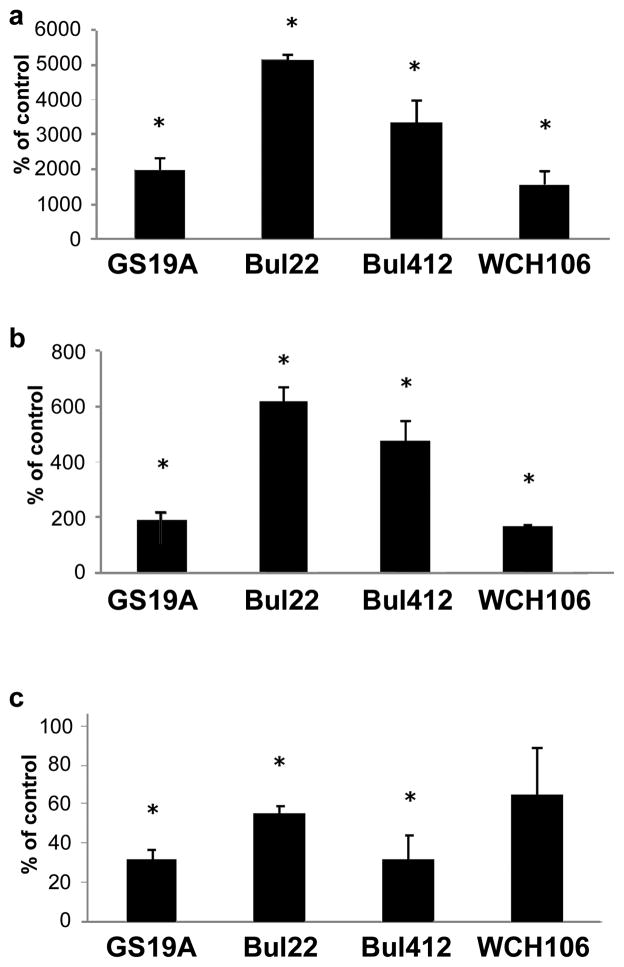
The significant variability of Malassezia culture extracts in their overall composition in indolic compounds with AhR activity complicates comparison of their relative potency. For this reason, in order to compare culture extracts of different M. furfur strains, we normalized extract dilutions for testing to 100nM ICZ. This was selected because besides being identified in all tested strains it is one of the most active AhR-inducers. However, since ICZ was only found in M. furfur strains, comparisons are restricted to M. furfur lesional skin isolates. Alterations in the expression of the AhR related genes (Cyp1a1, Cyp1b1, AhRR, Aldh3a1) as well as AhR-responsive Phase II genes (Gstp1, Gstt1) (Yu et al, 2009) by these indoles was assessed by Reverse Transcriptase Real-Time PCR. The extracts tested did not produce any obvious signs of toxicity in HaCaT cells when used at the equivalent of 100 nM ICZ (Fig. S2). Extracts at this ICZ equivalent concentration significantly induced expression of two prototypical markers of the AhR signaling pathway, namely Cyp1a1 and Cyp1b1, with the level of CYP1A1 mRNA approximately 7-fold greater than that of CYP1B1 (Fig. 2a,b). With the exception of WCH106, these extracts significantly down-regulated the level of AhRR mRNA (Fig. 2c) and both GSTP1 and GSTT1 mRNAs (p<0.05 compared to control) (Fig. S3).
FIGURE 2.
Effects of M. furfur extracts on AhR-dependent genes expression. Alterations in the relative levels of mRNA expression of the Cyp1a1 (Panel a), Cyp1b1 (Panel b), AhRR (Panel c) genes are shown [mean values ± standard deviations (bars) of 3 independent experiments]. The extracts were normalized to their ICZ content. The asterisk denotes statistically significant differences compared to control which is expressed as 100% (p<0.05; see also Fig. 3). Bul22, Bul412 and GS19 are strains isolated from seborrheic dermatitis (SD) and WCH106 from Malassezia-folliculitis.
