Abstract
It is widely accepted that the pathophysiology of hypertension involves autonomic nervous system dysfunction, as well as a multitude of immune responses. However, the close interplay of these systems in the development and establishment of high blood pressure and its associated pathophysiology remains elusive and is the subject of extensive investigation. It has been proposed that an imbalance of the neuro-immune systems is a result of an enhancement of the “pro-inflammatory sympathetic” arm in conjunction with dampening of the “anti-inflammatory parasympathetic” arm of the autonomic nervous system. In addition to the neuronal modulation of the immune system, it is proposed that key inflammatory responses are relayed back to the central nervous system and alter the neuronal communication to the periphery. The overall objective of this review is to critically discuss recent advances in the understanding of autonomic immune modulation, and propose a unifying hypothesis underlying the mechanisms leading to the development and maintenance of hypertension, with particular emphasis on the bone marrow, as it is a crucial meeting point for neural, immune, and vascular networks.
Keywords: Inflammation, Autonomic nervous system, ANS, Immune system, IS, Microglia, Neuroimmune modulation, Bone marrow, Vagal immune reflex, Hypertension, Cardiovascular disease, CVD
1. Introduction
Hypertension remains a global health concern despite significant advancements in its treatment in recent years. Approximately 10% of hypertensive patients suffer from resistant hypertension [1, 2], characterized by blood pressures that remain uncontrolled in spite of simultaneous administration of three antihypertensive agents of different classes [3]. The neurogenic component of resistant hypertension presents with a dysfunctional autonomic nervous system (ANS) [4-8], increased norepinephrine (NE) spillover and sympathetic nerve activity (SNA) and decreased cardiac parasympathetic tone [9-16]. Similar findings have been described in association with several of the hypertension comorbidities, including obesity, diabetes, and sleep apnea [17-20], thus complicating diagnosis and treatment [3, 21].
Multiple emerging therapies target autonomic dysfunction in patients with neurogenic hypertension [4, 8, 22, 23]. Renal denervation therapy [24-28] employs radiofrequency to specifically ablate the renal sympathetic nerves [22] resulting in a significant and long-lasting decrease in blood pressure and whole-body norepinephrine spillover in some patients [29-32]. While proof of concept has been established, the long term effectiveness of this strategy remains to be validated in view of recent evidence of reinnervation of the kidney following ablation [33]. Renal denervation may only be effective in lowering blood pressure and muscle sympathetic nerve activity in a small proportion of patients [34], possibly owing to low renin levels in some cases of neurogenic hypertension [35], amongst other issues. Similarly, chronic carotid baroreceptor activation has been shown to lower blood pressure and sympathetic activity in resistant patients [36-39], prompting initiation of clinical trials with promising long term results [40, 39]. Other more adventurous techniques such as the deep brain stimulation [41-47] and surgical relief of micro-vascular compression [48, 49] have demonstrated promising outcomes, but the invasive nature of these procedures decreases their general therapeutic use and renders them mainly experimental.
Present therapies that target the neurogenic component of hypertension highlight the need for novel therapeutic strategies for patients with resistant hypertension. However, the invasive nature of the procedures and the potential high cost and relatively low efficacy of treatment, coupled with a lack of understanding of underlying pathophysiological mechanisms of autonomic imbalance, further complicate the development of innovative strategies for the treatment of resistant hypertension. Recent advances have underscored the role of the immune system (IS) and the importance of neuro-immune pathways in autonomic regulation in hypertensive patients and animal models of hypertension. The aim of this review is to summarize latest advances in the field, review the current understanding of connections between the autonomic and immune systems, and discuss issues that remain to be addressed in the field of autonomic modulation of immunity in hypertension.
2. Immune system and neurogenic hypertension
It is well established that hypertension and cardiovascular disease (CVD) in humans are characterized by increased systemic inflammation [50-54]. Increased circulating levels of inflammatory markers have been reported even in pre-hypertensive patients [55-57], suggesting a causative role of the immune system in CVD. As a result, extensive investigation is underway to elucidate the role of innate and adaptive immunity in the development and maintenance of hypertension. Animal experiments have demonstrated that elimination of the immune response by thymus transplant or immunosuppressant drugs can delay and even arrest the progression of hypertension [58, 59]. David Harrison's group was among the first to show that T-lymphocytes are essential for the development of hypertension in several animal models [60, 61]. For example, RAG-/- mice lacking the mature B- and T-lymphocytes did not develop high blood pressure, but the adoptive transfer of T-cells, not B-cells, was able to restore the hypertensive phenotype [60, 61], suggesting an exclusive role of activated T-cells in hypertension. It is pertinent to note that not all types of T-cells behave similarly, as recent reports showed that the adoptive transfer of the T regulatory (Treg) lymphocytes (CD4+/CD25+), but not the T effector (helper) lymphocytes (Th, CD4+/CD25-), prevented both the angiotensin II (Ang II)- and aldosterone-dependent hypertension [62, 63]. Treg lymphocytes are thought to be able to suppress both the innate and adaptive immune responses by suppressing the pro-inflammatory actions of the effector Th lymphocytes, thereby playing a role in immune system homeostasis. These and other studies have led to the hypothesis that activation of the IS in hypertension depends on formation of specific neoantigens, which, through dendritic cell (DC)–dependent activation, lead to activation of naïve T-cells and their differentiation into effector Th lymphocytes [64]. Thus, Treg lymphocytes are able to protect against the development of hypertension by counter-acting renal vascular remodeling that is induced by effector Th cell activation [64]. However, the triggering mechanism that initiates this process remains to be identified.
Recent animal data suggest that, in addition to the increased pro-inflammatory pathways, anti-inflammatory pathways are dysfunctional in hypertension [65-67]. Francois Abboud's group has recently shown that the anti-inflammatory modulation of the innate IS in normotensive rats is reversed in pre-hypertensive SHRs [67]. They demonstrated that nicotine, a neurotransmitter of the cholinergic neurons, exerted an anti-inflammatory effect by suppressing a large population of myeloid DCs in the WKY, but had an opposite, pro-inflammatory effect in the SHR, exhibited in the activation of macrophages [67]. In another animal model, chronic vascular risk factors have been associated with decreases in cholinergic neurons [68]. Further evidence from a two-kidney one-clip hypertension model suggests that secondary hypertension induces cholinergic receptor down-regulation, which may ultimately contribute to the inflammatory processes [69]. Therefore, the importance of understanding the mechanism of the cholinergic anti-inflammatory pathway in hypertension is becoming increasing evident, and may provide a novel therapeutic target in the treatment of neurogenic hypertension. These pathways will be discussed in the section on autonomic regulation of the immune system.
3. Neuroinflammation and microglia
Involvement of brain inflammation in various CNS diseases such as Parkinson's and Alzheimer's disease and stroke has been well documented [70-72]. However, the role of brain inflammation in hypertension and CVD is less well understood and is a rapidly emerging field. It has been shown that increased inflammation in the cardioregulatory areas of the brain is associated with increased sympathetic nervous system activity and hypertension, and inhibition of inflammation in these brain regions attenuates the hypertension [73-78]. Furthermore, inhibition of pro-inflammatory oxidative stress by specific deletion of p22phox in the subfornical organ (SFO) attenuates hypertension and eliminates vascular inflammation in the chronic Ang II infusion model [74]. In addition, SHRs exhibit increased leukotriene B4 in the nucleus of tractus solitarius (NTS), which has been proposed to be pro-hypertensive in the SHR [79]. Pro-inflammatory pathways, such as NF-κB in the paraventricular nucleus of the hypothalamus (PVN) have been shown to enhance the hypertensive response to Ang II [80]. Taken together, these observations suggest that neuroinflammation plays an important role in the development and maintenance of hypertension.
