Abstract
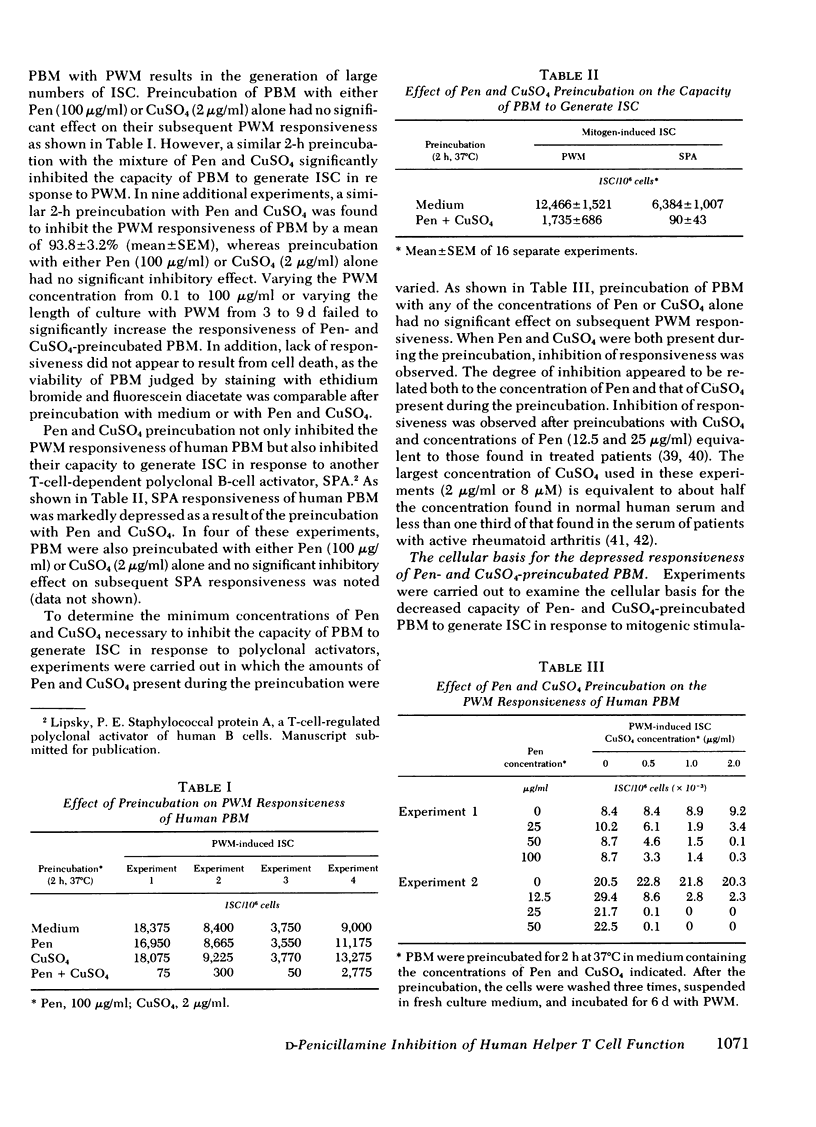
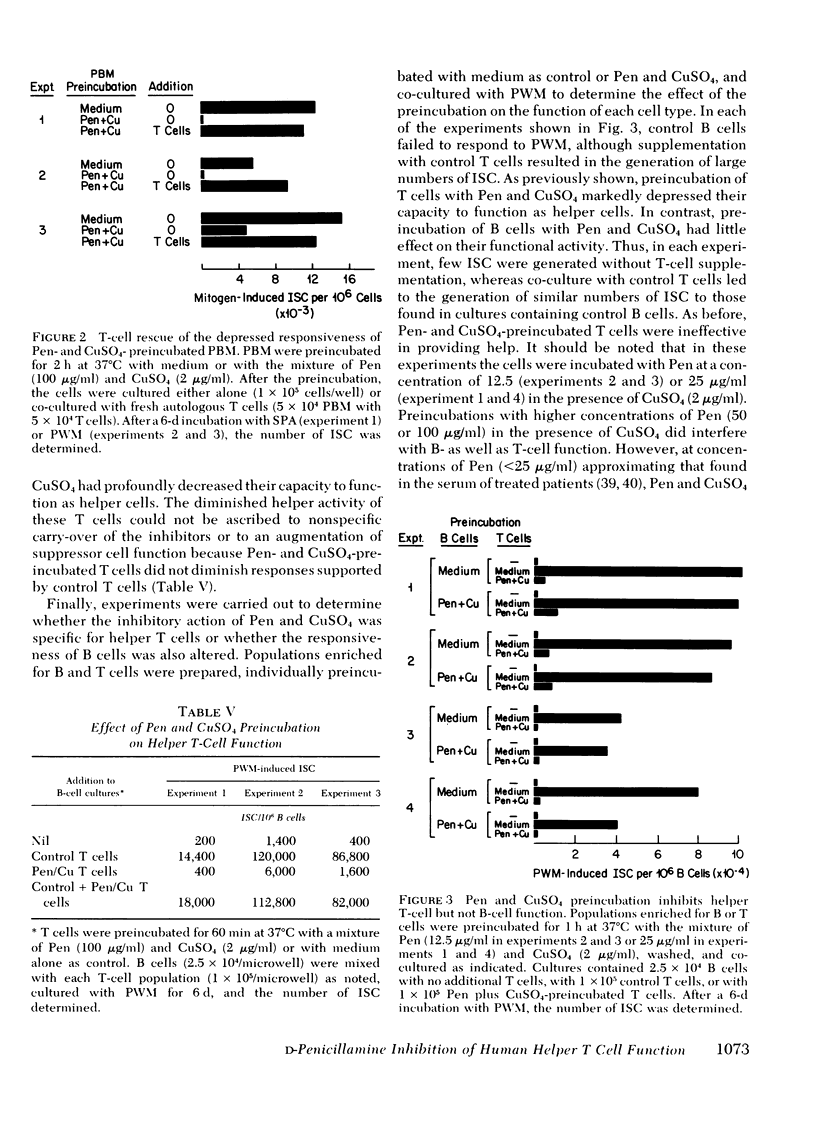
The effect of d-penicillamine (Pen) and mixtures of Pen and copper sulfate on the capacity of normal human peripheral blood mononuclear cells (PBM) to generate immunoglobulin-secreting cells (ISC) in response to the T-cell-dependent polyclonal B-cell activators pokeweed mitogen (PWM) and staphylococcal protein A (SPA) was examined. PBM obtained from normal individuals were incubated for 1-2 h at 37°C with medium alone, Pen, CuSO4, or a mixture of Pen and CuSO4. After washing, the cells were incubated for 6-7 d with PWM or SPA and then, with a reverse hemolytic plaque assay, assayed for the number of ISC generated. Preincubation of PBM with either Pen (100 μg/ml) or CuSO4 (2 μg/ml) did not alter the subsequent capacity of the cells to generate ISC in response to PWM or SPA. In contrast, responsiveness to both mitogens was nearly abolished when PBM were similarly preincubated with a mixture of Pen and CuSO4. Inhibition of responsiveness could not be ascribed to cell death, carry-over of the inhibitors, or an alteration in the concentration of PWM or the length of incubation yielding maximum responses. Co-culture experiments demonstrated that Pen and CuSO4 preincubation had not caused augmented suppressor cell function. Experiments in which PBM were separated into adherent and nonadherent populations indicated that Pen and CuSO4 preincubation inhibited the responsiveness of the nonadherent cells but did not alter the accessory cell function of monocytes. To determine whether Pen and CuSO4 preincubation effected T- or B-cell function, PBM were separated into B- and T-cell-enriched populations, individually preincubated with Pen and CuSO4, and then co-cultured with PWM. The results indicated that Pen and CuSO4 markedly inhibited helper T-cell function and had little effect on the capacity of B cells to generate ISC. The observation that in the presence of CuSO4 Pen inhibits helper T-cell activity may, in part, explain the therapeutic efficacy of Pen in rheumatoid arthritis and especially the capacity of Pen therapy to decrease antiglobulin titers in treated patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTMAN K., TOBIN M. S. SUPPRESSION OF THE PRIMARY IMMUNE RESPONSE INDUCED BY D-L PENICILLAMINE. Proc Soc Exp Biol Med. 1965 Feb;118:554–557. doi: 10.3181/00379727-118-29903. [DOI] [PubMed] [Google Scholar]
