Abstract
Precise control of uterine fluid pH, volume and electrolytes is important for the reproductive processes. In this study, we examined the functional involvement of multiple proteins including Cystic Fibrosis Transmembrane Regulator (CFTR), Cl-/HCO3- exchanger (SLC26A6), sodium-hydrogen exchanger-1 (NHE-1) and carbonic anhydrase (CA) in the regulation of these uterine fluid parameters. Methods: Adult female WKY rats were divided into intact, non-ovariectomised at different oestrous cycle phases and ovariectomised treated with sex-steroids. Following oestrous phase identification or sex-steroid treatment, in-vivo uterine perfusion was performed with and without the presence of these inhibitors: glibenclamide, DIDS, ACTZ and EIPA. The pH, volume, Cl-, HCO3- and Na+ concentrations of the perfusate from different groups were then analyzed. Meanwhile, the expression of CFTR, SLC26A6, NHE-1, CAII and CAXII was visualized by immunohistochemistry (IHC). Results: Parallel increase in the pH, volume, Cl-, HCO3- and Na+ concentrations was observed at estrus (Es), proestrus (Ps) and following 17β-oestradiol (E) treatment, which was inhibited by glibenclamide, DIDS and ACTZ while parallel reduction in these parameters was observed at diestrus (Ds) and following progesterone (P) treatment which was inhibited by ACTZ and EIPA. CFTR and SLC26A6 expression were up-regulated under E dominance, while NHE-1 expression was up-regulated under P dominance. Meanwhile, CA isoenzymes were expressed under both E and P influence. Conclusion: CFTR, SLC26A6 and CA were involved in mediating parallel increase in the uterine fluid volume, pH and electrolyte concentration under E while NHE and CA were involved in mediating the reduction of these parameters under P.
Keywords: uterus, CFTR, SLC26A6, CAII & XII, NHE-1, sex-steroid, oestrous cycle.
Introduction
Precise control of the uterine fluid volume, pH and electrolytes is important for a number of key reproductive events including sperm transport and capacitation and embryo transport and implantation 1. To date, several membrane transporters and enzymes have been proposed to participate in uterine fluid regulation which includes Cystic Fibrosis Transmembrane Regulator (CFTR) 2, Cl-/HCO3- exchanger (SLC26A6) 3, sodium-hydrogen exchanger (NHE) 4 and carbonic anhydrase (CA) 3. The expression of CFTR, a cAMP activated Cl- channel has been reported in the uterus in mice 5, rats 6 and humans 7. Additionally, CFTR activity has also been documented in the endometrial epithelia in-vitro 3. SLC26A6, a Cl-/HCO3-exchanger family has been reported to be expressed in the uterus and was shown to participate in the pH increase under the influence of sex-steroid 3, 8. Meanwhile, the expression of NHE, which is involved in the pH regulation 9, has been reported in the uterus of immature mice 10. CA, which is responsible for a reversible hydration of CO2 to H+ and HCO3- 11 has also been reported to be expressed in the human 12, rat 13 and mouse 14 endometrium.
Few independent studies have reported the changes in one or two of the above parameters under the influence of sex-steroids. Salleh et al 6 reported that sex-steroid affects the volume, Na+ and Cl- concentrations of the uterine fluid as well as the expression of epithelial sodium channel (ENaC) and CFTR which were increased following P and E treatment respectively. This study however did not directly confirm the involvement of these proteins in mediating these changes. In another study, Salleh et al, 4 investigated the effect of sex-steroids on uterine fluid pH and the expression of NHE-1 which was thought to participate in this pH changes. Although this study showed that the pH increased under E and reduced under P, the use of amiloride, a non-specific inhibitor for NHE could limit result interpretation. Furthermore, pH changes were only documented in the steroid replaced ovariectomised rats but not in rats at different phases of the oestrous cycle.
He et al 3 performed an in-vitro study to investigate the involvement of CFTR, SLC26A6 and CA in endometrial surface pH changes under the effect of E and at Es. Using forskolin to stimulate endometrial HCO3- secretion, this study has shown that the surface pH increase was inhibited by antagonists for CFTR, SLC26A6 and CA respectively. The limitation of this in-vitro study was that it may not reflect the dynamic changes that occur in the uterus under the influence of sex-steroids. The effect of P on surface pH changes was not reported while changes that occur during Ds were minimally documented. In addition to this, few other in-vitro studies have also documented the involvement of CFTR in forskolin-induced increase in the surface pH of the endometrial epithelia in culture 10.