Increased activity of the renin-angiotensin system (RAS) in hypertensive models [81-83] has a role in driving pro-inflammatory responses in the periphery as well as the brain [80, 84, 85]. For example, chronic knockdown of AT1 receptors in the NTS of SHR animals increases peripheral inflammation and vascular dysfunction [86]. As mentioned earlier, T-lymphocytes are essential for the development of hypertension [60, 61], and central Ang II appears critical for T-lymphocyte activation [87]. A study by Marvar et al. showed that lesioning the anteroventral third cerebral ventricle (AV3V) eliminates the Ang II-induced blood pressure increase, T-cell activation, and vascular leukocyte infiltration. The authors postulated a feed-forward mechanism that includes modest increases in blood pressure promoting inflammation, further raising blood pressure, and eventually culminating in severe hypertension [87]. These studies emphasize the link between the central RAS and the immune system. Whether the initial pro-inflammatory trigger comes from the brain remains the topic of many studies, and will be discussed in detail in the following sections.
Investigation of the role of microglia and neuroinflammation in hypertension may provide valuable insight into this problem. Microglia are the immune cells of the central nervous system and make up approximately 12% of the brain cell population [88, 72]. Within the healthy CNS, microglia are tightly regulated in order to continuously monitor the brain environment [89, 90]. Their phenotype can rapidly change in response to alterations of homeostasis, pathological insults, and even systemic inflammation in an attempt to repair injury at inflammatory sites [88, 91, 92]. However, over-activation of microglia is detrimental, as they release pro-inflammatory cytokines and generate ROS, thereby contributing to the inflammation in the brain [77, 76, 93]. Neurogenic hypertension involves activation of microglia in the PVN in both the Ang II-dependent and SHR models [94, 77]. Moreover, close interactions between the microglia, astrocytes, and neurons can lead to the modulation of neuronal activity in the PVN, enhancing the neuronal response to Ang II [95]. On the other hand, inhibition of the brain mitochondrial ROS, but not the peripheral ROS, is able to attenuate hypertension, inhibit microglial activation in the PVN, and normalize peripheral IC levels [78]. Therefore, microglia are emerging as a novel therapeutic target for the treatment of resistant hypertension, although further understanding of the molecular mechanisms underlying these processes is needed.
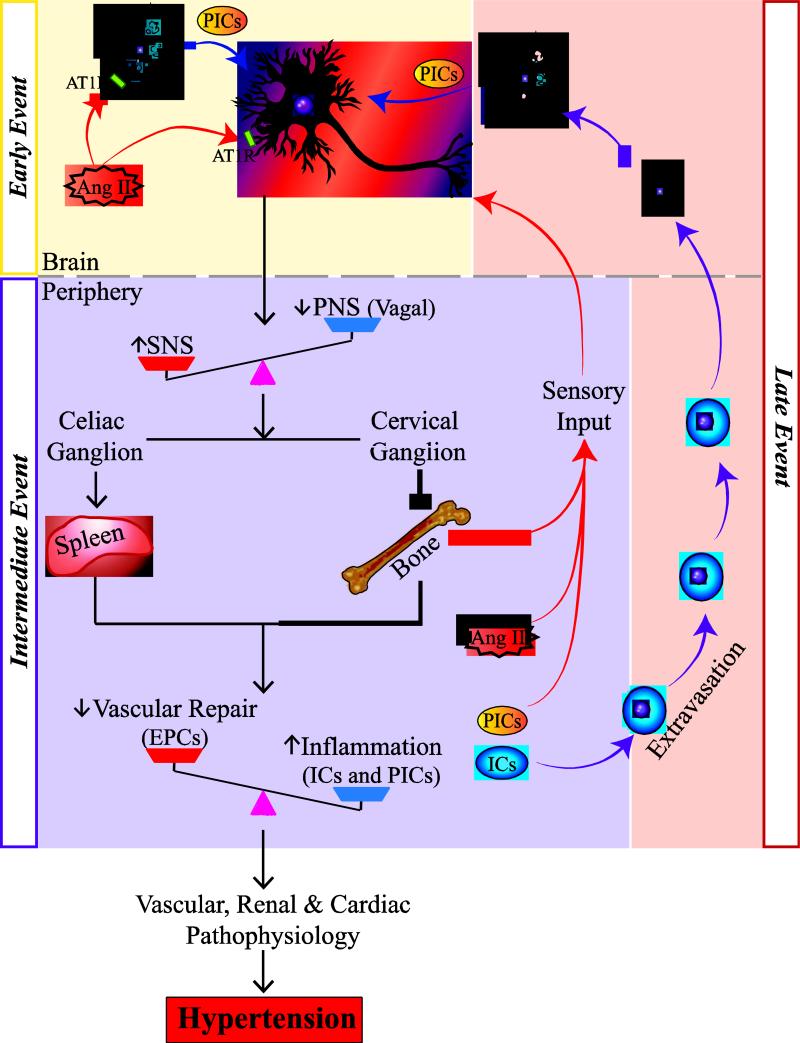
Taking all this into consideration, we propose that activation of endogenous microglia in cardioregulatory areas of the brain is an early event in the development of hypertension (Fig. 1). Ang II and other pro-hypertensive stimuli directly activate the microglia and stimulate the microglial release of the pro-inflammatory cytokines. These cytokines, in addition to the pro-hypertensive stimuli, may influence neuronal activity, particularly in the autonomic areas of the brain (Fig. 1). These central responses then give rise to intermediate effects in the periphery, which include autonomic dysregulation, increased inflammation and vascular dysfunction. Furthermore, we propose that the maintenance of hypertension involves a late event, in which bone marrow-derived inflammatory progenitors extravasate in brain and differentiate into microglia, further enhancing neuroinflammation in the autonomic areas of the brain. Our model highlights the importance of central pro-hypertensive stimuli, proposing that these processes are crucial for the development of hypertension. Furthermore, we make the novel inclusion of bone marrow sensory inputs which may signal important information about the inflammatory status of the periphery. The role of autonomic dysregulation in the control of inflammatory responses in hypertension will be discussed in the following section.
Fig. 1. Proposed hypothesis of a dysfunctional brain-bone marrow communication in the development and establishment of hypertension.
Activation of microglia in cardioregulatory areas of the brain is an early event in the development of hypertension. AngII and other pro-hypertensive stimuli activate both microglia and neurons, thus altering neuronal activity in the hypothalamus and the brainstem. These central responses give rise to intermediate events in the periphery, which include increased sympathetic and decreased parasympathetic signaling to the spleen and bone marrow, leading to increased inflammation and impairment in vascular repair. Moreover, the bone marrow sensory afferents may relay information about the inflammation to the brain. Finally, the late event involves the extravasation of bone marrow-derived inflammatory progenitors in brain that differentiate into microglia, further enhancing neuroinflammation in the autonomic regulatory areas of brain, thus facilitating the development of hypertension.
Abbreviations- PICs: pro-inflammatory cytokines; ICs: inflammatory cells; EPCs: endothelial progenitor cells; SNS: sympathetic nervous system; PNS: parasympathetic nervous system
4. Autonomic regulation of the immune system
The concept that the central nervous system (CNS) can regulate the IS has existed for several decades. However, the notion that heightened emotional stress can exacerbate inflammatory responses has only recently been supported by scientific evidence [96-99]. It is now known that specific cell types such as lymphocytes, myeloid cells and endothelial cells respond to changes in hormones and neurotransmitters of CNS origin [100]. Specifically, activation of the sympathetic arm of the ANS has been shown to play a major role in regulation of inflammatory responses [101]. This is particularly manifested in CVD and diabetes, where a direct link between elevated sympathetic drive and exaggerated inflammation has been demonstrated [50, 78, 102]. In SHR, the pro-inflammatory innate immune response is exaggerated even before the development of the high blood pressure [67], due to elevated central sympathetic drive preceding the blood pressure increase [103]. Furthermore, specific T-lymphocyte responses are crucial in development of Ang II hypertension [61], which is characterized by elevated central sympathetic drive to the spleen [104] and the bone marrow [78], where it directly regulates the activity of pro-inflammatory and other hematopoietic cells [105, 78, 106].