- Aaseth J., Munthe E., Førre O., Steinnes E. Trace elements in serum and urine of patients with rheumatoid arthritis. Scand J Rheumatol. 1978;7(4):237–240. doi: 10.3109/03009747809095662. [DOI] [PubMed] [Google Scholar]
- Arrigoni-Martelli E., Bramm E., Huskisson E. C., Willoughby D. A., Dieppe P. A. Pertussis vaccine oedema: an experimental model for the action of penicillamine-like drugs. Agents Actions. 1976 Sep;6(5):613–617. doi: 10.1007/BF01971579. [DOI] [PubMed] [Google Scholar]
- Bailey K. R., Sheffner A. L. The reduction of experimentally induced inflammation by sulfhydryl compounds. Biochem Pharmacol. 1967 Jul 7;16(7):1175–1182. doi: 10.1016/0006-2952(67)90149-9. [DOI] [PubMed] [Google Scholar]
- Berry H., Liyanage S. P., Durance R. A., Barnes C. G., Berger L. A., Evans S. Azathioprine and penicillamine in treatment of rheumatoid arthritis: a controlled trial. Br Med J. 1976 May 1;1(6017):1052–1054. doi: 10.1136/bmj.1.6017.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binderup L., Bramm E., Arrigoni-Martelli E. D-penicillamine and macrophages: modulation of lymphocyte transformation by concanavalin A. Scand J Immunol. 1978 Apr;7(4):259–264. doi: 10.1111/j.1365-3083.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Blackham A., Radziwonik H. The effect of drugs in established rabbit monoarticular arthritis. Agents Actions. 1977 Oct;7(4):473–480. doi: 10.1007/BF01966856. [DOI] [PubMed] [Google Scholar]
- Bluestone R., Goldberg L. S. Effect of D-penicillamine on serum immunoglobulins and rheumatoid factor. Ann Rheum Dis. 1973 Jan;32(1):50–52. doi: 10.1136/ard.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucknall R. C., Dixon A St J., Glick E. N., Woodland J., Zutshi D. W. Myasthenia gravis associated with penicillamine treatment for rheumatoid arthritis. Br Med J. 1975 Mar 15;1(5958):600–602. doi: 10.1136/bmj.1.5958.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalinska-Sadowska H., Baum J. The effect of D-penicillamine on polymorphonuclear leukocyte function. J Clin Invest. 1976 Oct;58(4):871–879. doi: 10.1172/JCI108540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. M., Ford-Hutchinson A. W., Oliver A. M., Smith M. J., Walker J. R. The effects of D-pencillamine and levamisole on leucocyte chemotaxis in the rat. Br J Pharmacol. 1978 May;63(1):119–123. doi: 10.1111/j.1476-5381.1978.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A. J., Davies J., Dormandy T. L., Hamilton E. B., Holt P. J., Mason R. M., Thompson M., Weber J. C., Zutshi D. W. Synthetic D(-)penicillamine in rheumatoid arthritis. Double-blind controlled study of a high and low dosage regimen. Ann Rheum Dis. 1975 Oct;34(5):416–421. doi: 10.1136/ard.34.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. A rapid, quantitative fluorescence assay for cell damage by cytotoxic antibodies. J Immunol. 1970 May;104(5):1303–1306. [PubMed] [Google Scholar]