So far, no in-vivo studies have been performed to investigate concomitant changes in the pH, volume and electrolyte concentration of this fluid throughout phases of the oestrous cycle and under the effect of exogenous sex-steroids. We hypothesized that parallel changes in these parameters occurred under the influence of sex-steroid which were mediated via common transporters and enzyme including CFTR, SLC26A6, NHE and CA. These were based on the following observations: (i) CFTR was found to be involved in endometrial HCO3- 10, Cl- 15, 16 and fluid 2 secretion in-vitro, (ii) NHE has been reported to participate in the uterine fluid pH changes under P influence 4, although no evidences suggest its involvement in mediating changes in other uterine parameters and (iii) SLC26A6 and CA have been reported to cause changes in the endometrial surface pH although their role in mediating changes in other parameters of interest were relatively unknown. In view of these, this study aimed to investigate the concomitant changes in the uterine fluid pH, volume and electrolyte concentration under the influence of sex-steroid in-vivo. In addition, this study also aimed to confirm the known role of these proteins and to investigate their yet unknown roles in mediating the changes in these uterine fluid parameters.
Materials and Method
Animal and hormones treatment
Adult female WKY rats, weighing 200±25g were obtained from the Animal House, Faculty of Medicine, University of Malaya (UM). The animals were caged in a group of six, in a clean and well ventilated animal room, Department of Physiology, UM and were maintained with a standard suitable environment of 12 hours light: dark cycle. The room temperature was kept at 25±2 oC, with a humidity of 30-70%. The animals were provided with soy-free diet (Gold Coin Pellet) and tap water ad libitum. All experimental procedures were approved by the Faculty of Medicine Animal Care and Use Committee (ACUC), UM. Bilateral ovariectomy was performed under isoflurane anesthesia. After surgery, the animals were given intramuscular injection of 0.1 ml Kombitrim antibiotic to prevent post- surgical wound infection. Sex-steroids were administered via subcutaneous injection behind the neck cuff ten days after ovariectomy as had been previously described 17. The animals were divided into four groups which received the following sex-steroid regimes: (i) 3 days treatment with 0.2µg E, (ii) 3 days treatment with 4mg P, (iii) 3 days treatment with 0.2µg E followed by another 3 days with 4mg P (E+P), which was intended to mimic the normal sex-steroid profile throughout the reproductive cycle and (iv) 3 days treatment with peanut oil (vehicle) which acts as a control.
Oestrous phase identification
Vaginal secretion was collected using a plastic pipette pre-filled with 10µl saline solution (NaCl 0.9%). The saline was flushed into the vagina and immediately withdrawn. The unstained vaginal secretion was collected and placed onto a glass slide which was then observed under a light microscope. Phases of the oestrous cycle were identified from the proportion of different cell types in the vaginal smear according to the description by Marcondez et al, 18.
In-vivo Uterine perfusion
In-vivo uterine perfusion was performed according to the method by Salleh et al 6 to investigate changes in the volume (rate of fluid secretion), pH and electrolytes concentration of the uterine fluid under different sex-steroid treatment and at different phases of the oestrous cycle. A day after the last drug administration or following identification of oestrous cycle phase, the animals were anesthetized with intraperitoneal (i.p.) injection of xylazine HCl (8mg/kg) and ketamine (80 mg/kg). The animal was placed on a heat pad to maintain a constant body temperature at 37oC. An incision was made at both flanks to expose the abdominal cavity and an in-going tube (fine polythene tubing ID 0.38mm, OD 1.09mm, pre-filled with perfusate) was inserted at the distal end of the uterine horns. Meanwhile, a midline anterior incision was made in the abdomen to insert an out-going tube which was tied in-situ at the uterocervical junction. A syringe-driven infusion pump (Harvard Apparatus) was used to deliver perfusion medium into the lumen at a constant rate of 0.75μl/min. The in-going tube, animal and out-going tube were placed at the same level to minimize gravitational effect. The perfused fluid was collected into a small, pre-weighed polythene tubes with covered tops to minimize evaporation.