We have recently demonstrated that the chronic Ang II-dependent increase in central sympathetic drive stimulates the release of bone marrow-derived pro-inflammatory lymphocytes. This effect was abolished by blockade of the Ang II effect on the brain, particularly in the PVN [78]. Furthermore, our anatomical tracing studies revealed that the brain-bone marrow connections involve a direct sympathetic neuronal input to the bone marrow, which is enhanced in hypertension [78]. This enhanced ANS-IS communication is also present in other forms of CVD, such as myocardial infarction (MI), where elevated SNS is a major signal for recruitment of hematopoietic cells from the bone marrow [107]. Further, the abundance of adrenergic receptors in metabolic tissues such as fat supports a role for elevated SNS-dependent initiation of inflammatory responses [108-110] in diseases such as diabetes and obesity [61]. The role of SNS is further highlighted by the observation that the release of hematopoietic progenitor cells from the bone marrow depended on circadian oscillations of expression of certain clock genes which were governed by adrenergic stimulation from the sympathetic nerves innervating the bone marrow [111, 112]. Furthermore, blood leukocyte numbers exhibit circadian oscillations [113, 101], with their peak activation and migration from the bone marrow occurring at night [101], corresponding to the highest sympathetic drive in mice. This effect is completely dependent on the presence of the bone marrow sympathetic innervation, as well as the presence of beta 2 and 3 adrenergic receptors on the bone marrow hematopoietic cells, including the EPCs [101]. Since the circadian rhythms are entrained by the suprachiasmatic hypothalamic nuclei [114-116], any changes in neuronal activity within the hypothalamus, such as those seen in neurogenic hypertension [78, 77, 95], may affect the circadian control of the bone marrow activity.
Epidemiological evidence also suggests that these diurnal oscillations are clinically significant. Some inflammatory and immune diseases present a diurnal pattern of onset and progression [117]. For example, in models of sickle cell anemia and septic shock, where increased leukocyte inflammatory response contributes to the pathology of the disease, survival was appreciably compromised when inflammation was stimulated at night compared to the day [101]. In patients with MI, there is a significant increase in onset in the morning (9 am) compared to night (11pm), which is reduced in those receiving beta adrenergic blockers [118]. The diurnal oscillatory pattern of hematopoietic progenitor and immune cell regulation should also be considered when designing bone marrow and organ transplant protocols [112], as evidence suggests differential survival rates of recipients depending on the time of the day of the transplant [101]. In summary, it appears that the SNS plays a pivotal role in regulation of inflammatory responses in health and disease, and that the bone marrow may be a major if not the main contributor to the immune cell pool.
The balancing arm of the sympathetic influence is the parasympathetic arm of the ANS which regulates the “cholinergic anti-inflammatory pathway” or the “vagal immune reflex” [52, 94, 119-121]. Substantial evidence exists supporting the beneficial anti-inflammatory effect of vagal activation in inflammatory and immune conditions [122-128]. Vagal nerve stimulation is effective in reducing the peripheral release of cytokines such as TNF-alpha, IL-6, and IL-1, which is dependent on acetylcholine (ACh)-mediated reduction of macrophage activation via nicotinic acetylcholine receptor (nAChR)-dependent inhibition of NF-κB signaling in these cells [119, 129]. The afferent vagal fibers are also able to sense changes in the peripheral inflammatory status; for example, the glomus cells located in close proximity to the vagal nerve fibers possess IL-1beta binding sites, and therefore the sensory portion of the inflammatory reflex is able to sense changes in IL-1beta levels in the periphery [130]. The sensory message is then relayed to the brainstem, particularly the NTS and the dorsal vagal motor nucleus, where the signal is processed, integrated and relayed back to the periphery via the vagal efferents [121, 131].
It has been proposed that the spleen serves as an end-organ of the cholinergic anti-inflammatory pathway, albeit indirectly [132], as the vagal parasympathetic efferents communicate with the splenic postganglionic nerves via the celiac-superior mesenteric plexus [133, 134]. Therefore, the cholinergic anti-inflammatory vagal-splenic reflex requires activation of the splenic sympathetic (catecholaminergic) postganglionic nerves and possibly release of NE. This reflex could exert its anti-inflammatory effects either directly, via the anti-inflammatory subset of beta adrenergic receptors on macrophages [135], or indirectly, by stimulating a subset of CD4+ T-cells thought to be capable of releasing acetylcholine [132, 136], which could in turn activate the anti-inflammatory nAChRs within the spleen.
Importantly, the cholinergic anti-inflammatory pathway may not be confined to the spleen, and the anti-inflammatory actions of the vagus may also be exerted via other peripheral lymphoid tissues such as the gut [136-138] or the bone marrow. The effect of vagus on the bone marrow could be particularly pertinent in hypertension and other forms of CVD, which are characterized by inflammation and a dampened vagal reflex. This view is supported by our preliminary data indicating that direct administration of NE into the bone marrow increases mobilization of pro-inflammatory T-cells, which can be attenuated by ACh, suggesting that the bone marrow itself may be a beneficiary of the anti-inflammatory cholinergic pathway. The bone marrow, like the spleen, does not have a direct vagal input; however, the vagal message may be relayed through the superior cervical ganglion, as it contains both the vagal input [139] and the sympathetic output innervating the bone marrow [101]. Therefore, the vagal anti-inflammatory input to the bone marrow could be similar to that described in the spleen [136, 140].
Francois Abboud's group recently demonstrated that the cholinergic input to the spleen was pro-inflammatory in the pre-hypertensive SHR, whereas there was a pronounced anti-inflammatory cholinergic modulation of the innate IS in the WKY [67], suggesting that a dysfunctional ANS-IS communication may precede the development of hypertension. It is attractive to propose that the anti-inflammatory cholinergic input to the bone marrow could also be processed in a similar dysfunctional fashion, causing pro-inflammatory effects in the SHR and anti-inflammatory effects in the WKY. Alternatively, reduced cholinergic release in the periphery due to dampened vagal activity in hypertension may have a direct anti-inflammatory effect on the bone marrow, as suggested by our preliminary data showing that the activation of the bone marrow ICs can be inhibited by direct application of ACh.
In summary, recent findings have underscored the importance of sympathetic and parasympathetic modulation of IS responses. We propose that an imbalance between sympathetic and parasympathetic control could be associated with several pathologies, including hypertension, CVD and diabetes. Furthermore, the anti-inflammatory parasympathetic axis may present as a potential therapeutic target in hypertension. The role of the vagus and of the bone marrow in modulating IS responses is an area of emerging interest.
5. Possible role of sensory input
A plethora of evidence describes how the ANS controls immune responses, as discussed in the previous section. There is also good evidence of a bidirectional communication between the CNS and the IS. As mentioned before, the vagal sensory afferents are able to detect cytokine levels in the periphery and relay the message to the brainstem nuclei, subsequently activating the vagal efferents in an immune reflex loop.
Whether the bone marrow is able to relay messages back to the brain in a similar fashion may be of great interest in relation to CVD. Many of the fibers innervating the bone marrow, in addition to the sympathetic, are primary afferent sensory fibers [141-143]. Pain studies have demonstrated that the periosteum, mineralized bone, and the bone marrow are innervated by various sensory nerve fibers, including thickly and thinly myelinated A-fibers, as well as the peptide-rich C-fibers [144, 145]. These fibers are able to detect multiple environmental factors, including the inflammatory cytokines, which enhance the excitability of the sensory nerve fibers [146]. In hypertensive patients who have increased levels of systemic inflammation, it is possible that the bone marrow can directly relay the pro-inflammatory message back to the CNS. In line with this, the nociceptive afferent sensory message from the periphery is processed in brainstem pre-sympathetic nuclei such as the NTS [147], in close proximity to the vagus. Nociception has been shown to attenuate the parasympathetic, but not the sympathetic, arm of the baroreflex within the NTS [148, 149] by dampening the vagal activity. Moreover, direct electrical stimulation of the bone marrow increases blood pressure [150], supporting a direct afferent neuronal input to the pre-sympathetic areas of the brain. Both sensory and sympathetic nerve fibers in the limbs are able to sprout in response to inflammation [151, 152], suggesting high responsiveness of the sensory fibers to pro-inflammatory signals and providing additional support for the concept of bidirectional communication in the system. The increased inflammatory responses seen in hypertension may be directly related to the increased sympathetic activity and originating in the bone marrow.
