Summary
Aging is a degenerative process characterized by declining molecular, cell and organ functions, and accompanied by the progressive accumulation of oxidatively damaged macromolecules. This increased oxidative damage may be causally related to an age-associated dysfunction of defense mechanisms, which effectively protect young individuals from oxidative insults. Consistently, older organisms are more sensitive to acute oxidative stress exposures than young ones. In studies on the Drosophila Nrf2 transcription factor CncC, we have investigated possible causes for this loss of stress resistance and its connection to the aging process. Nrf2 is a master regulator of antioxidant and stress defense gene expression with established functions in the control of longevity. Here we show that the expression of protective Nrf2/CncC target genes in unstressed conditions does not generally decrease in older flies. However, aging flies progressively lose the ability to activate Nrf2 targets in response to acute stress exposure. We propose that the resulting inability to dynamically adjust the expression of Nrf2 target genes to the organism’s internal and external conditions contributes to age-related loss of homeostasis and fitness. In support of this hypothesis, the Drosophila small Maf protein, MafS, an Nrf2 dimerization partner, is critical to maintain responsiveness of the Nrf2 system: overexpression of MafS in older flies preserves Nrf2/CncC signaling competence and improves measures of age-associated functional decline. The maintenance of acute stress resistance, motor function, and heart performance in aging flies over expressing MafS supports a critical role for signal responsiveness of Nrf2 function in promoting youthful phenotypes.
Keywords: aging, Drosophila, small Maf, oxidative stress, Nrf2, Keap1
Introduction
The accumulation of macromolecules that have been damaged by oxidative insults is widely regarded as a consequence and possibly a cause of functional decline in aging organisms (for a recent review see (Sohal and Orr, 2011).Such oxidation-driven degeneration is counteracted by a number of cellular systems that serve to control redox balance and to repair oxidative damage. Many of the genes mediating these defense functions are tightly regulated in response to environmental and metabolic signals. An important mediator of this regulation is the transcription factor Nrf2 (nuclear factor E2-related factor 2). Nrf2-regulated genes encode antioxidants, phase-2 detoxification enzymes, mediators of glutathione synthesis, chaperones, and other protective gene products (Sykiotis and Bohmann, 2010). In addition to averting acute oxidative stress, well-controlled redox homeostasis is thought to support a number of critical cellular and organism functions such as signal transduction (Burhans and Heintz, 2009), proteostasis (Balch et al., 2008; Christians and Benjamin, 2011), and stem cell maintenance. Furthermore, recent evidence supports a role of Nrf2 signaling in the control of energy metabolism (reviewed in Sykiotis et al., 2011). It appears therefore that Nrf2 signaling has several functions that are critical for the maintenance of organism integrity. Correlative evidence generated in studies on several species shows that Nrf2 signaling can decline during aging and disease (Du et al., 2009; Przybysz et al., 2009; Suh et al., 2004; Suzuki et al., 2008), yet the question of how declining Nrf2 function may be causally related to aging or disease progression is not sufficiently resolved.
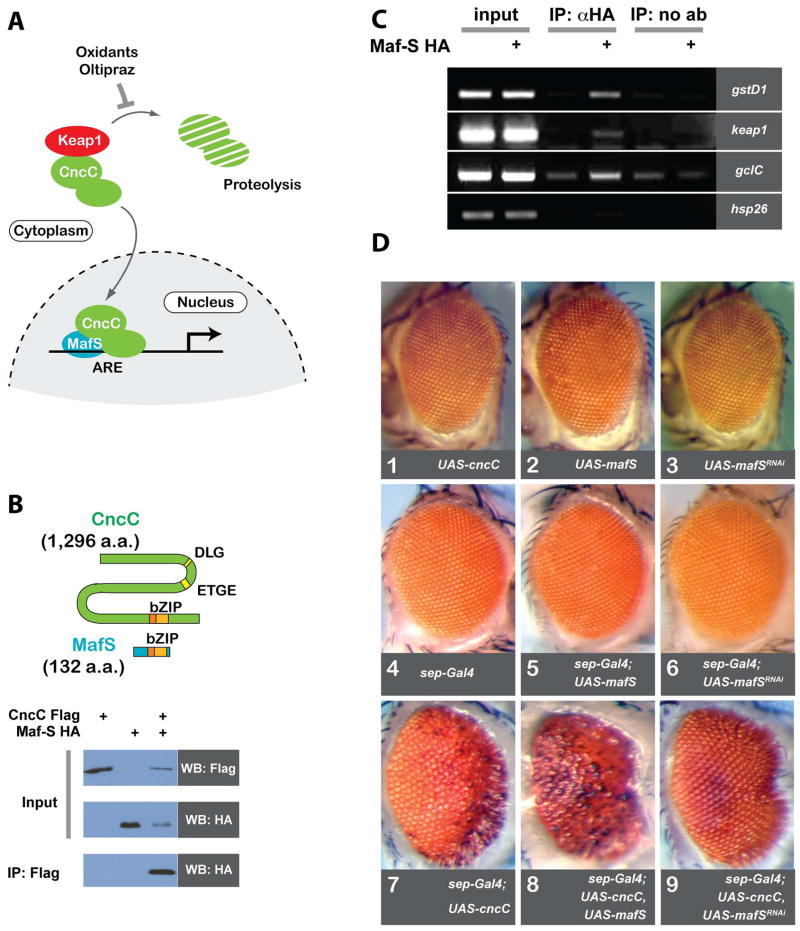
Our previous work established that central components of the Nrf2 pathway are conserved between vertebrate sand flies (Sykiotis and Bohmann, 2008). The Drosophila homolog of Nrf2 is encoded by the cap’n’collar (cnc)gene. The protein product of the “C” splice form, CncC, is structurally and functionally homologous to vertebrate Nrf2, and mechanisms that regulate Nrf2 are also conserved between vertebrates and flies(Grimberg et al., 2011; Sykiotis and Bohmann, 2008) (Fig. 1). In basal conditions, Nrf2 activity is limited by its cognate inhibitor Keap1, which serves as an adaptor protein for a Cul3-based ubiquitin ligase, thereby targeting Nrf2 for proteolysis (Motohashi and Yamamoto, 2004)(Fig 1A). In this uninduced state, antioxidant response elements (AREs) in Nrf2 target gene promoters are quiescent due to occupancy by small Maf (musculo aponeurotic fibrosarcoma) proteins(Blank, 2008). Like Nrf2, small Mafs are leucine zipper proteins, but unlike Nrf2 they lack transcription activation domains. Upon exposure to stress, Keap1 is modified such that it can no longer target Nrf2 for degradation, allowing the latter to accumulate in the nucleus and to form transcriptionally competent MafS/Nrf2 heterodimers. Therefore, Maf homodimers not only repress their associated transcription units and shield them from spurious activation by neighboring regulatory regions(Blank, 2008), but they can also be viewed as “genomic placeholders” that mark Nrf2 responsive genes and presumably keep them accessible for rapid Nrf2-mediated activation. Small Mafs are the prevalent dimerization partners for Nrf2 and are critical for Nrf2-mediated transcription. The sole Drosophila homolog of small Maf proteins is the product if the CG9954 gene, MafS (Veraksa et al., 2000). Here we show that MafS is a CncC dimerization partner in Drosophila required for CncC signaling and target gene activation in cell culture and in vivo.