Perfusion was conducted over a period of 3 hours. At the end of the experiment, the anaesthetized animals were sacrificed by cervical dislocation. The perfusate contains the following compositions: 110.0 NaCl mmol/L, 14.3 Na2HCO3, 1.0 Na2HPO4 , 15 KCl, 0.8 MgSO4, 10.0 HEPES, 1.8 CaCl2 and 5.5 glucose at pH 7.34 were selected to closely mimic normal uterine fluid composition 6. In order to investigate the functional involvement of the proteins of interest, the following inhibitors were dissolved into the perfusion fluid and were then perfused into the uterine horn: acetazolamide (ACTZ), (CA inhibitor) (Sigma) at 100µM 19, glibenclamide (CFTR inhibitor) (Sigma) at 200 µM 10, 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate (DIDS) (SLC26A6 inhibitor) (Sigma) at 500 µM and 5-(N-Ethyl-N-isopropyl)-amiloride (EIPA) (NHE inhibitor) (Sigma) at 100 µM 20. The pH of the collected samples (usually less than 500 µl) was directly measured using HI 8424 NEW micropH meter from Hanna instrument (Singapore). The collected samples were briefly exposed to air to equilibrate the dissolved CO2 with the atmosphere 21. In order to measure the rate of fluid secretion, net weight of the collected fluid was divided by the total perfusion time (180minutes). HCO3- concentration was determined by enzymatic assay using phosphoenolpyruvate carboxylase (PEPC) and malate dehydrogenase (MDH) in which the end product was measured by spectrophotometer at the absorbance wavelength of 405 or 415 nm, which was directly proportional to the HCO3- concentration in the samples. Na+ and Cl- concentrations were measured directly using Ion Selective Electrode (ISE), which was voltage-dependent on the levels of ion in the solution.
Detection of protein distribution by immunohistochemistry (IHC)
A day after the last day of sex-steroid treatment or following identification of the oestrous phase, the animals were humanely sacrificed and the uterine horns were immediately removed for IHC. The horns were fixed in a 4% paraformaldehyde for 4 hours at 4oC, which were then processed through to wax blocks. The tissues were then cut into 5μm sections, which were dewaxed in xylene and re-hydrated. The tissues were incubated in 10% H2O2 in methanol to quench the activity of the endogenous peroxidase and then incubated overnight with primary antibodies in 5%BSA at the following concentrations (1:1000, 0.2 µg/ml for CFTR and SLC26A6, CAII 1:3000, 0.06 µg/ml and CAXII 1:100, 2µg/ml, NHE1 1:200, 1 µg/ml) at room temperature in a humidified chamber. The tissues were then incubated with biotinylated secondary antibodies (1:1000, 0.1µg/ml CFTR, 1:2000, 0.05µg/ml SLC26A6, 1:4000, 0.025µg/ml CAII, 1:200, 0.05µg/ml CAXII and NHE1,1:1000, 0.1µg/ml) for 30 minutes at room temperature followed by 30 minutes incubation with Avidin and biotinylated HRP in PBS. The sites of antibody binding were visualized using DAB (Diaminobenzidine HCl), which gave dark-brown stain. The intensity of staining was proportional to the amount of proteins in the tissue. The sections were counterstained with hematoxylin for nuclear staining. Goat polyclonal primary antibodies were used against CFTR, NHE1 and SLC26A6, while mouse monoclonal and rabbit polyclonal primary antibodies were used against CAII and CAXII respectively. Anti-goat, anti-mouse and anti-rabbit biotinylated secondary antibodies were used for the respective primary antibodies. All antibodies were purchased from Santa Cruz, (USA). Non-immune normal serum was used as a negative control.
Evaluation of immunostaining
The relative intensity of the products of immune reaction at the luminal and glandular epithelia were evaluated and graded blindly by three independent observers using a light microscope (Olympus, Japan) at 100X magnification. The staining intensity was estimated semi-quantitatively on a scale of 0-4 as: 0- no detectable stain; 1- faint; 2- moderate; 3- strong; and 4- very intense, as were previously reported 22, 23.
Statistical analysis
The results were presented as mean± SEM. Statistical analysis was performed using IBM SPSS 19 software. One-way ANOVA with Tukey's post-hoc test were used to compare between the treatment groups and the level of significance was at p value of less than 0.05.