In light of the evidence presented here, we propose that the dysfunctional cholinergic anti-inflammatory reflex stimulates inflammation in the bone marrow, and thus contributes to the pathophysiology of neurogenic hypertension. Understanding the sensory input from the bone marrow may hold the answers to the mechanisms underlying the establishment and maintenance of hypertension.
The recent discovery of a new RAS member by Robson Santos's group poses many interesting questions about the role of the RAS in sensory afferent nerve function. Alamandine is a heptapeptide formed from angiotensin A or angiotensin-(1-7) and is present in vivo [153]. It acts through the MrgD receptor, a member of the Mas-related gene receptor family that is found in many sensory structures, particularly in skin [154, 155]. Although the fibers expressing this novel RAS-related receptor are not present in the bone marrow [144], these sensory fibers could be important in other peripheral organs and even the vasculature. Recently, MrgD receptors have been found to regulate mast cell activation during intestinal inflammation [156, 157]. Therefore, it is possible that there is a direct link between the peripheral RAS communicating to the brain through afferent nerves and the inflammatory response in hypertension. Interestingly, sensory dorsal root ganglion neurons also express functional Ang II receptors [158, 159], suggesting a role for multiple members of the RAS in this novel bidirectional communication loop.
6. Role of bone marrow in hypertension and CVD
As inferred in the previous section, the importance of bone marrow in neurogenic hypertension is extremely undervalued. Studies suggest that the bone marrow harbors memory T-cells, and most importantly, it is a site for the initiation of T-cell activation responses [160]. Given the importance of T-cell activation in hypertension, discussed in earlier sections, the bone marrow may be a key organ in neurogenic hypertension. The bone marrow is also the primary source of EPCs [161], which play a particularly important role in endothelial repair in the setting of arterial and renal injury following inflammatory and other pro-hypertensive stimuli [162-164]. Compromising the ability of the EPCs to repair endothelial damage may perpetuate the pathophysiology of hypertension.
In the bone marrow, EPCs are localized in the stem cell niche. This niche is particularly important for controlling the ability of these cells to mobilize and differentiate [165], and has recently been shown to have immune privilege provided by Treg cells [166]. It is important, however, to note that stem cells and lymphoid progenitors occupy separate bone marrow compartments [167]. EPCs respond differently to various stimuli. For example, acute inflammatory stimuli trigger EPC mobilization, while chronic inflammation can have the opposite effect and actually decrease the number of circulating EPCs [168]. In addition to inflammation, EPCs are also able to respond to autonomic stimulation. Recently, it has been suggested that beta-2 adrenergic receptor stimulation can improve EPC function [169]. However, chronic elevation in bone marrow NE may impair the function of EPCs, and this may be important in the context of hypertension. These observations are strengthening the link between the nervous system and the bone marrow.
There is evidence linking bone pathologies with vascular lesions in chronic kidney disease, including end-stage kidney disease [170-172]. Further, bone vascularization is of particular interest in the context of hypertension and other forms of CVD, since the cross-talk between the ANS and the bone marrow vasculature could be a key mechanism of hypertension pathophysiology. As in other vascular beds, NE acts as a vasoconstrictor in the bone marrow and plays an important role in controlling blood flow [173]. Within the bone marrow, there is an oxygen gradient responsible for maintaining healthy cell environments. Hematopoietic stem cells are found in the osteoblastic niche, which is hypoxic, and upon maturation, travel to the vascular niche where they are able to differentiate [174]. It is possible that in the context of neurogenic hypertension, which presents with increased circulating NE, there may be extensive vasoconstriction in the bone marrow, creating a hypoxic environment that could negatively modulate stem and progenitor cell function, as well as enhance local inflammatory responses. These ideas merit further investigation in the context of hypertension, as improvement of the bone marrow blood flow may present a novel therapeutic target for that condition.
7. Conclusion
In the present review we have discussed the interplay between the ANS and the IS. Both systems hold particular importance in the pathophysiology of hypertension. We propose that the relevance of bone marrow in the field of CVD is greatly undervalued. The bone marrow is a crucial meeting point for neural, immune, and vascular networks. Afferent sensory fibers may relay important inflammatory signals to the brain, and sympathetic efferent fibers could affect both inflammatory and progenitor cells in the bone marrow niches. The interplay among these systems in the bone marrow is likely complex. It is important to determine the nature and origins of the signals transmitted to the brain by the bone marrow afferents, as well as to determine how the vasculature within the bone marrow is regulated in hypertensive subjects. .
There is current interest in studying these systems in subjects with pre-hypertension. The finding of increased inflammation and autonomic dysfunction in pre-hypertensive patients and animals has led to emerging interest in examining the time course of the development of hypertension and identifying novel biomarkers that may yield new therapeutic targets for prevention of high blood pressure. While increasing amounts of data suggest that both increasing sympathetic activity and inflammatory responses precede increases in blood pressure, the exact timing of these events and their specific roles in the development of hypertension remain to be elucidated. Therefore, the question remains: which comes first, inflammation or hypertension?
We propose the following unifying hypothesis to summarize our discussion (Fig. 1). Hypertensive stimuli, including Ang II, work centrally to activate both microglia and neurons. Changes in neuronal activity lead to dysfunction of ANS signaling to the periphery, including decreased parasympathetic efferent signaling through the vagus and increase sympathetic efferent signaling through the sympathetic chain to key lymphoid organs, such as the spleen and bone marrow. Autonomic dysfunction in these organs works to increase inflammatory responses, such as the production of pro-inflammatory cytokines and cells, as well as to inhibit EPC function and ultimately vascular repair. Elevation in the pro-inflammatory factors may be detected by the sensory afferents in the periphery, including those within the bone marrow, which may relay the message to the brainstem cardioregulatory areas, ultimately contributing to dampening of the vagal anti-inflammatory reflex and perpetuating the pro-inflammatory responses via the feed-forward loop. These increases in peripheral inflammation and endothelial dysfunction ultimately lead to the vascular, renal, and cardiac lesions of hypertension (Fig. 1).
Despite important recent advances in the field, several key questions remain. First, what are the underlying mechanisms triggering both peripheral and central inflammation? Can the vagus relay messages to the bone marrow? Can bone marrow afferent signals modify function in cardioregulatory regions of the brain? Better understanding of the balance between the pro-inflammatory sympathetic system and the anti-inflammatory parasympathetic system will provide many answers to these questions.