Figure 1. Physical and genetic interactions between MafS and CncC.
A. The CncC pathway in Drosophila. In non-stress conditions the Drosophila ortholog of the Nrf2 transcription factor, CncC, is sequestered in the cytoplasm and targeted for ubiquitin-dependent proteasomal degradation by its negative regulator Keap1. Oxidative stress, electrophiles, or drugs like oltipraz cause inhibition of Keap1-mediated CncC degradation. As a result, CncC accumulates in the nucleus and heterodimerizes with the small Maf protein, MafS. MafS/CncC dimers bind to antioxidant response elements (AREs) and activate the transcription of stress response genes.
B. MafS and CncC associate in S2 cells. The cartoon shows a schematic representation of CncC and MafS proteins; the positions of their respective bZIP dimerization domains are indicated. The DLG and ETGE motifs in CncC resemble Keap1 interaction domains that have been characterized in vertebrate Nrf2. Epitope-tagged versions of MafS and CncC (3xHA and 3xFlag, respectively) were expressed in S2 cells as indicated. Western blots (WB) with the denoted antibodies demonstrate that MafS was co-immunoprecipitated with CncC from a lysate of cells in which the two proteins were expressed together.
C. MafS can bind to CncC target gene promoters. 3xHA-tagged MafS was expressed in transgenic flies under the control of the ubiquitously activearm-Gal4 driver. Chromatin from larvae of this genotype (arm-Gal4 / UAS-mafS)and corresponding controls (arm-Gal4/+) was processed for chromatin immunoprecipitation (ChIP) using anti-HA antibody. Semi-quantitative PCR analyses were conducted to assess MafS binding to ARE-containing sequences from the promoters of the CncC target genes,gstD1, keap1 and gclC. The lanes labeled “input” show PCR assays on total genomic DNA. The promoter region of the hsp26gene, which does not harbor ARE consensus sequences, serves as negative control. In control experiments omitting the anti-HA antibody, no MafS binding was detected.
D. MafS can interact genetically with CncC. Transgenic Drosophila lines carrying UAS-cncC, UAS-mafS, UAS-mafSRNAior the photoreceptor-specificsep-Gal4 driver display normal eye morphology (panels 1–4). Similarly, ectopic expression of MafS or mafSRNAi with sep-Gal4 does not cause any morphological defect in fly eyes (panels 5 and 6). In contrast, ectopic expression of CncC causes a rough eye phenotype, as previously reported (Sykiotis and Bohmann, 2008). This abnormal eye phenotype isenhanced by co-expression of MafS (panel 8) and is suppressed by co-expression of a MafS-targeting dsRNA (panel 9). The images shown in these panels are representative of phenotypes that for a broadly uniform and consistent for a given genotype.
In mammals, Nrf2-dependent gene expression programs counteract a number of pathologies that are caused or exacerbated by oxidative stress. Studies on mouse Nrf2 mutants have shown the factor to be protective against cancer, respiratory disease, neurodegeneration, inflammatory and autoimmune conditions, and several other pathologies (Osburn and Kensler, 2008). Chemical compounds that stimulate Nrf2 activity have efficacy in numerous mouse models of disease (Yu and Kensler, 2005). In addition, evidence published by us and others suggests Nrf2 as an evolutionarily conserved modulator of longevity (Onken and Driscoll, 2010; Sykiotis and Bohmann, 2008; Tullet et al., 2008). Genetic activation of Nrf2 signaling can extend lifespan in flies and worms; and the C. elegans Nrf2 homologis required for the life-extending effects of caloric restriction (Bishop and Guarente, 2007; Onken and Driscoll, 2010). The question of whether and how the anti-aging function of Nrf2 becomes ineffective in old organisms is not resolved. Here we present evidence that MafS plays a critical role in maintaining oxidative stress resistance and extending health span in aging flies.
Results
MafS interacts physically with CncC
In a previous study we had identified CncC, the signal responsive Drosophila Nrf2 homolog, and its cognate inhibitor, the Drosophila Keap1 (Sykiotis and Bohmann, 2008). Once released from Keap1-mediated repression, mammalian Nrf2 accumulates in the nucleus and binds DNA as an obligate heterodimer with members of the small Maf protein family, such as MafF, MafG, and MafK (Blank, 2008). In contrast, the C. elegans Nrf2 homolog Skn-1functions as a monomer (Blackwell et al., 1994). We therefore asked whether the Drosophila Nrf2 homolog, CncC, requires a dimerization partner, and whether such a protein might make functional contributions to stress defense and maintenance of organism homeostasis.