Differential potency of Malassezia produced indoles to induce AhR pathway in HaCaT cells
A concentration-response protocol was applied to expose HaCaT cells to different concentrations of ICZ, malassezin, indirubin, FICZ and tryptanthrin. A clear concentration-dependent increase in mRNA levels of the AhR related genes Cyp1A1 and Cyp1b1 was observed in addition to Aldh3a1 (Fig. 3 and Fig. S4). FICZ, ICZ and indirubin proved to be more potent AhR agonists than malassezin and tryptanthrin (Fig. 3 and Fig. S4). This was also applicable for ALDH3A1 mRNA. Interestingly, Cyp1a1 induction by FICZ, ICZ and indirubin was higher at 24h as compared to 3 and 6h, a situation that is typically reversed for these compounds given that they are readily metabolized in intact cells (Fig. S5).
FIGURE 3.
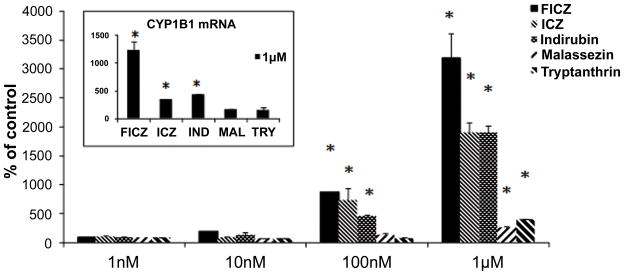
Comparative induction of CYP1A1 and CYP1B1 (insert) mRNA by FICZ, ICZ, indirubin (IND), malassezin (MAL) and tryptanthrin (TRY) at 24h [mean values ± standard deviations (bars) of 3 independent experiments]. Statistically significant induction of Cyp1a1 is noted at 100 nM for FICZ, ICZ and indirubin. At 1 μM FICZ is a significantly more potent Cyp1a1 inducer than ICZ or indirubin. This also applies for Cyp1b1 at 1 μM (insert). The asterisk denotes statistically significant differences compared to control (p<0.05).
Discussion
The data presented herein demonstrate the identification of an array of indolic metabolites possessing potent AhR activity in skin scales of patients with SD and PV and attribute their origin to the skin commensal and pathogen Malassezia. In addition to the previously found ICZ and malassezin, three more compounds, indirubin, FICZ and tryptanthrin were identified as Malassezia metabolites. Up to now only tryptanthrin has been shown to be produced by the yeast species Yarrowia (Candida) lipolytica (Shrenk et al, 1997) and Candida glabrata (Mayser et al, 2007). Both FICZ (Wincent et al, 2009; 2012) and indirubin (Prochazkova et al, 2011) are proposed as endogenous AhR ligands and have multiple biological functions. Indirubin inhibits CDKs as well as the GSK-3 (Hoessel et al, 1999) and can modulate key cellular signaling pathways like Wnt and NF-kB (Tian et al, 1999). Also bacteria can cause the purple urine syndrome through the synthesis of indirubin (Kang et al, 2011). FICZ has drawn attention as it has been implicated in the mediation of UV damage through AhR activation (Fritche et al, 2007). However both could also cause indirect skin damage by the local leak of free radicals after AhR induced hyperactivation of CYP enzymes (Park et al, 1996). Presently, the identification of malassezin and pityriacitrin exclusively in lesional skin scale extracts points to Malassezia as the source of origin. Malassezin is uniquely produced by Malassezia species, and can be considered as a chemical marker of this genus. In humans, pityriacitrin has been shown to be produced also by Candida glabrata (Mayser et al, 2007), the colon yeast equivalent of Malassezia. Cross-contamination only of the SD and PV samples with pityriacitrin originating from Candida strains residing in the colon is highly improbable and thus isolation or this indole from skin scales, further corroborates towards its Malassezia origin.