Acknowledgments
This work is supported in part by the NIH grant HL 33610. Special thanks to Sara L. Croft for help with editing, and Mina S. Hanna for help with the figure.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Monica M. Santisteban, Jasenka Zubcevic, David M. Baekey, and Mohan K. Raizada declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Recently published papers of importance have been highlighted as:
• Of importance
•• Of major importance
- 1.Roberie DR, Elliott WJ. What is the prevalence of resistant hypertension in the United States? Curr Opin Cardiol. 2012;27(4):386–91. doi: 10.1097/HCO.0b013e328353ad6e. doi:10.1097/HCO.0b013e328353ad6e. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87. doi: 10.1097/HJH.0b013e3281fc975a. doi:10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. doi:10.1161/hypertensionaha.108.189141. [DOI] [PubMed] [Google Scholar]
- 4••.Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012;26(8):463–75. doi: 10.1038/jhh.2011.66. doi:10.1038/jhh.2011.66. [This excellent review focuses on the neuro-adrenergic causes of human hypertension, clinical implications and therapeutic targeting.] [DOI] [PubMed] [Google Scholar]
- 5.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–46. doi: 10.1038/nrn1902. doi:10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 6.Cates MJ, Dickinson CJ, Hart EC, Paton JF. Neurogenic hypertension and elevated vertebrobasilar arterial resistance: is there a causative link? Curr Hypertens Rep. 2012;14(3):261–9. doi: 10.1007/s11906-012-0267-6. doi:10.1007/s11906-012-0267-6. [DOI] [PubMed] [Google Scholar]
- 7.Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010;108(2):227–37. doi: 10.1152/japplphysiol.00832.2009. doi:10.1152/japplphysiol.00832.2009. [DOI] [PubMed] [Google Scholar]
- 8•.Dibona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61(3):556–60. doi: 10.1161/HYPERTENSIONAHA.111.00633. doi:10.1161/hypertensionaha.111.00633. [This review is an excellent guide to our current understanding of the role of the sympathetic nervous system in hypertension, both human and animal models.] [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11(1):3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Colombo M, Seravalle G, Spaziani D, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31(1):64–7. doi: 10.1161/01.hyp.31.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens Suppl. 1990;8(7):S53–7. [PubMed] [Google Scholar]
- 12.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14(2):177–83. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31(1):68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32(2):293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 15•.Thiyagarajan R, Pal P, Pal GK, Subramanian SK, Bobby Z, Das AK, et al. Cardiovagal Modulation, Oxidative Stress, and Cardiovascular Risk Factors in Prehypertensive Subjects: Cross-Sectional Study. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt025. doi:10.1093/ajh/hpt025. [This study in human subjects underlies the importance of several risk factors in the development of hypertension.] [DOI] [PubMed] [Google Scholar]
- 16.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43(2):169–75. doi: 10.1161/01.HYP.0000103160.35395.9E. doi:10.1161/01.hyp.0000103160.35395.9e. [DOI] [PubMed] [Google Scholar]
- 17.Baum P, Petroff D, Classen J, Kiess W, Bluher S. Dysfunction of autonomic nervous system in childhood obesity: a cross-sectional study. PLoS One. 2013;8(1):e54546. doi: 10.1371/journal.pone.0054546. doi:10.1371/journal.pone.0054546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javorka M, Trunkvalterova Z, Tonhajzerova I, Lazarova Z, Javorkova J, Javorka K. Recurrences in heart rate dynamics are changed in patients with diabetes mellitus. Clin Physiol Funct Imaging. 2008;28(5):326–31. doi: 10.1111/j.1475-097X.2008.00813.x. doi:10.1111/j.1475-097X.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24(10):1793–8. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 20.Toschi-Dias E, Trombetta IC, Dias da Silva VJ, Maki-Nunes C, Cepeda FX, Alves MJ, et al. Time delay of baroreflex control and oscillatory pattern of sympathetic activity in patients with metabolic syndrome and obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00848.2012. doi:10.1152/ajpheart.00848.2012. [DOI] [PubMed] [Google Scholar]
- 21.Solini A, Ruilope LM. How can resistant hypertension be identified and prevented? Nat Rev Cardiol. 2013 doi: 10.1038/nrcardio.2013.23. doi:10.1038/nrcardio.2013.23. [DOI] [PubMed] [Google Scholar]
- 22.Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. 2012;380(9841):591–600. doi: 10.1016/S0140-6736(12)60825-3. doi:10.1016/s0140-6736(12)60825-3. [DOI] [PubMed] [Google Scholar]
- 23.Fisher JP, Fadel PJ. Therapeutic strategies for targeting excessive central sympathetic activation in human hypertension. Exp Physiol. 2010;95(5):572–80. doi: 10.1113/expphysiol.2009.047332. doi:10.1113/expphysiol.2009.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet. 1953;1(6757):403–8. doi: 10.1016/s0140-6736(53)91589-x. [DOI] [PubMed] [Google Scholar]
- 25.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152(16):1501–4. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 26.Peet MM. Results of bilateral supradiaphragmatic splanchnicectomy for arterial hypertension. N Engl J Med. 1947;236(8):270–7. doi: 10.1056/NEJM194702202360802. doi:10.1056/nejm194702202360802. [DOI] [PubMed] [Google Scholar]
- 27.Polimeni A, Curcio A, Indolfi C. Renal sympathetic denervation for treating resistant hypertension. Circ J. 2013;77(4):857–63. doi: 10.1253/circj.cj-13-0297. [DOI] [PubMed] [Google Scholar]
- 28.Froeschl M, Hadziomerovic A, Ruzicka M. Renal Sympathetic Denervation for Resistant Hypertension. Can J Cardiol. 2013 doi: 10.1016/j.cjca.2013.02.019. doi:10.1016/j.cjca.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. doi: 10.1016/S0140-6736(09)60566-3. doi:10.1016/s0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 30.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. doi: 10.1016/S0140-6736(10)62039-9. doi:10.1016/s0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 31.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361(9):932–4. doi: 10.1056/NEJMc0904179. doi:10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 32••.Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14(3):247–53. doi: 10.1007/s11906-012-0264-9. doi:10.1007/s11906-012-0264-9. [This review discusses recent and promising advancements in renal denervation therapy in human hypertension.] [DOI] [PubMed] [Google Scholar]
- 33.Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal Sensory and Sympathetic Nerves Reinnervate the Kidney in a Similar Time Dependent Fashion Following Renal Denervation in Rats. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00599.2012. doi:10.1152/ajpregu.00599.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60(6):1485–90. doi: 10.1161/HYPERTENSIONAHA.112.201186. doi:10.1161/hypertensionaha.112.201186. [DOI] [PubMed] [Google Scholar]
- 35.Esler M, Randall O, Bennett J, Zweifler A, Julius S, Rydelek P. Suppression of sympathetic nervous function in low-renin essential hypertension. Lancet. 1976;2(7977):115–8. doi: 10.1016/s0140-6736(76)92844-0. [DOI] [PubMed] [Google Scholar]
- 36.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, et al. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54(3):530–6. doi: 10.1161/HYPERTENSIONAHA.109.134023. doi:10.1161/hypertensionaha.109.134023. [DOI] [PubMed] [Google Scholar]
- 37.Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010;56(15):1254–8. doi: 10.1016/j.jacc.2010.03.089. doi:10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 38.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–26. doi: 10.1161/HYPERTENSIONAHA.109.140665. doi:10.1161/hypertensionaha.109.140665. [DOI] [PubMed] [Google Scholar]
- 39.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58(7):765–73. doi: 10.1016/j.jacc.2011.06.008. doi:10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev. 2011;19(2):52–7. doi: 10.1097/CRD.0b013e3181f87921. doi:10.1097/CRD.0b013e3181f87921. [DOI] [PubMed] [Google Scholar]
- 41.Green AL, Wang S, Owen SL, Xie K, Liu X, Paterson DJ, et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16(16):1741–5. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- 42.Green AL, Wang S, Bittar RG, Owen SL, Paterson DJ, Stein JF, et al. Deep brain stimulation: a new treatment for hypertension? J Clin Neurosci. 2007;14(6):592–5. doi: 10.1016/j.jocn.2006.04.015. doi:10.1016/j.jocn.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Carter HH, Dawson EA, Cable NT, Basnayake S, Aziz TZ, Green AL, et al. Deep brain stimulation of the periaqueductal grey induces vasodilation in humans. Hypertension. 2011;57(5):e24–5. doi: 10.1161/HYPERTENSIONAHA.111.170183. doi:10.1161/hypertensionaha.111.170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira EA, Wang S, Paterson DJ, Stein JF, Aziz TZ, Green AL. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci. 2010;17(1):124–7. doi: 10.1016/j.jocn.2009.02.041. doi:10.1016/j.jocn.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 45.Patel NK, Javed S, Khan S, Papouchado M, Malizia AL, Pickering AE, et al. Deep brain stimulation relieves refractory hypertension. Neurology. 2011;76(4):405–7. doi: 10.1212/WNL.0b013e3182088108. doi:10.1212/WNL.0b013e3182088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyam JA, Kringelbach ML, Silburn PA, Aziz TZ, Green AL. The autonomic effects of deep brain stimulation--a therapeutic opportunity. Nat Rev Neurol. 2012;8(7):391–400. doi: 10.1038/nrneurol.2012.100. doi:10.1038/nrneurol.2012.100. [DOI] [PubMed] [Google Scholar]