The sole Drosophila member of the small Maf family is the 132-amino acid MafS protein. MafS has previously been shown to form DNA-binding heterodimers with CncB, a splice variant produced from the same locus as CncC (Veraksa et al., 2000). The CncB isoform controls aspects of embryonic development, but unlike CncC, lacks DLG or ETGE motifs which mediate the interaction with Keap1 (see Fig. 1B). As CncC and CncB share the same leucine zipper dimerization domain, it seemed plausible that MafS would also interact with CncC. To test this prediction, epitope-tagged versions of MafS (MafS3xHA) and CncC (CncC3xFlag) were expressed in Drosophila S2 cells. Co-immunoprecipitation experiments confirmed that the two proteins could bind to each other, as their mammalian homologues do (Fig. 1B).The physical interaction between MafS and CncC suggests that heterodimers of the two proteins might bind to target gene promoters. Consistent with this prediction, chromatin immunoprecipitation (ChIP) experiments showed that MafS can associate with DNA sequences comprising predicted AREs upstream of several CncC-regulated genes (Fig. 1C).
MafS interacts genetically with CncC
To test whether MafS and CncC might cooperate functionally in vivo, we conducted genetic interaction experiments in the Drosophila eye, a system in which we previously documented the regulatory relationship between CncC and Keap1 (Sykiotis and Bohmann, 2008). Overexpression of CncC under the control of the eye-specific sep-Gal4driver severely disrupts eye development, causing ommatidial loss and a rough eye phenotype (Fig. 1D, panel 7). This effect, presumably caused by the inappropriate activation of CncC-dependent gene expression, abated when MafS activity was suppressed by the simultaneous expression of a MafS-specific RNAi construct in the developing eye (Fig. 1D, panel 9). Conversely, over-expression of MafS along with CncC caused a more severe ommatidial loss and rough eye phenotype (Fig. 1D, panel 8). MafS overexpression or knock-down, or the presence of the sep-Gal4 driver or any of the UAS transgenes by themselves did not elicit any noticeable phenotypic effects in the eye (Fig. 1D, panels 1–6). We conclude that MafS is required for CncC-dependent phenotypes in vivo in a manner that recapitulates the described properties of mammalian Nrf2 and small Maf proteins.
MafS is required for CncC-dependent induction of gene expression
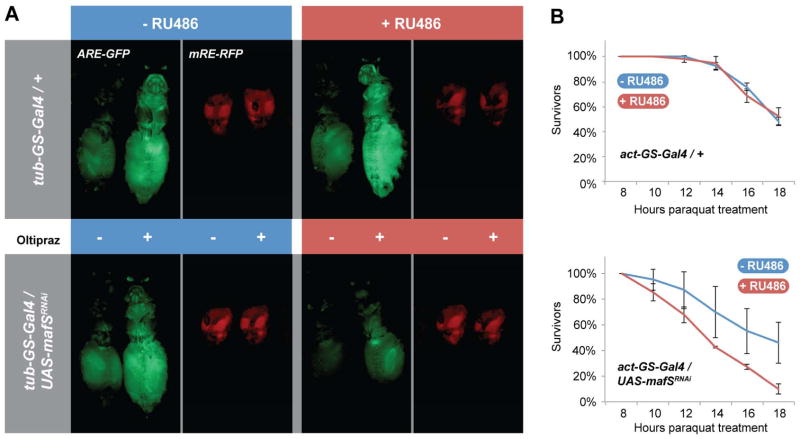
The results described above suggested cooperation between MafS and CncC in ARE-mediated target gene activation. To directly test this prediction, we used Drosophila lines carrying a CncC-responsive GFP reporter transgene (Chatterjee and Bohmann, 2012). In this system, activation of the CncC pathway, for example by feeding the cancer chemopreventive drug oltipraz, causes increased fluorescence that can be monitored in live flies (Fig. 2A). To assess the contribution of MafS to this response, a MafS knock down transgene was expressed under the control of the tub-GS-Gal4 driver, which is ubiquitously inducible by RU486. Knock down of MafS upon exposure of the flies to dietary RU486 decreased ARE activity under both normal and oltipraz-stimulated conditions (Fig. 2A). RU486 itself had no apparent effect (Fig. 2A). We conclude that MafS contributes to both basal and signal-dependent activation of ARE-mediated transcription.
Figure 2. MafS is required for CncC-target gene expression and oxidative stress resistance.
A. Knock-down of MafS decreases basal and inducible CncC reporter gene activity in adult flies. mafSRNAi was ubiquitously expressed in Drosophila adults under the control of the RU486-inducible driver tubulin Gene Switch Gal4 (tub-GS-Gal4). The flies also carried the CncC-responsive ARE-GFP reporter (Sykiotis and Bohmann, 2008). Animals were treated, as indicated, with oltipraz to stimulate CncC activity and/or with 300 μM RU486 to induce the expression of mafSRNAi. Both basal and induced activities of the CncC reporter were suppressed in the presence of mafSRNAi. In a control experiment flies carrying the tub-GS-Gal4 driver, but not the UAS-mafSRNA itransgene showed that RU486 itself has no effect on oltipraz-induced CncC activity (upper panels). A parallel experiment was conducted using a control reporter in which the ARE is mutated to render it unresponsive to CncC (mRE-RFP, red images). The low level of control reporter gene activity arising from this reporter was not affected by mafSRNAi expression, thus ruling out an unspecific effect that might have been caused by general suppression of gene activity or viability.
B. MafS knock down increases sensitivity to paraquat. MafSRNAi was expressed in Drosophila adults under the control of actin-GS-Gal4 driver, causing a knockdown of mafS, but no lethality. The presence of 300 μM RU 486 in the diet had no effect on paraquat sensitivity in flies carrying the actin-GS-Gal4 driver only (upper graph). In contrast, flies carrying both actin-GS-Gal4 driver and UAS-mafSRNAi showed increased paraquat sensitivity when the expression of the RNAi construct was induced by RU486. Error bars indicate standard deviations of three biologically independent replicates (each replicate with a cohort of ~25 flies) arising from three independent crosses. p-values were calculated by two-way ANOVA using Qi Macros software.