- Epstein O., De Villiers D., Jain S., Potter B. J., Thomas H. C., Sherlock S. Reduction of immune complexes and immunoglobulins induced by D-penicillamine in primary biliary cirrhosis. N Engl J Med. 1979 Feb 8;300(6):274–278. doi: 10.1056/NEJM197902083000602. [DOI] [PubMed] [Google Scholar]
- GUBLER C. J., LAHEY M. E., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. IX. The transportation of copper in blood. J Clin Invest. 1953 May;32(5):405–414. doi: 10.1172/JCI102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- HUEBNER K. F., GENGOZIAN N. DEPRESSION OF THE PRIMARY IMMUNE RESPONSE BY DL-PENICILLAMINE. Proc Soc Exp Biol Med. 1965 Feb;118:561–565. doi: 10.3181/00379727-118-29905. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Sjoerdsma A. Effect of penicillamine on human collagen and its possible application to treatment of scleroderma. Lancet. 1966 Nov 5;2(7471):996–999. doi: 10.1016/s0140-6736(66)92926-6. [DOI] [PubMed] [Google Scholar]
- Hunneyball I. M., Stewart G. A., Stanworth D. R. The effects of oral D-penicillamine treatment on experimental arthritis and the associated immune response in rabbits. II. The effects on cellular parameters. Immunology. 1978 Jul;35(1):159–166. [PMC free article] [PubMed] [Google Scholar]
- Hunneyball I. M., Stewart G. A., Stanworth D. R. The effects of oral D-penicillamine treatment on experimental arthritis and the associated immune response in rabbits. Immunology. 1978 Jun;34(6):1053–1061. [PMC free article] [PubMed] [Google Scholar]
- JAFFE I. A. Comparison of the effect of plasmapheresis and penicillamine on the level of circulating rheumatoid factor. Ann Rheum Dis. 1963 Mar;22:71–76. doi: 10.1136/ard.22.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFE I. A. Intra-articular dissociation of the rheumatoid factor. J Lab Clin Med. 1962 Sep;60:409–421. [PubMed] [Google Scholar]
- Jaffe I. A. Penicillamine treatment of rheumatoid arthritis: effect on immune complexes. Ann N Y Acad Sci. 1975 Jun 13;256:330–337. doi: 10.1111/j.1749-6632.1975.tb36059.x. [DOI] [PubMed] [Google Scholar]