- 47.Green AL, Hyam JA, Williams C, Wang S, Shlugman D, Stein JF, et al. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation. 2010;13(3):174–81. doi: 10.1111/j.1525-1403.2010.00274.x. doi:10.1111/j.1525-1403.2010.00274.x. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki S, Tanda S, Hatta T, Morimoto S, Takeda K, Kizu O, et al. Neurovascular decompression of the rostral ventrolateral medulla decreases blood pressure and sympathetic nerve activity in patients with refractory hypertension. J Clin Hypertens (Greenwich) 2011;13(11):818–20. doi: 10.1111/j.1751-7176.2011.00522.x. doi:10.1111/j.1751-7176.2011.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geiger H, Naraghi R, Schobel HP, Frank H, Sterzel RB, Fahlbusch R. Decrease of blood pressure by ventrolateral medullary decompression in essential hypertension. Lancet. 1998;352(9126):446–9. doi: 10.1016/s0140-6736(97)11343-5. [DOI] [PubMed] [Google Scholar]
- 50.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–51. doi: 10.1001/jama.290.22.2945. doi:10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 51.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19(2):149–54. doi: 10.1038/sj.jhh.1001785. doi:10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 52.Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, et al. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156(4):759, e1–7. doi: 10.1016/j.ahj.2008.07.009. doi:10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–40. doi: 10.1161/HYPERTENSIONAHA.110.163576. doi:10.1161/hypertensionaha.110.163576. [This review discusses recent findings on CNS control of inflammation and hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27(2):174–82. doi: 10.1016/j.cjca.2010.12.040. doi:10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 55.Celik T, Yuksel UC, Fici F, Celik M, Yaman H, Kilic S, et al. Vascular inflammation and aortic stiffness relate to early left ventricular diastolic dysfunction in prehypertension. Blood Press. 2013;22(2):94–100. doi: 10.3109/08037051.2012.716580. doi:10.3109/08037051.2012.716580. [DOI] [PubMed] [Google Scholar]
- 56.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17(7):568–73. doi: 10.1016/j.amjhyper.2004.03.675. doi:10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 57.Navarro-Gonzalez JF, Mora C, Muros M, Garcia J, Donate J, Cazana V. Relationship between inflammation and microalbuminuria in prehypertension. J Hum Hypertens. 2013;27(2):119–25. doi: 10.1038/jhh.2011.118. doi:10.1038/jhh.2011.118. [DOI] [PubMed] [Google Scholar]
- 58.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol. 1982;128(3):1211–6. [PubMed] [Google Scholar]
- 59.Khraibi AA. Association between disturbances in the immune system and hypertension. Am J Hypertens. 1991;4(7 Pt 1):635–41. doi: 10.1093/ajh/4.7.635. [DOI] [PubMed] [Google Scholar]
- 60••.Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, et al. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71(9):774–82. doi: 10.1016/j.biopsych.2012.01.017. doi:10.1016/j.biopsych.2012.01.017. [In this and reference [61•], authors determine that T-lymphocytes are essential in multiple animal models of hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60. doi: 10.1084/jem.20070657. doi:10.1084/jem.20070657. [In this and reference [60••], authors determine that T-lymphocytes are essential in multiple animal models of hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57(3):469–76. doi: 10.1161/HYPERTENSIONAHA.110.162941. doi:10.1161/hypertensionaha.110.162941. [This report elucidates the roles of different T-lymphocyte populations, especifically regulatory T cell's ability to prevent hypertension.] [DOI] [PubMed] [Google Scholar]
- 63.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59(2):324–30. doi: 10.1161/HYPERTENSIONAHA.111.181123. doi:10.1161/hypertensionaha.111.181123. [DOI] [PubMed] [Google Scholar]
- 64.Schiffrin EL. The Immune System: Role in Hypertension. Can J Cardiol. 2012 doi: 10.1016/j.cjca.2012.06.009. doi:10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, et al. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension. 2011;57(2):298–307. doi: 10.1161/HYPERTENSIONAHA.110.160077. doi:10.1161/hypertensionaha.110.160077. [DOI] [PubMed] [Google Scholar]
- 66.Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vazquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm (Lond) 2010;7:21. doi: 10.1186/1476-9255-7-21. doi:10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res. 2012;111(9):1190–7. doi: 10.1161/CIRCRESAHA.112.277475. doi:10.1161/circresaha.112.277475. [The authors demonstrate that anti-inflammatory modulation of the innate immunes system in normotensive rats is reversed in pre hypertensive rats, attributed to increase sympathetic drive preceding the rise in blood pressure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehrlich D, Humpel C. Chronic vascular risk factors (cholesterol, homocysteine, ethanol) impair spatial memory, decline cholinergic neurons and induce blood-brain barrier leakage in rats in vivo. J Neurol Sci. 2012;322(1-2):92–5. doi: 10.1016/j.jns.2012.07.002. doi:10.1016/j.jns.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen JK, Zhao T, Ni M, Li DJ, Tao X, Shen FM. Downregulation of alpha7 nicotinic acetylcholine receptor in two-kidney one-clip hypertensive rats. BMC Cardiovasc Disord. 2012;12:38. doi: 10.1186/1471-2261-12-38. doi:10.1186/1471-2261-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37(4):1087–93. doi: 10.1161/01.STR.0000206281.77178.ac. doi:10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 71.Liu B. Modulation of microglial pro-inflammatory and neurotoxic activity for the treatment of Parkinson's disease. AAPS J. 2006;8(3):E606–21. doi: 10.1208/aapsj080369. doi:10.1208/aapsj080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. doi:10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 73.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, et al. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55(2):277–83. 6, 83. doi: 10.1161/HYPERTENSIONAHA.109.142646. doi:10.1161/hypertensionaha.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH Oxidases in the Subfornical Organ in Angiotensin II-Induced Hypertension. Hypertension. 2013;61(2):382–7. doi: 10.1161/HYPERTENSIONAHA.111.00546. doi:10.1161/hypertensionaha.111.00546. [This report indicates a critical role for central oxidative stress in the CNS modulation of hypertension and peripheral inflammation using a genetic p22phox mouse model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol. 2011;11(2):156–61. doi: 10.1016/j.coph.2010.12.001. doi:10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37(2):e52–7. doi: 10.1111/j.1440-1681.2009.05234.x. doi:10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56(2):297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. doi:10.1161/hypertensionaha.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, et al. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension. 2012;60(5):1316–23. doi: 10.1161/HYPERTENSIONAHA.112.199547. doi:10.1161/hypertensionaha.112.199547. [This study indicates that specific inhibition of central oxidative stress is able to attenuate hypertension, inhibit microglial activation in the PVN, and normalize the release of peripheral bone marrow-derived inflammatory cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, et al. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61(1):194–201. doi: 10.1161/HYPERTENSIONAHA.112.192252. doi:10.1161/hypertensionaha.112.192252. [In this report, investigators elucidate brain stem inflammatory reactions to be mechanistically related to neurogenic hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80••.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension. 2012;59(1):113–21. doi: 10.1161/HYPERTENSIONAHA.111.182154. doi:10.1161/hypertensionaha.111.182154. [This report strengthens the hypothesis that inflammatory responses in the PVN, in this case specifically NF-κB, play an important pro-hypertensive role via modulation of the renin angiotensin system.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–8. doi: 10.1016/j.amjcard.2006.01.059. doi:10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 82.Patel BM, Mehta AA. Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur J Pharmacol. 2012;697(1-3):1–12. doi: 10.1016/j.ejphar.2012.09.034. doi:10.1016/j.ejphar.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 83.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: implications for new treatment. Prog Neurobiol. 2011;95(2):89–103. doi: 10.1016/j.pneurobio.2011.06.006. doi:10.1016/j.pneurobio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3beta in neuronal culture. Br J Pharmacol. 2013 doi: 10.1111/bph.12177. doi:10.1111/bph.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zubcevic J, Jun JY, Lamont G, Murca TM, Shi P, Yuan W, et al. Nucleus of the Solitary Tract (Pro)Renin Receptor-Mediated Antihypertensive Effect Involves Nuclear Factor-kappaB-Cytokine Signaling in the Spontaneously Hypertensive Rat. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.111.199836. doi:10.1161/hypertensionaha.111.199836. [DOI] [PubMed] [Google Scholar]