Like Nrf2 factors in other species, CncC protects flies against acute oxidative insults by regulating the expression of antioxidants and other stress defense proteins (Sykiotis and Bohmann, 2008). Based on the requirement of MafS for ARE activation, we expected that loss of MafS function would cause a stress sensitive phenotype. Indeed, when mafSRNAi was ubiquitously expressed in adult flies under the control of the actin-GS-Gal4 driver, survival upon exposure to paraquat, are active oxygen species-generating chemical, was markedly reduced compared to controls (Fig. 2B).
MafS counteracts age-associated functional decline
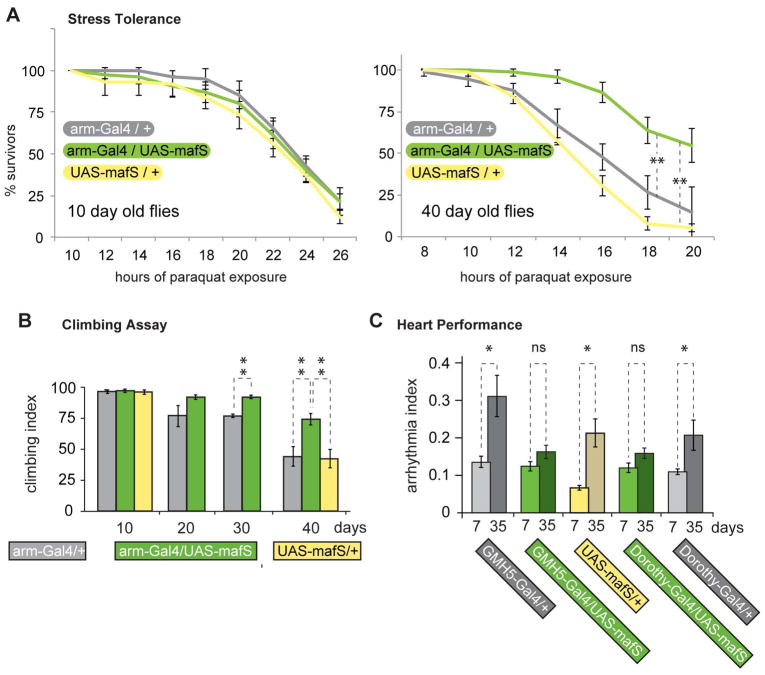
One reason for the age-associated accumulation of oxidative damage is that the molecular defense mechanisms against xenobiotic insults lose effectiveness as organisms become older (Supplemental Fig. 1, Ben-Zvi et al., 2009; Heydari et al., 2000; Kourtis and Tavernarakis, 2011; Przybysz et al., 2009). It is likely that this effect contributes to age-associated frailty and disease. To investigate whether declining stress resistance is connected to a loss of Nrf2 function, we asked whether boosting Nrf2 activity might delay age-associated functional decline. Thus, we measured the effect of MafS over expression on the sensitivity to paraquat exposure in young and old flies (Fig. 3A). We exposed animals in which MafS was transgenically over-expressed (genotype: arm-Gal4 / UAS-mafS) as well as matching controls without transgenic MafS expression (UAS-mafS/+ or arm-Gal4/+) to semi-lethal doses of paraquat. In young animals (10 days after hatching) MafS expression had no measurable effect on surviving paraquat-induced stress, nor did it cause any other noticeable phenotypic difference between arm-Gal4 / UAS-mafS and control flies. However, MafS expression had a marked protective effect in 40-day-old animals (Fig. 3A). Evidently, the expression of MafS counteracted the decline in the resistance to acute paraquat toxicity over the organism’s lifespan.
Figure 3. MafS over-expression counteracts the loss of stress resistance, motor function, and heart performance in aging flies.
A. Ubiquitous over-expression of MafS increases paraquat resistance in old, but not in young flies. Flies of different ages over-expressing MafS (arm-Gal4 / UAS-mafS) or controls carrying the driver or the UAS construct alone (arm-Gal4/+ and UAS-mafS / +) were treated with paraquat and their survival was recorded over a period of up to 26 hours, as detailed in Experimental Procedures. Percent survival over time is plotted for each genotype. Error bars indicate standard deviations of three biologically independent replicates (each with a cohort of ~25 flies) in which flies were collected from separate crosses. Statistical significance was calculated by two-way ANOVA using Qi Macros software. The two asterisks represent p-values less than 0.003.Note that the protective effect of MafS overexpression in old flies is evident and statistically significant relative to both controls, the driver or the UAS construct alone. This indicates that the effect is not influenced by different genetic backgrounds.
B. MafS expression delays the loss of motor function in aging flies. 10, 20, 30 and 40 day old flies of the genotypes arm-Gal4/+, arm-Gal4 / UAS-mafS and UAS-mafS/+were scored for their climbing ability as described in Experimental Procedures. This assay detected no difference between the three genotypes when young flies were tested. Older arm-Gal4 / UAS-mafS flies, however, performed better than the two control groups of matched ages, with progressively larger differences. Error bars indicate standard deviations of three biologically independent replicates. Statistical significance was calculated using two-way ANOVA. The asterisks represent p-values less than 0.002.
C. MafS expression ameliorates the age-associated decline in heart performance. Heart rhythmicity was analyzed in young flies (7 days after hatching) or in 35-day-old flies of the indicated genotypes. In control flies (Gal4 drivers or UAS-mafS transgene alone, grey bars) the heartbeats became irregular with age, i.e. the arrhythmia index increased, as previously reported (Ocorr et al., 2007). In contrast, overexpression of MafS, either in the myocardium (GMH5-Gal4 / UAS-mafS), or in the pericardial cells (Dorothy-Gal4 / UAS-mafS) prevented the decline in cardiac performance as manifested by an incremental, not statistically significant increase of age-associated arrhythmias (green bars).
We next investigated whether the salutary effect of MafS over-expression on paraquat resistance in aging flies might be paralleled by a more general gain in fitness. First, control and MafS-expressing flies of different ages were examined in a climbing assay, a standardized test for motor function. The performance of wild type flies in this test declines in the later weeks of life (Barone et al., 2011). Similar to the effects on paraquat toxicity, MafS over-expression did not benefit newly hatched flies, but significantly delayed the loss of motor function with age (Fig. 3B).