Regarding the clinical significance of AhR ligand production by Malassezia yeasts we have two important complementary findings: 1)These ligands are produced in vitro by the majority of Malassezia species and in particular by the ubiquitous M. globosa (Tajima et al, 2008) and 2)their presence was confirmed on the skin scales of PV and SD by both analytical and biological assays. The quantitative differences in the identified indoles from the skin scales could represent variations in the activity of the yet unknown biosynthetic pathway or in loco modification by environmental factors as is the skin pH and/or solar radiation (Fritsche et al, 2007). Thus ICZ has been proposed to be easily produced through transformation of malassezin (Wille et al, 2001), yet in our samples we could find either one, or both of these two substances. We cannot attribute the production of the AhR ligands in the skin scales solely to M. furfur as it was isolated only in 2 of our present SD patients (data not shown). Probably, as shown from our results, additional Malassezia species, as is M. globosa (Tajima et al, 2008), participate in the in vivo production of these ligands in SD and PV skin. For M. furfur the ability to produce or not these ligands in vitro is a stable biological trait, as we found out by repeated sub-cultures of our clinical strains and is exemplary highlighted by the reference strain M. furfur CBS 6094. The latter is a healthy skin isolate and the inability to assimilate L-tryptophan for indole production was noted in 2 independent studies with at least 4 years difference (Mayser et al, 2004; Gaitanis et al, 2008).
The biological effects of the Malassezia-associated ligands found in skin scale extracts were evaluated in the human hepatoma cell line (CALUX assay) and correlated well with the respective indole quantification. The potency order of the pure indoles and TCDD when incubated for 6 h was measured as follows: indirubin>FICZ>TCDD>ICZ>malassezin> tryptanthrin. Regarding skin scale extracts, the lowest EC50s for gene induction were recorded for those containing the highest concentrations of indirubin with or without FICZ. For example, in samples SD1 and SD2 where only indirubin was found, the AhR activity could satisfactorily be explained by the levels of indirubin. In the case of SD2 the concentration of indirubin corresponding to the EC50 of the extract is 1.2×10−11M which is very close to the EC50 of pure indirubin (2.6×10−11M). Overall the EC50 for gene induction from the lesional skin extracts was significantly lower than that measured for the control skin extracts. The observed notable activation of the AhR from some healthy skin extracts, without identification of Malassezia indoles in the chemical analysis, could be attributed to environmental pollutants or inherent chemical factors with AhR activity found in human skin.
The clinical significance of the Malassezia produced indoles was further confirmed in the immortalized epidermal cell HaCaT by examining the AhR inducing ability either of the pure indoles or the M. furfur culture extracts. The tested AhR agonists showed clear ligand, time and dose-dependent effects on CYP1A1, CYP1B1 and ALDH3A1 mRNA expression (Fig. 3 and Fig. S4). FICZ in addition to ICZ and indirubin were found to be the most potent AhR agonists as suggested from previous reports (Flaveny et al, 2009; Wincent et al, 2009). The culture extracts induced statistically significant alterations in AhRR, CYP1A1, CYP1B1 and ALDH3A1, but also in GSTT1 and GSTP1 mRNA expression (Fig. 2, Fig. S3). Interestingly, AhRR and GSTs mRNA expression was decreased by the extracts while Aldh3a1 showed variable expression. AhRR down-regulation points towards the existence of additional bioactive substances while down regulation of Gstp could be attributed to tryptanthrin (Yu et al, 2009).
Our findings do not demonstrate any causal associations, yet this the next step, as the existence of potent AhR ligands should be complemented in future research with the assessment of the in vivo AhR activation level in the epidermis of Malassezia-associated skin diseases, as is SD and PV. Indeed, in an experimental model in mice, constitutively activated AhR resulted in inflammatory skin lesions (Tauchi et al., 2005). In PV and SD, these lipophilic ligands could cross the defective epidermis, reach the granular and spinous layers, and activate the AhR (Swanson HI, 2004) and cause the respective downstream effects. The corresponding, AhR mediated local metabolic and immune aberrations have been proposed as the pathophysiological background of the suggested Malassezia indoles and basal cell carcinoma association (Gaitanis et al, 2011). It is noteworthy that ICZ, like TCDD, has been considered as a tumor promoter (Herrmann et al, 2002). Another interesting issue that rises from our findings is the impact these indoles could have on the local induction of P450 enzymes that metabolize antifungal azoles. Although the main azole metabolizing enzyme CYP3A4 is not directly controlled by AhR (Swanson HI, 2004) there is increasing evidence that there is a cross-talk between AhR, PXR and CYP3A4 (Gerbal-Chaloin et al, 2006) that could affect the skin metabolism of the commonly used antifungal azoles. Currently, AhR is also under intense investigation as it can modulate the function of T-regs and Th17 lymphocytes (Quintana et al, 2008) as well as dendritic cells (Vlachos et at, 2012; Voorhis et al, 2012) in a ligand dependent manner. Morevorer, the Malassezia produced indirubin and ICZ have been recently shown to inhibit Toll-like receptor induced maturation of dendritic cells, a finding that coincides with the need of a skin commensal to ameliorate local immune reactions in its niche (Vlachos et al, 2012).