- 86••.Shan Z, Zubcevic J, Shi P, Jun JY, Dong Y, Murca TM, et al. Chronic Knockdown of the Nucleus of the Solitary Tract AT1 Receptors Increases Blood Inflammatory-Endothelial Progenitor Cell Ratio and Exacerbates Hypertension in the Spontaneously Hypertensive Rat. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.111.00156. doi:10.1161/hypertensionaha.111.00156. [This study indicates a connection between central RAS and the peripheral immune response in hypertension pathophysiology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107(2):263–70. doi: 10.1161/CIRCRESAHA.110.217299. doi:10.1161/circresaha.110.217299. [The central effects of angiotensin II are critical for peripheral inflammatory responses, including T cell activation. This study proposes a feed-forward mechanisms underlying the connection between central RAS and peripheral inflammation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. doi:10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 89.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. doi:10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 90••.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339(6116):156–61. doi: 10.1126/science.1227901. doi:10.1126/science.1227901. [This excellent review focuses on the role of microglia, ranging from the resting state to the different states of activation and neurotoxicity. The authors discuss microglia in several diseases and emphasize both the beneficial and detrimental roles of these cells in the CNS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–24. doi: 10.1038/nature09788. doi:10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 92.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100(26):15983–8. doi: 10.1073/pnas.2237050100. doi:10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–31. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 94.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57(6):1026–33. doi: 10.1161/HYPERTENSIONAHA.111.169748. doi:10.1161/hypertensionaha.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: A new therapeutic angle? Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.02.005. doi:10.1016/j.pharmthera.2013.02.005. [This review focuses on the interaction between the immune, renin-angiotensin, and autonomic nervous systems.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marvar PJ, Harrison DG. Stress-dependent hypertension and the role of T lymphocytes. Exp Physiol. 2012;97(11):1161–7. doi: 10.1113/expphysiol.2011.061507. doi:10.1113/expphysiol.2011.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ippoliti F, Canitano N, Businaro R. Stress and obesity as risk factors in cardiovascular diseases: a neuroimmune perspective. J Neuroimmune Pharmacol. 2013;8(1):212–26. doi: 10.1007/s11481-012-9432-6. doi:10.1007/s11481-012-9432-6. [DOI] [PubMed] [Google Scholar]
- 98.Lu XT, Zhao YX, Zhang Y, Jiang F. Psychological stress, vascular inflammation and atherogenesis: potential roles of circulating cytokines. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e3182858fac. doi:10.1097/FJC.0b013e3182858fac. [DOI] [PubMed] [Google Scholar]
- 99.Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun. 2013;27(1):174–84. doi: 10.1016/j.bbi.2012.10.013. doi:10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong K, Park HT, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 2002;12(5):583–91. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 101••.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37(2):290–301. doi: 10.1016/j.immuni.2012.05.021. doi:10.1016/j.immuni.2012.05.021. [This report underlies the clinical importance of autonomic modulation on circadian function of immune cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, Shaw LC, Carnegie D, Caballero S, et al. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2012;31(5):481–94. doi: 10.1016/j.preteyeres.2012.04.005. doi:10.1016/j.preteyeres.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587(Pt 3):597–610. doi: 10.1113/jphysiol.2008.165902. doi:10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, et al. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289(4):H1683–91. doi: 10.1152/ajpheart.00125.2005. doi:10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 105.Crowley SD. The Cooperative Roles of Inflammation and Oxidative Stress in the Pathogenesis of Hypertension. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5258. doi:10.1089/ars.2013.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106•.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension. 2012;59(4):755–62. doi: 10.1161/HYPERTENSIONAHA.111.186833. doi:10.1161/hypertensionaha.111.186833. [This excellent review focuses on the autonomic modulation of the immune system, emphasizing that the autonomic system is a powerful regulator of the immune system in the “death” and “survival” triangles circuitry of cardiovascular disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107••.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. doi: 10.1038/nature11260. doi:10.1038/nature11260. [This intriguing report provides novel insight into the progression of cardiovascular disease, particularly the role of the sympathetic nervous system in recruitment of hematopoietic cells from the bone marrow.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang YY, Lin SY, Chuang YH, Chen CJ, Tung KC, Sheu WH. Adipose proinflammatory cytokine expression through sympathetic system is associated with hyperglycemia and insulin resistance in a rat ischemic stroke model. Am J Physiol Endocrinol Metab. 2011;300(1):E155–63. doi: 10.1152/ajpendo.00301.2010. doi:10.1152/ajpendo.00301.2010. [DOI] [PubMed] [Google Scholar]
- 109.Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol Behav. 2007;91(4):343–51. doi: 10.1016/j.physbeh.2007.04.002. doi:10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48(8):1655–72. doi: 10.1194/jlr.R700006-JLR200. doi:10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 111.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–7. doi: 10.1038/nature06685. doi:10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 112.Muller WA. Regulate globally, act locally: adrenergic nerves promote leukocyte recruitment. Immunity. 2012;37(2):189–91. doi: 10.1016/j.immuni.2012.08.004. doi:10.1016/j.immuni.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- 114.Beaule C, Arvanitogiannis A, Amir S. Light suppresses Fos expression in the shell region of the suprachiasmatic nucleus at dusk and dawn: implications for photic entrainment of circadian rhythms. Neuroscience. 2001;106(2):249–54. doi: 10.1016/s0306-4522(01)00313-x. [DOI] [PubMed] [Google Scholar]
- 115.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. Oxford University Press; USA: 1991. [Google Scholar]
- 116.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 117.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106(3):447–62. doi: 10.1161/CIRCRESAHA.109.208355. doi:10.1161/circresaha.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315–22. doi: 10.1056/NEJM198511213132103. doi:10.1056/nejm198511213132103. [DOI] [PubMed] [Google Scholar]
- 119••.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–68. doi: 10.1084/jem.20120571. doi:10.1084/jem.20120571. [This review cohesively focuses on recent findings underlying the neural immune reflex in health and disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kiguchi N, Kobayashi Y, Maeda T, Tominaga S, Nakamura J, Fukazawa Y, et al. Activation of nicotinic acetylcholine receptors on bone marrow-derived cells relieves neuropathic pain accompanied by peripheral neuroinflammation. Neurochem Int. 2012;61(7):1212–9. doi: 10.1016/j.neuint.2012.09.001. doi:10.1016/j.neuint.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Thayer JF, Loerbroks A, Sternberg EM. Inflammation and cardiorespiratory control: the role of the vagus nerve. Respir Physiol Neurobiol. 2011;178(3):387–94. doi: 10.1016/j.resp.2011.05.016. doi:10.1016/j.resp.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 122.Yamakawa K, Matsumoto N, Imamura Y, Muroya T, Yamada T, Nakagawa J, et al. Electrical vagus nerve stimulation attenuates systemic inflammation and improves survival in a rat heatstroke model. PLoS One. 2013;8(2):e56728. doi: 10.1371/journal.pone.0056728. doi:10.1371/journal.pone.0056728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123•.Lujan HL, Dicarlo SE. Physical activity, by enhancing parasympathetic tone and activating the cholinergic anti-inflammatory pathway, is a therapeutic strategy to restrain chronic inflammation and prevent many chronic diseases. Med Hypotheses. 2013 doi: 10.1016/j.mehy.2013.01.014. doi:10.1016/j.mehy.2013.01.014. [Authors postulate an interesting hypothesis, suggesting that exercise enhances parasympathetic tone and can dampen and even prevent chronic inflammatory diseases.] [DOI] [PubMed] [Google Scholar]
- 124.Zhao YX, He W, Jing XH, Liu JL, Rong PJ, Ben H, et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. doi: 10.1155/2012/627023. doi:10.1155/2012/627023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125••.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–54. doi: 10.1038/nrendo.2012.189. doi:10.1038/nrendo.2012.189. [This review focuses on the interaction between the dysregulation of the vagal inflammatory relfex and obesity-related inflammation and complications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Erin N, Duymus O, Ozturk S, Demir N. Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul Pept. 2012;179(1-3):101–8. doi: 10.1016/j.regpep.2012.08.001. doi:10.1016/j.regpep.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Dustin ML. Signaling at neuro/immune synapses. J Clin Invest. 2012;122(4):1149–55. doi: 10.1172/JCI58705. doi:10.1172/jci58705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huston JM. The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg Infect (Larchmt) 2012;13(4):187–93. doi: 10.1089/sur.2012.126. doi:10.1089/sur.2012.126. [DOI] [PubMed] [Google Scholar]