As a second, independent test for the effect of MafS on health span we conducted assays of heart function. As in other species, the performance of the heart declines in Drosophila with increasing age (Wessells and Bodmer, 2004). The deterioration of heart function can be assessed quantitatively by measuring parameters such as contraction, heart rate, or arrhythmia (reviewed in Nishimura et al., 2011). To test if MafS might protect the heart from the sequelae of aging, we expressed MafS under the control myocardium- or pericardium-specific drivers. As expected, control flies displayed a significant increase in arrhythmia susceptibility with progressive age; MafS-expressing flies, however, showed a much milder increase in arrhythmia susceptibility with age (Fig. 3C). As in the other functional assays (Fig.3A, 3B), expression of MafS in young flies had no notable effect, indicating that the over-expression of the protein counteracts an age-dependent degenerative process.
MafS restores the inducibility of antioxidant transcriptional responses
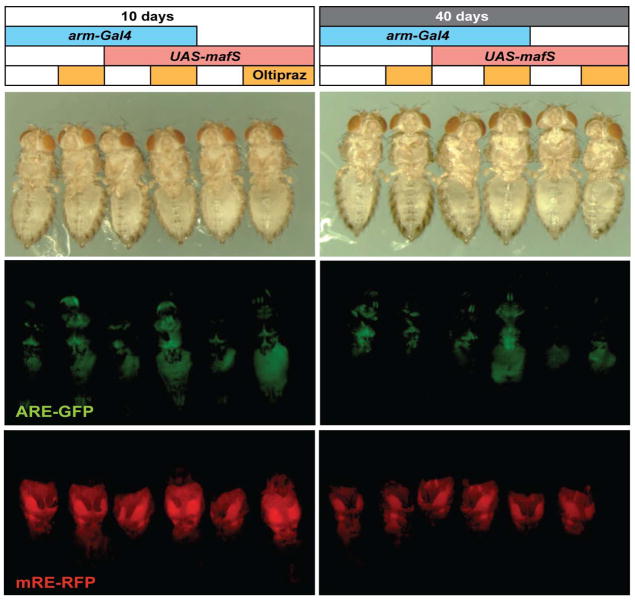
To explore how MafS over-expression might protect aging organisms against oxidative stress and functional decline, we assayed CncC/MafS-regulated gene expression in flies of different ages. As shown previously, in young animals CncC reporter transgene activity is strongly inducible by oxidative stress or by the cancer chemopreventive agent oltipraz (Sykiotis and Bohmann, 2008). In control flies (arm-Gal4/+ or UAS-mafS/+),this signal-dependent activation of CncC became blunted or was completely abolished with increasing age (Fig. 4).However, over-expressing MafS markedly ameliorated this age-associated loss of gene activation; in old arm-Gal4 / UAS-mafS flies the inducibility of the CncC reporter was largely preserved. We conclude that elevated levels of MafS can maintain ARE sin a signal-responsive state in aging animals.
Figure 4. Age-associated decline of CncC reporter gene activation by oltipraz is rescued by MafS over-expression.
Control flies (arm-Gal4/+ or UAS-mafS/+) or flies over expressing MafS (arm-Gal4 / UAS-mafS), were maintained on standard food for 10 days (left panels) or 40 days (right panels). All flies carried a CncC-responsive GFP reporter (ARE-GFP, (Chatterjee and Bohmann, 2012). Young flies (10days old, left panels) of all genotypes showed efficient reporter activation after transfer to food containing 1 mM of the Nrf2 inducer oltipraz for 48 hours. This response was lost in 40dayold UAS-mafS/+andarm-Gal4 / + flies, which failed to efficiently induce ARE-GFP reporter activity in response to oltipraz feeding (middle). In contrast, 40 day old arm-Gal4 / UAS-mafS flies retained the ability to efficiently induce the reporter in response to oltipraz (middle right panel, central two flies). The lower panels show a parallel experiment using the control reporter which is not responsive to CncC. The mRE reporter was constructed with the DSred.T4 gene which can be visualized by its red fluorescence (mRE-RFP). The activity of this mutant reporter did not change with age or oltipraz exposure. Images of the mRE reporter flies where taken at a higher gain because the activity of the mutant reporter is much lower than that of the intact ARE reporter.
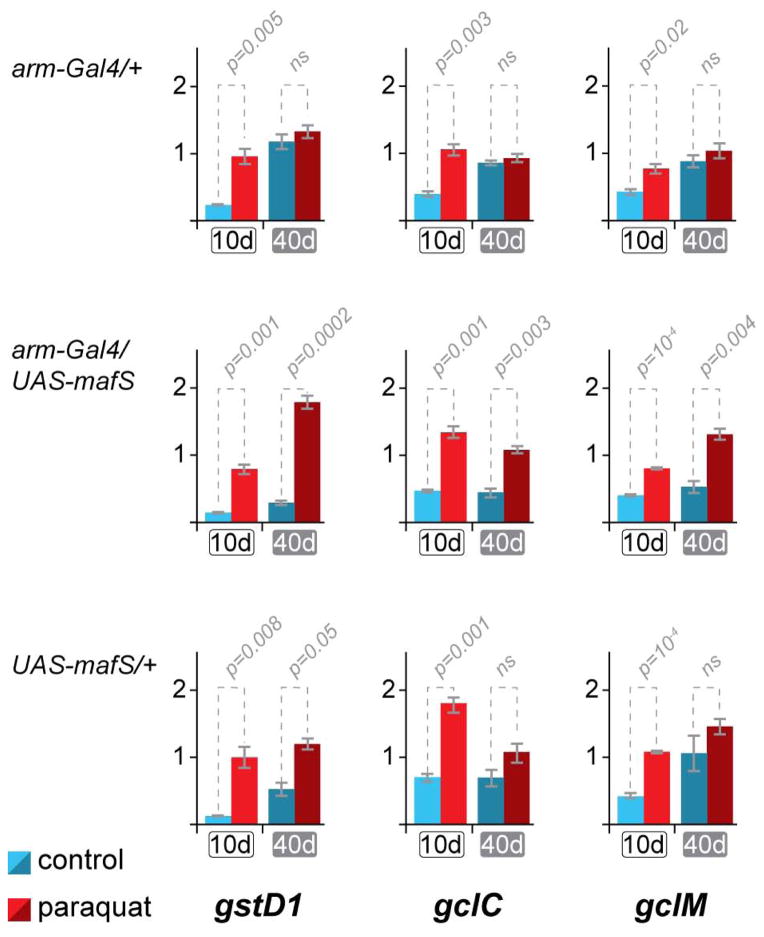
Next, we tested whether MafS over-expression might affect the age-dependent response profiles of endogenous CncC target genes in the same manner. These experiments employed paraquat, a strong activator of Nrf2-inducible RNAs (Ishii et al., 2000; Sykiotis and Bohmann, 2008). Consistent with the reporter gene analyses (Fig. 4), the expression of three conserved CncC/Nrf2 target genes (gstD1, gclc and gclm)was strongly induced by oxidative stress in young flies: the expression levels of these genes differed significantly when comparing mock and paraquat-treated samples. However, the same genes were only weakly responsive in 40 day old flies, where the induction by paraquat was found to be marginal or not statistically significant (Fig. 5). Notably, this loss of responsiveness was substantially prevented in MafS over-expressing flies. Flies over expressing Maf-S throughout their lives retain significant paraquat responsiveness of Nrf2 target gene expression, even at an age of 40 days. Qualitatively similar results were observed using oltipraz as an inducer (not shown). We conclude that over-expression of MafS throughout the lifespan of Drosophila ameliorates the loss of signal-responsive Nrf2 target gene expression and the age-associated decline of fitness and stress resistance.