In conclusion, this article describes the synthesis of bioactive indoles by the majority of the currently accepted Malassezia species and the preferential production of all the most active known natural indolic AhR ligands in culture extracts of M. furfur pathogenic strains. These findings coupled by the presence of the AhR ligands in skin scales of Malassezia-associated diseased skin corroborates indole production as a virulence factor in this yeast. Although the role of Malassezia yeasts in human skin is commonly neglected or underestimated, based on the observed strong activation of AhR in HaCaT cells by Malassezia extracts -and the pure indolic constituents- we can propose that the presence on the human skin of a microorganism able to continually synthesize highly potent AhR ligands plays a crucial role in skin homeostasis and in the development of skin diseases, like SD and PV.
Materials & Methods
Skin sample collection and extraction
Skin scales (50–100 mg) from ten patients (6 SD and 4 PV) were collected and stored at −80°C until extraction. For control samples, scraping of healthy facial skin (N=3) and normally thicker skin from the elbows, palms and soles (N=3) was performed. All patients gave a written informed consent. All samples were extracted with EtOAc (10 mL) under sonication (30 min). The resulting solution was filtrated and evaporated under reduced pressure. The residue was dissolved in DMSO to give a stock solution (100 mg/mL skin extract). Subsequently, a series of diluted solutions to 1 μg/mL were prepared for the AhR test.
Malassezia growth conditions
Type strains of 12 Malassezia species and clinical strains of M. furfur were grown in L-tryptophan agar as previously (Gaitanis et al, 2008).
Chemical synthesis
Malassezin, FICZ, ICZ, indirubin and tryptanthrin were synthesized following literature methods described in supporting information.
Extraction and isolation of Malassezia metabolites from M. furfur cultures
Indirubin, FICZ and tryptanthrin were isolated from the M. furfur Bul22 strain employing column chromatography with Sephadex LH-20 and preparative HPLC (column: Nucleosil 100-7, RP-18, 7 μm, 250×21 mm) with a gradient system from 100% H2O to 100% acetonitrile in 180 min, with flow 5 mL/min.
Extraction and HPLC screening
The tested Malassezia strains (Tables 2,3) were grown, extracted and submitted to analytical HPLC as previously (Gaitanis et al, 2008). The newly identified substances were detected with UV as follows: indirubin at 540 nm, ICZ and malassezin at 331 nm, FICZ at 454 nm and tryptanthrin and pityriacitrin at 390 nm.
LCMS analysis
The LC/MS/MS analysis was performed using an Agilent 6460 QQQ operating on ESI mode. A portion of the stock solution (1 μL) was submitted to LCMS (RP Poroshell 120, 150×2.1 mm) using a gradient system from 95% H2O/5% Acetonitrile to 100% acetonitrile in 10 min, with flow 0.3 mL/min (Fig. S1).
HG2L7.5c1 CALUX cell bioassay
HG2L7.5c1 cells were trypsinized and resuspended in 20 mL α-MEM. An aliquot (100 μL) of the suspension was added into 96-well tissue culture plates and incubated for 24h. Subsequently they were incubated with controls and test compounds for 6h or 24h at 37°C. Luciferase activity was measured as previously (Baston and Denison 2010).