- Jaffe I. A. The effect of penicillamine on the laboratory parameters in rheumatoid arthritis. Arthritis Rheum. 1965 Dec;8(6):1064–1079. doi: 10.1002/art.1780080606. [DOI] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kendall P. A., Hutchins D. The effect of thiol compounds on lymphocytes stimulated in culture. Immunology. 1978 Jul;35(1):189–201. [PMC free article] [PubMed] [Google Scholar]
- Klamer B., Kimura E. T., Makstenieks M. Effects of oral cysteine, penicillamine and N-acetyl-penicillamine on adjuvant arthritis in rats. Pharmacology. 1968 May;1(5):283–288. doi: 10.1159/000135976. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. The effect of D-penicillamine on mitogen-induced human lymphocyte proliferation: synergistic inhibition by D-penicillamine and copper salts. J Immunol. 1978 Mar;120(3):1006–1013. [PubMed] [Google Scholar]
- Liyanage S. P., Currey H. L. Failure of oral D-penicillamine to modify adjuvant arthritis or immune response in the rat. Ann Rheum Dis. 1972 Nov;31(6):521–521. doi: 10.1136/ard.31.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Nicholls A. C., Jayson M. I., Bailey A. J. Changes in the collagen of synovial membrane in rheumatoid arthritis and effect of D-penicillamine. Clin Sci Mol Med. 1978 Jul;55(1):31–40. doi: 10.1042/cs0550031. [DOI] [PubMed] [Google Scholar]
- Marsden R. A., Vanhegan R. I., Walshe M., Hill H., Mowat A. G. Pemphigus foliaceus induced by penicillamine. Br Med J. 1976 Dec 11;2(6049):1423–1424. doi: 10.1136/bmj.2.6049.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Dawkins R. L., Zilko P. J., Simpson J. A., Leedman R. J. Penicillamine-associated myasthenia gravis, antiacetylcholine receptor and antistriational antibodies. Am J Med. 1977 Nov;63(5):689–694. doi: 10.1016/0002-9343(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Mery C., Delrieu F., Ghozlan R., Saporta L., Simon F., Amor B., Menkes C. J., Delbarre F. Controlled trial of D-penicillamine in rheumatoid arthritis. Dose effect and the role of zinc. Scand J Rheumatol. 1976;5(4):241–247. doi: 10.3109/03009747609099913. [DOI] [PubMed] [Google Scholar]
- Mohammed I., Barraclough D., Holborow E. J., Ansell B. M. Effect of penicillamine therapy on circulating immune complexes in rheumatoid arthritis. Ann Rheum Dis. 1975 Oct;35(5):458–462. doi: 10.1136/ard.35.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G. Neutrophil chemotaxis in rheumatoid arthritis. Effect of D-penicillamine, gold salts, and levamisole. Ann Rheum Dis. 1978 Feb;37(1):1–8. doi: 10.1136/ard.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muijsers A. O., Van de Stadt R. J., Henrichs A. M., Van der Korst J. K. Determination of D-penicillamine in serum and urine of patients with rheumatoid arthritis. Clin Chim Acta. 1979 Jun 1;94(2):173–180. doi: 10.1016/0009-8981(79)90010-x. [DOI] [PubMed] [Google Scholar]
- Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. I. Metabolic and functional abnormalities in soft tissues. J Biol Chem. 1968 Apr 10;243(7):1457–1466. [PubMed] [Google Scholar]
- Nimni M. E., Bavetta L. A. Collagen defect induced by penicillamine. Science. 1965 Nov 12;150(3698):905–907. doi: 10.1126/science.150.3698.905. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Schumacher K., Maerker-Alzer G., Schaaf W. Influence of D-penicillamine on the immune response of mice. Arzneimittelforschung. 1975 Apr;25(4):600–603. [PubMed] [Google Scholar]
- Scudder P. R., Al-Timimi D., McMurray W., White A. G., Zoob B. C., Dormandy T. L. Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis. 1978 Feb;37(1):67–70. doi: 10.1136/ard.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., McMurray W., White A. G., Dormandy T. L. Synovial fluid copper and related variables in rheumatoid and degenerative arthritis. Ann Rheum Dis. 1978 Feb;37(1):71–72. doi: 10.1136/ard.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa Y., Horiuchi Y., Honma M., Kageyama T., Okada T., Azuma T. Clinical evaluation of D-penicillamine by multicentric double-blind comparative study in chronic rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1464–1472. doi: 10.1002/art.1780200804. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J Biol Chem. 1977 Jan 10;252(1):254–259. [PubMed] [Google Scholar]
- Sorenson J. R. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976 Jan;19(1):135–148. doi: 10.1021/jm00223a024. [DOI] [PubMed] [Google Scholar]
- TOBIN M. S., ALTMAN K. ACCELERATED IMMUNE RESPONSE INDUCED BY D-L PENICILLAMINE. Proc Soc Exp Biol Med. 1964 Jan;115:225–228. doi: 10.3181/00379727-115-28876. [DOI] [PubMed] [Google Scholar]
- Zuckner J., Ramsey R. H., Dorner R. W., Gantner G. E., Jr Penicillamine in rheumatoid arthritis. Arthritis Rheum. 1970 Mar-Apr;13(2):131–138. doi: 10.1002/art.1780130205. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., Muijsers A. O., Henrichs A. M., van der Korst J. K. D-penicillamine: biochemical, metabolic and pharmacological aspects. Scand J Rheumatol Suppl. 1979;(28):13–20. doi: 10.3109/03009747909108229. [DOI] [PubMed] [Google Scholar]