Figure 5. MafS restores the age-associated loss of inducibility of endogenous CncC target genes by oxidative stress.
10 day old and 40 day old flies over expressing MafS (arm-Gal4/UAS-mafS) and control flies (arm-Gal4/+ or UAS-mafS/+) of the same ages were exposed to paraquat(red bars) or mock treated (blue bars). After 12 hours of treatment 8 flies from each group were collected for quantitative RT-PCR analysis to measure the mRNA expression levels of the CncC target genesgstD1, gclC, and gclM. These genes are known to be inducible by pharmacological or genetic activation of CncC and their promoters harbor CncC binding sites as mapped by the Mod Encode project. The y-axis indicates the quantification of the respective mRNA relative to the internal reference, the mRNA of the rp49 gene, which is not regulated by CncC. The fold induction of each gene’s expression in response to paraquat decreases in the controls flies when they age (top and bottom panels), but the over-expression of MafS restores the inducibility (middle panels). Error bars indicate standard deviations of three biologically independent replicates arising from three independent crosses. Statistical significance (p-values) was calculated using Student’s T-Test.
Discussion
A central question in the aging field is why oxidative damage accumulates in the cells and tissues of older organisms. One possible cause for this phenomenon would be that old organisms might express lower levels of stress defense and antioxidant proteins than young ones. However, published genomic analyses, in which gene expression profiles between young and old organisms were compared, as well as our own unpublished studies do not support this explanation (Girardot et al., 2004; Pletcher et al., 2002). While the steady state levels of some of the relevant transcripts are low in old compared to young animals, this trend is not consistent across studies or uniform among genes. Antioxidant and Nrf2 target genes do not stand out as a group in which consistent changes of steady state RNA levels become manifest throughout the lifespan (Girardot et al., 2004; Pletcher et al., 2002). However, the data presented here in concert with other recently published evidence indicate that older organisms lose the ability to regulate Nrf2 target genes in response to stress (Przybysz et al., 2009; Ungvari et al., 2011). The loss of stress-responsive expression is a more striking and consistent age-associated change among Nrf2 target genes than any differences in steady state RNA levels.
Interestingly, a loss of stress responsiveness with age has also been noted in other signaling pathways such as HSF1, Hif-1 and p38 (Ben-Zvi et al., 2009; Frenkel-Denkberg et al., 1999; Hsieh et al., 2003). Such data suggest that declining signaling competence limits health span and contributes to the increased oxidative damage and loss of vital functions during aging. The loss of stress-inducible Nrf2 target gene activation also manifests in a paradoxical phenomenon that has been observed in a number of diseases associated with increased oxidative load. In spite of the increased oxidative stress in neurons from Alzheimer’s patients’ brains or in alveolar macrophages from lungs of COPD patients, the levels of Nrf2 activity are low, suggesting that these diseases coincide with a state in which Nrf2 can no longer be properly activated. To what extent this defect is cause or/and consequence of such pathologies is an important unanswered issue (reviewed in Sykiotis and Bohmann, 2010).
The phenomenon of age-associated loss of stress responsive gene expression raises a number of questions: at which level is the signal flow between extracellular stimulus and gene activation interrupted in old animals? Can this decline in signaling competence be reversed? How important is this phenomenon for the senescent phenotype? The data presented here suggest a potential nuclear mechanism by which stress signaling can break down. We presented evidence that the expression levels of MafS are critical for the maintenance of stress resistance in older flies. Supplementing the system with transgenic MafS can counteract the decline both of Nrf2 target gene responsiveness and of fitness.
Our findings suggest that MafS expression can extend health span as indicated by beneficial effects on climbing activity or heart rhythmicity. However, it should be noted that these are indicators only, and not substitutes for actual measurements of the many other aspects of both mobility and cardiac function, which may or may not respond the same way as the indicators chosen.
The expression levels of the endogenous MafS mRNA do not decline with age, at least when measured at the whole organism level (Supplemental Fig. 2). A possible explanation for the beneficial effect of MafS over-expression specifically in older animals would be that higher levels of MafS expression counteract a stochastic decline of Nrf2 functionality or chromatin occupancy. Future studies should investigate whether the function of MafS as a genomic placeholder for Nrf2 is compromised in older flies causing epigenetic disorganization, which might explain the loss of regulated stress defenses. Such a loss of epigenetic organization as a cause for aging has been discussed before (Munoz-Najar and Sedivy, 2010) and it will be interesting to investigate a possible function for MafS in this context.