HaCaT cell analysis
HaCaT cells were grown in DMEM supplemented with 10% FBS, L-glutamine and streptomycin/penicillin. The extracts from the clinical M. furfur strains GS19A, Bul22, Bul412 and WCH106 were normalized at 100 nM ICZ. HaCaT cells were grown to nearly confluence and treated with the respective extract for 24h. The AhR ligands were also tested for dose and time response effects on HaCaT cells accordingly. Total RNA was isolated employing the Nucleospin kit (Macherey-Nagel, Germany).
qRT-PCR
mRNA of the AhR, AhR repressor (AhRR) genes and AhR regulated genes (CYP1A1, CYP1B1, ALDH3A1, GSTT1 and GSTP1) was measured with a CFX-96-quantitative RT-PCR system (BioRad Lab) with Taqman® Universal PCR master mix (4304437, AB, NJ, USA) using inventoried 20x assay mixes of the respective primer/probe sets as detailed, also for qRT-PCR conditions in Supporting material. Relative quantification was performed using the ΔCt method which results in ratios between the target genes and the house keeping reference gene β-actin (4352935E, AB, NJ, USA).
Statistical analysis
For statistical analysis Mann-Whitney U-test and Student’s t-test were employed using SPSS17 (Chicago, Ill) for analysis. Two-sided p<0.05 values were considered significant.
Supplementary Material
Acknowledgments
We thank Prof. Alyson Mitchell and Prof. Susan Ebeler for providing Eleni Melliou the opportunity to run the LCMS experiments in the Food safety Laboratory at UC Davis. We also thank Stravroula Kritikou for expert technical assistance in Malassezia cultures, Guochun He for assistance in CALUX assays, Nikos Lemonakis and Evagelos Gikas for their help in some LCMS experiments. This work was supported in part by the General Secretariat of Research and Technology of Greece (Program PENED), the National and Kapodistrian University of Athens (Program Kapodistrias) and the National Institutes of Environmental Health Sciences (ES007685 and ES04699).
Abbreviations
- AD
atopic dermatitis
- AhR
Aryl hydrocarbon receptor
- AHRR
AhR repressor
- CALUX
Chemical Activated Luciferase gene eXpression
- FICZ
formyl-indolo[3,2-b]carbazole
- ICZ
indolo[3,2-b]carbazole
- MS
Mass Spectroscopy
- NMR
Nuclear Magnetic Resonance
- PV
pityriasis versicolor
- SD
Seborrheic Dermatitis
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin)
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Additional detailed descriptions are provided in Supporting information.
References
- Ashbee HR. Update on the genus Malassezia. Med Mycol. 2007;45:287–303. doi: 10.1080/13693780701191373. [DOI] [PubMed] [Google Scholar]
- Barouti N, Fontao L, Pardo B, et al. AhR pathway as a novel pharmacological target for wound healing. J Invest Dermatol. 2009;129:S80. (Abstr.) [Google Scholar]
- Baston DS, Denison MS. Considerations for potency equivalent calculations in the Ah receptor-based CALUX bioassay: normalization of superinduction results for improved sample potency estimation. Talanta. 2010;83:1415–1421. doi: 10.1016/j.talanta.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem. 2009;390:1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- Denison MS, Zhao B, Baston DS, et al. Recombinant cell bioassay systems for the detection and relative quantitation of halogenated dioxins and related chemicals. Talanta. 2004;63:1123–1133. doi: 10.1016/j.talanta.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Chiaro CR, et al. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E, Schäfer C, Calles C, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmic target fro ultravioliet B radiation. Proc Nat Acad Sc USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis G, Magiatis P, Stathopoulou K, et al. AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J Invest Dermatol. 2008;128:1620–1625. doi: 10.1038/sj.jid.5701252. [DOI] [PubMed] [Google Scholar]
- Gaitanis G, Magiatis P, Hantschke M, et al. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis G, Velegraki A, Magiatis P, et al. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med Hypotheses. 2011;77:47–51. doi: 10.1016/j.mehy.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Gerbal-Chaloin S, Pichard-Garcia L, Fabre JM, et al. Role of CYP3A4 in the regulation of the aryl hydrocarbon receptor by omeprazole sulphide. Cell Signal 2006. 2006;18:740–750. doi: 10.1016/j.cellsig.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Batra R, Bluhm R, et al. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51:785–798. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Herrmann S, Seidelin M, Bisgaard HC, et al. Indolo[3,2-b]carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumor promoter. Carcinogenesis. 2002;23:1861–1868. doi: 10.1093/carcin/23.11.1861. [DOI] [PubMed] [Google Scholar]