- 129.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265(6):663–79. doi: 10.1111/j.1365-2796.2009.02098.x. doi:10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43(3):357–64. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 131.Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24(8):1223–8. doi: 10.1016/j.bbi.2010.07.247. doi:10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–13. doi: 10.1073/pnas.0803237105. doi:10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42(2):153–69. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 134.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35(1):80–6. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. doi:10.1002/(sici)1097-0029(19960901)35:1<80::aid-jemt7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 135.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145(1-2):77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 136.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248(1):188–204. doi: 10.1111/j.1600-065X.2012.01138.x. doi:10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2012 doi: 10.1136/gutjnl-2012-302550. doi:10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wehner S, Vilz TO, Sommer N, Sielecki T, Hong GS, Lysson M, et al. The novel orally active guanylhydrazone CPSI-2364 prevents postoperative ileus in mice independently of anti-inflammatory vagus nerve signaling. Langenbecks Arch Surg. 2012;397(7):1139–47. doi: 10.1007/s00423-012-0989-6. doi:10.1007/s00423-012-0989-6. [DOI] [PubMed] [Google Scholar]
- 139.Okamoto T, Kurahashi K, Fujiwara M. Cholinergic transmission in the superior cervical ganglion reinnervated by peripheral vagal stump cut below the nodose ganglion in cats. J Pharmacol Exp Ther. 1988;245(3):990–4. [PubMed] [Google Scholar]
- 140.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64(1):28–32. doi: 10.1016/j.neuron.2009.09.039. doi:10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996;98(4):453–7. doi: 10.1016/S0065-1281(96)80013-4. doi:10.1016/s0065-1281(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 142.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 143.Amadesi S, Reni C, Katare R, Meloni M, Oikawa A, Beltrami AP, et al. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation. 2012;125(14):1774–86, S1-19. doi: 10.1161/CIRCULATIONAHA.111.089763. doi:10.1161/circulationaha.111.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144•.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46(2):306–13. doi: 10.1016/j.bone.2009.09.013. doi:10.1016/j.bone.2009.09.013. [This study indicates the profile of primary afferent nerve fibers innervating the bone and marrow.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. doi:10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84. doi: 10.1016/j.cell.2009.09.028. doi:10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–36. doi: 10.1038/nrn2947. doi:10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pickering AE, Boscan P, Paton JF. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003;551(Pt 2):589–99. doi: 10.1113/jphysiol.2003.046615. doi:10.1113/jphysiol.2003.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boscan P, Kasparov S, Paton JF. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002;16(5):907–20. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- 150••.Suzuki A, Shimura M. Changes in blood pressure induced by electrical stimulation of the femur in anesthetized rats. Auton Neurosci. 2010;158(1-2):39–43. doi: 10.1016/j.autneu.2010.05.012. doi:10.1016/j.autneu.2010.05.012. [This report indicates a valuable connection between the bone marrowprimary afferents and blood pressure control.] [DOI] [PubMed] [Google Scholar]
- 151.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14(3):R101. doi: 10.1186/ar3826. doi:10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, et al. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum. 2012;64(7):2223–32. doi: 10.1002/art.34385. doi:10.1002/art.34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153••.Lautner RQ, Villela DC, Fraga-Silva RA, Silva NC, Verano-Braga T, Costa-Fraga F, et al. Discovery and Characterization of Alamandine, a Novel Component of the Renin-Angiotensin System. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301077. doi:10.1161/circresaha.113.301077. [This is the first report of the novel component of the RAS: alamandine. The authors describe its similarity to Ang(1-7) and postulate the therapeutic value of this novel peptide.] [DOI] [PubMed] [Google Scholar]
- 154.Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, et al. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29(26):8612–9. doi: 10.1523/JNEUROSCI.1057-09.2009. doi:10.1523/jneurosci.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. doi:10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 156.Avula LR, Buckinx R, Alpaerts KH, Adriaensen D, Nassauw LV, Timmermans J-P. 879 The Mas-Related Gene Receptor MrgD Modulates Mucosal Mast Cell Infiltration During Intestinal Inflammation. Gastroenterology. 2012;142(5):S-152. [Google Scholar]
- 157.Avula LR, Buckinx R, Favoreel H, Cox E, Adriaensen D, Van Nassauw L, et al. Expression and distribution patterns of Mas-related gene receptor subtypes A-H in the mouse intestine: inflammation-induced changes. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1086-9. doi:10.1007/s00418-013-1086-9. [DOI] [PubMed] [Google Scholar]
- 158.Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, et al. Angiotensin II type 2 receptor (AT(2) R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00269.x. doi:10.1002/j.1532-2149.2012.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patil J, Schwab A, Nussberger J, Schaffner T, Saavedra JM, Imboden H. Intraneuronal angiotensinergic system in rat and human dorsal root ganglia. Regul Pept. 2010;162(1-3):90–8. doi: 10.1016/j.regpep.2010.03.004. doi:10.1016/j.regpep.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9(9):1151–7. doi: 10.1038/nm914. doi:10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 161.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 162.Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100(4):590–7. doi: 10.1161/01.RES.0000259043.42571.68. doi:10.1161/01.res.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 163.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31(1):117–25. doi: 10.1002/stem.1263. doi:10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ. Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol. 2004;24(5):834–8. doi: 10.1161/01.ATV.0000124891.57581.9f. doi:10.1161/01.ATV.0000124891.57581.9f. [DOI] [PubMed] [Google Scholar]
- 165.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45(3):321–5. doi: 10.1161/01.HYP.0000154789.28695.ea. doi:10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 166••.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–9. doi: 10.1038/nature10160. doi:10.1038/nature10160. [The authors use an interesting technique to image individual populations and demonstrate the importance of regulatory T cells in maintaining the stem-cell niche in the bone marrow.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. doi:10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189(2):247–54. doi: 10.1016/j.atherosclerosis.2006.06.021. doi:10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 169••.Galasso G, De Rosa R, Ciccarelli M, Sorriento D, Del Giudice C, Strisciuglio T, et al. beta2-Adrenergic Receptor Stimulation Improves Endothelial Progenitor Cells Mediated Ischemic Neoangiogenesis. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.111.300152. doi:10.1161/circresaha.111.300152. [To our knowledge, this is the first evidence of functional beta-2 adrenergic receptors being expressed by endothelial progenitor cells. This study provides novel insight into the mechanisms of central control of EPC function.] [DOI] [PubMed] [Google Scholar]
- 170.London GM, Marchais SJ, Guerin AP, Boutouyrie P, Metivier F, de Vernejoul MC. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19(9):1827–35. doi: 10.1681/ASN.2007050622. doi:10.1681/asn.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23(2):586–93. doi: 10.1093/ndt/gfm660. doi:10.1093/ndt/gfm660. [DOI] [PubMed] [Google Scholar]
- 172.Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, et al. Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):450–5. doi: 10.2215/CJN.01870408. doi:10.2215/cjn.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feitelson JB, Kulenovic E, Beck DJ, Harris PD, Passmore JC, Malkani AL, et al. Endogenous norepinephrine regulates blood flow to the intact rat tibia. J Orthop Res. 2002;20(2):391–6. doi: 10.1016/S0736-0266(01)00121-8. doi:10.1016/s0736-0266(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 174.Lafage-Proust MH, Prisby R, Roche B, Vico L. Bone vascularization and remodeling. Joint Bone Spine. 2010;77(6):521–4. doi: 10.1016/j.jbspin.2010.09.009. doi:10.1016/j.jbspin.2010.09.009. [DOI] [PubMed] [Google Scholar]