Experimental Procedures
Fly stocks and maintenance
In all experiments, 0 to 1 day old flies were collected, allowed to mate overnight, and then separated by sex. The flies were maintained on standard cornmeal- and molasses-based food at 25°C, and were transferred to fresh food every two days until they reached the age at which the experiments were to be conducted. UAS-mafS and UAS-mafSRNAi transgenic flies were generated by standard P element-mediated transgenesis on a w1118 background. The mafS coding sequence (NM_137656.3) was amplified by PCR from embryonic cDNA and inserted into the XbaI site of pUAST-3xHA (Sykiotis and Bohmann, 2008) to generate pUAS-mafS, which expresses MafS fused at its C-terminus to a 3xHA epitope tag. The same amplicon was also cloned into the AvrII and NheI sites of the pWiz vector (Lee and Carthew, 2003) in opposite orientations (sense and antisense, respectively) to generate UAS-mafSRNAi; this construct expresses a hairpin encompassing the whole coding sequence of mafS. The UAS-cncC, UAS-cncCRNAi, and gstD:GFP flies have been described (Sykiotis and Bohmann, 2008). Flies carrying thesep-Gal4driver in which the sevenless gene enhancer and promoter confer eye specific expression were obtained from MarekMlodzik; tubGS10 flies were gifts from Scott Pletcher. arm-Gal4 and D42-Gal4 were procured from Bloomington Stock Center (BL-1560 and BL-8816 respectively). For the analysis of heart function, GMH5 (myocardial driver,(Wessells and Bodmer, 2004) and Dorothy Gal4 (pericardial driver,(Kimbrell et al., 2002) were used.
To induce gene expression using gene switch drivers, flies were transferred to food containing 300 μM RU486 or a solvent control for 4 days to induce transgene expression prior to conducting stress experiments or scoring reporter activity. 10 mM RU486 was prepared in 80% ethanol and mixed with standard fly food at 50°C to a final concentration of 300 μM. A corresponding amount of 80% ethanol was added to control food.
Cell culture, transfection, co-immunoprecipitation and western blot
S2 cells were cultured and HA tagged MafS and Flag tagged CncC were expressed using a calcium phosphate transient transfection protocol. Co-immunoprecipitation and western blot were carried out using anti-HA (Roche Diagnostics GmbH, Mannheim, Germany) and anti-Flag (Sigma-Aldrich Co, St. Louis, MO) antibodies.
Reporter induction and stress sensitivity assays
To stimulate CncC activity, flies were maintained for48 hours on food containing 1mMoltipraz. Oltipraz was first dissolved in DMSO before mixing with food; control flies were given food with 2% DMSO. Wings and legs were dissected for easier inspection before observing the flies under a fluorescence microscope. For stress sensitivity assays, flies were subjected to dry starvation for 1 hour and then exposed to different concentrations of paraquat dissolved in a 5% sucrose solution. Because the sensitivity to paraquat increases with age (Supplemental Fig. 1), its dose was adjusted such that ~50% of treated animals died within 24 hrs (25 mM for 10 day old flies and 10 mM for 40 day old flies). Flies were scored as live or dead at multiple time points over 24 hrs.
Transcript quantification
RNA extraction, cDNA preparation and quantitative RT-PCR were performed as described previously(Sykiotis and Bohmann, 2008). The following primer pairs were used for amplifying specific transcripts:mafS, 5′-agcaggtagagaggaagaagaagaca-3′ and 5′-acatggcgaaagtggtgcct-3′;gclC, 5′-ataccgaccataacgaagaagtaccaga-3′ and 5′-atacttatctcattccgtccattctccgt-3′;gstD1, 5′-ccgtgggcgtcgagctgaaca-3′ and 5′-gcgcgaatccgttgtccacca-3′;gstE1, 5′-cgggcgagcacctgagcgag-3′ and 5′-ggcggcaatggcatgggagt-3′;rp49, 5′-caagatgaccatccgcccagca-3′ and 5′-gcttgcgccatttgtgcgaca-3′.
Climbing assay
The negative geotactic climbing assay was performed as described previously with minor modifications (Barone et al., 2011). Briefly, cohorts of 20 flies of the indicated age and genotype were transferred into two empty vials, which were connected at their opening with adhesive tape, creating an enclosed transparent tube, approximately 15 cm tall. After the transfer, flies were allowed to rest for 30 minutes at room temperature. The tubes were then tapped to bring all the flies to the bottom. The movement of the flies during the experiments was recorded with a digital camera. The number of flies passing a 5 cm mark on the vial in 20 seconds was counted. For each cohort of 20 flies the experiment was repeated 10 times. A total of three cohorts from separate crosses were analyzed and scored for each age group and genotype.
Heart performance/arrhythmia assay
Flies were briefly anesthetized with fly nap (Carolina Biol., Corp., Burlington, NC) and dissected as previously described (Fink et al., 2009; Ocorr et al., 2007). Movies at the rate of up to200 frames per second were taken for 30 seconds by using a Hamamatsu CCD digital camera (McBain Instruments, Chatsworth, CA) on a Leica DM LFSA microscope with a 10x water immersion lens and HC Image imaging software. The Images were analyzed using Optical Heart Beat Analysis software (Fink et al., 2009). Arrhythmia Index (AI) was calculated as the standard deviations of heart period (HP) normalized by the median HP in each fly. Same numbers of males and females were analyzed and the average values of AI are represented.
Chromatin immunoprecipitation (ChIP)
Third in star larvae were collected, dried on blotting paper, frozen in liquid nitrogen, and crushed with a chilled mortar and pestle on dry ice. The resulting powder was processed for chromatin immunoprecipitation. The primer pairs employed flank ARE consensus sequences in the promoter regions of known CncC responsive genes: gstD1,5′-agagggggctgctagttgtt-3′ and 5′-atcaatcgtctccacacacc-3′; gclC,5′-atgacatggcacaatccaaa-3′ and 5′-tcgttaattgcaacgtcacc-3′; keap1, 5′-ctacgcgtgcttttggtaaa-3′ and 5′-tacattgccaaccggaattt-3′ hsp26, 5′-ttaataaagaggaaaaccag-3′ and 5′-aaaaataaaactaactaacctt-3′.