- Hoessel R, Leclerc S, Endicott JA, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol. 2011;75:557–559. doi: 10.5414/cn106615. [DOI] [PubMed] [Google Scholar]
- Mayser P, Töws A, Krämer HJ, Weiss R. Further characterization of pigment-producing Malassezia strains. Mycoses. 2004;47:34–39. doi: 10.1046/j.1439-0507.2003.00957.x. [DOI] [PubMed] [Google Scholar]
- Mayser P, Wenzel M, Krämer HJ, et al. Production of indole pigments by Candida glabrata. Med Mycol. 2007;45:519–524. doi: 10.1080/13693780701411557. [DOI] [PubMed] [Google Scholar]
- Mayser P, Gaitanis G. Physiology and Biochemistry. In: Boekhout T, Gueho E, Mayser P, Velegraki A, editors. Malassezia and the Skin. Science and Clinical Practice. Springer; Berlin: 2010. [Google Scholar]
- Panteleyev AA, Bickers DR. Dioxin-induced chloracne-reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp Dermatol. 2006;15:705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Park JYK, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA. 1996;93:2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova J, Kozubik A, Machala M, et al. Differential effects of indirubin and 2,3,7,8-tetrachlorodibenzo-p-dioxin on the aryl hydrocarbon receptor (AhR) signalling in liver progenitor cells. Toxicology. 2011;279:146–154. doi: 10.1016/j.tox.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Schrenk D, Riebniger D, Till M, et al. Typtanthrins: a novel class of agonists of the aryl hydrocarbon receptor. Biochem Pharmacol. 1997;54:165–171. doi: 10.1016/s0006-2952(97)00150-0. [DOI] [PubMed] [Google Scholar]
- Sorg O, Zennegg M, Schmid P, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: identification and measurement of TCDD metabolites. Lancet. 2009;374:1179–1185. doi: 10.1016/S0140-6736(09)60912-0. [DOI] [PubMed] [Google Scholar]
- Sugita T, Boekhout T, Velegraki A, et al. Epidemiology of Malassezia-related skin diseases. In: Boekhout T, Gueho E, Mayser P, Velegraki A, editors. Malassezia and the skin. Science and clinical practice. Springer; Berlin: 2010. pp. 65–120. [Google Scholar]
- Swanson HI. Cytochrome P450 expression in human keratinocytes: an aryl hydrocarbon receptor perspective. Chem Biol Interact. 2004;149:69–79. doi: 10.1016/j.cbi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Tajima M, Sugita T, Nishikawa A, et al. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: comparison with other diseases and healthy subjects. J Invest Dermatol. 2008;128:345–351. doi: 10.1038/sj.jid.5701017. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma W, Kramer HJ, Mayser P. Pityriasis versicolor alba. J Eur Acad Dermatol Venereol. 2005;19:147–152. doi: 10.1111/j.1468-3083.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Denison M, et al. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- Vlachos C, Schulte BM, Magiatis P, et al. Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit Toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br J Dermatol. 2012;167:496–505. doi: 10.1111/j.1365-2133.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- Voorhis MV, Fechner JH, Zhang X, et al. The Aryl Hydrocarbon Receptor: A Novel Target for Immunomodulation in Organ Transplantation. Transplantation. 2012 Dec 21; doi: 10.1097/TP.0b013e31827a3d1d. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille G, Mayser P, Thoma W, et al. Malassezin-A novel agonist of the arylhydrocarbon receptor from the yeast Malassezia furfur. Bioorg Med Chem. 2001;9:955–960. doi: 10.1016/s0968-0896(00)00319-9. [DOI] [PubMed] [Google Scholar]
- Wincent E, Bengtsson J, Mohammadi Bardbori A, et al. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2012;109:4479–4484. doi: 10.1073/pnas.1118467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- Yu ST, Chen TM, Chern JW, et al. Downregulation of GSTpi expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-jun NH2-terminal kinase-mediated apoptosis. Anticancer Drugs. 2009;20:382–388. doi: 10.1097/CAD.0b013e32832a2cd4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.