Supplementary Material
Groups of 10, 20, 30 or 40 day old wild type flies were fed with 5% sucrose solution with or without 18mMparaquat. After 15 hours flies were scored as live or dead. The bars in the histogram represent the ratios of surviving flies after paraquat treatment versus mock treatment at different ages. Paraquat sensitivity increases significantly with age. Error bars indicate standard deviations of three biologically independent replicates. Statistical significance was calculated using Student’s t-test.
Total RNA was harvested from Groups of 10, 20, 30, 40 and50 day old male flies for cDNA preparation. Quantitative real-time PCR analysis show ednegligible/marginal differences in expression levels of cncC, keap1 and mafS mRNAs among the different samples. Gene expression levels were normalized to the ribosomal gene rp49.Error bars indicate standard deviations of three biologically independent replicates where each replicate contains a cohort of ~10 flies.
Acknowledgments
We are grateful to Henri Jasper, Mirka Uhlirova, and Nirmalya Chatterjee for helpful discussion and reagents, and to Christine Sommers for excellent technical assistance at various stages of the work. We thank Juliann Beaulieu and Andrew Pitoniak for comments on the manuscript. MarekMlodzik, William McGinnis, and Scott Pletcher kindly contributed Drosophila stocks. This work was supported by NIH grant R01 AG039753-01 and NYSTEM grant N08G-048 to DB and HL54732, HL098053 and AG033456 to RB.
Footnotes
Author contributions
MMR participated in designing the study, performed most experiments, interpreted data, and co-wrote the manuscript; GPS generated reagents, designed and performed experiments, interpreted data, and co-wrote the manuscript; MN and RB designed, performed, and interpreted the heart experiments. DB designed and oversaw the study, interpreted data, and wrote the manuscript.
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science (New York, NY. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Barone MC, Sykiotis GP, Bohmann D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis Model Mech. 2011;4:701–707. doi: 10.1242/dmm.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Bowerman B, Priess JR, Weintraub H. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science (New York, NY. 1994;266:621–628. doi: 10.1126/science.7939715. [DOI] [PubMed] [Google Scholar]
- Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? Journal of molecular biology. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Burhans WC, Heintz NH. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free radical biology & medicine. 2009;47:1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Bohmann D. A versatile φC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS ONE. 2012 doi: 10.1371/journal.pone.0034063. in press, w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians ES, Benjamin IJ. Proteostasis and REDOX state in the heart. Am J Physiol Heart Circ Physiol. 2011;302:H24–37. doi: 10.1152/ajpheart.00903.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free radical biology & medicine. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. BioTechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- Girardot F, Monnier V, Tricoire H. Genome wide analysis of common and specific stress responses in adult drosophila melanogaster. BMC genomics. 2004;5:74. doi: 10.1186/1471-2164-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg KB, Beskow A, Lundin D, Davis MM, Young P. Basic leucine zipper protein Cnc-C is a substrate and transcriptional regulator of the Drosophila 26S proteasome. Molecular and cellular biology. 2011;31:897–909. doi: 10.1128/MCB.00799-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Experimental cell research. 2000;256:83–93. doi: 10.1006/excr.2000.4808. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Rosenblatt JI, Papaconstantinou J. Age-associated changes in SAPK/JNK and p38 MAPK signaling in response to the generation of ROS by 3-nitropropionic acid. Mechanisms of ageing and development. 2003;124:733–746. doi: 10.1016/s0047-6374(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. The Journal of biological chemistry. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Kimbrell DA, Hice C, Bolduc C, Kleinhesselink K, Beckingham K. The Dorothy enhancer has Tinman binding sites and drives hopscotch-induced tumor formation. Genesis. 2002;34:23–28. doi: 10.1002/gene.10134. [DOI] [PubMed] [Google Scholar]
- Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. The EMBO journal. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in molecular medicine. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Munoz-Najar UM, Sedivy JM. Antioxidants & redox signaling. 2010. Epigenetic Control of Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Ocorr K, Bodmer R, Cartry J. Drosophila as a model to study cardiac aging. Experimental gerontology. 2011;46:326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Health span via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutation research. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Przybysz AJ, Choe KP, Roberts LJ, Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mechanisms of ageing and development. 2009;130:357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. Free radical biology & medicine. 2011. The redox stress hypothesis of aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Developmental cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-Activated Cap’n’collar Transcription Factors in Aging and Human Disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JMA, Hertweck M, An JH, Baker J, Oliviera RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity promoting factor SKN-1 by Insulin like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraksa A, McGinnis N, Li X, Mohler J, McGinnis W. Cap ‘n’ collar B cooperates with a small Maf subunit to specify pharyngeal development and suppress deformed homeotic function in the Drosophila head. Development (Cambridge, England) 2000;127:4023–4037. doi: 10.1242/dev.127.18.4023. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Bodmer R. Screening assays for heart function mutants in Drosophila. BioTechniques. 2004;37:58–60. 62, 64. doi: 10.2144/04371ST01. passim. [DOI] [PubMed] [Google Scholar]
- Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutation research. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Groups of 10, 20, 30 or 40 day old wild type flies were fed with 5% sucrose solution with or without 18mMparaquat. After 15 hours flies were scored as live or dead. The bars in the histogram represent the ratios of surviving flies after paraquat treatment versus mock treatment at different ages. Paraquat sensitivity increases significantly with age. Error bars indicate standard deviations of three biologically independent replicates. Statistical significance was calculated using Student’s t-test.
Total RNA was harvested from Groups of 10, 20, 30, 40 and50 day old male flies for cDNA preparation. Quantitative real-time PCR analysis show ednegligible/marginal differences in expression levels of cncC, keap1 and mafS mRNAs among the different samples. Gene expression levels were normalized to the ribosomal gene rp49.Error bars indicate standard deviations of three biologically independent replicates where each replicate contains a cohort of ~10 flies.