Results
The effect of protein inhibitors on uterine fluid pH, volume, HCO3-, Cl- & Na+ concentrations
Effects on uterine fluid pH
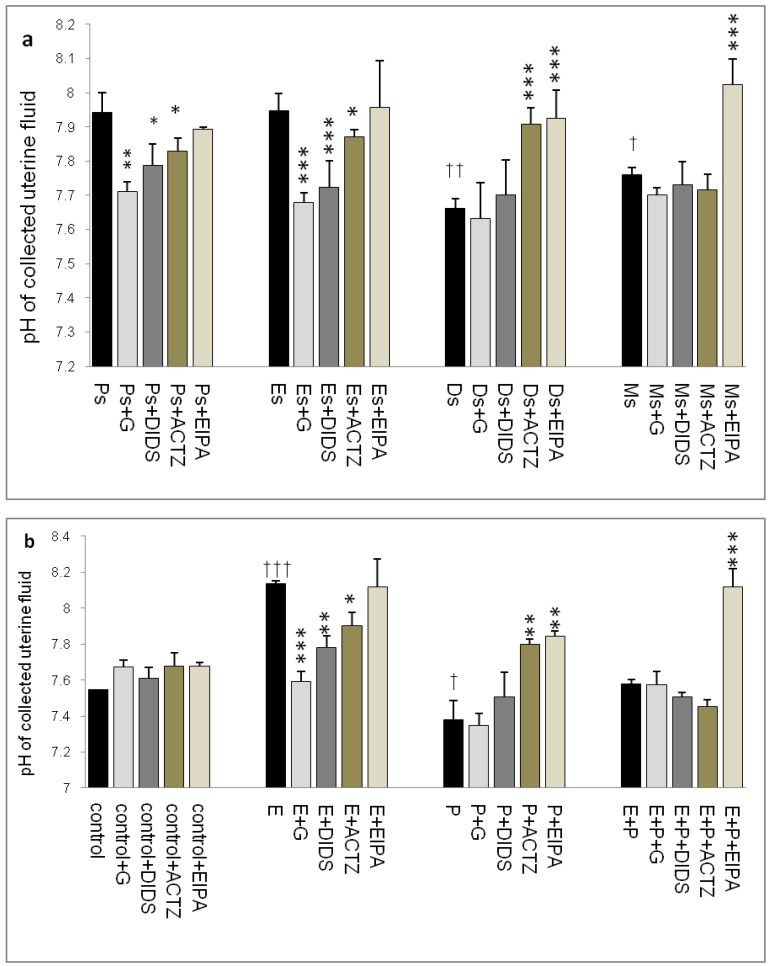
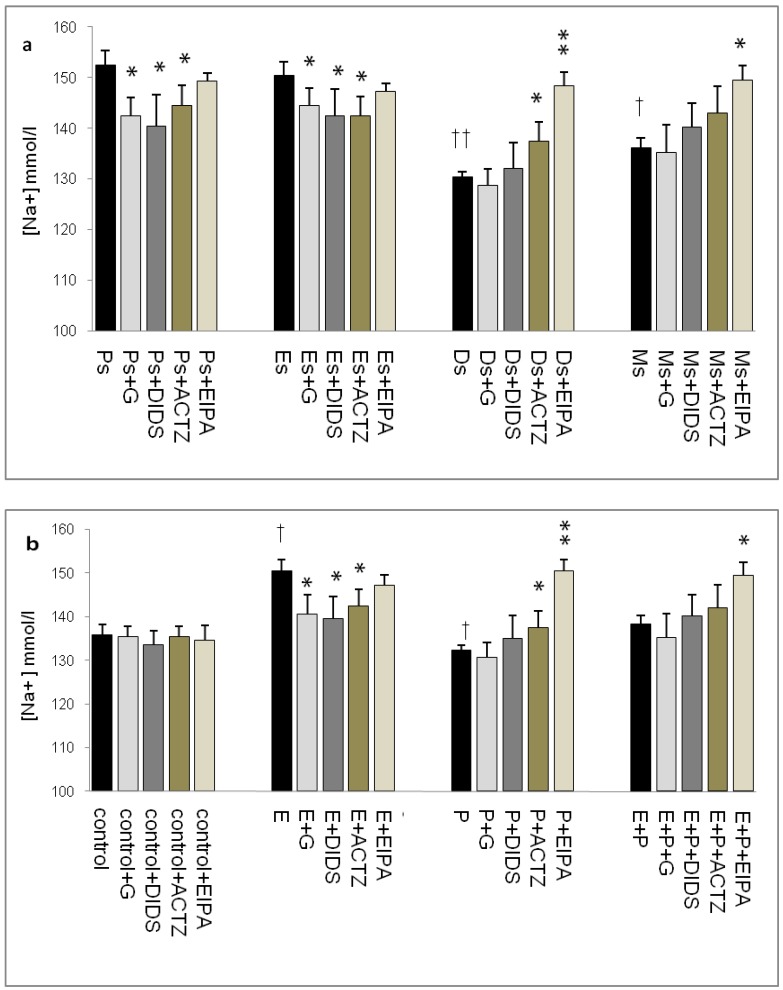
Throughout the oestrous cycle, the highest pH was observed at Es (7.95) followed by Ps (7.93) while the lowest pH was observed at Ds (7.67) (figure 1 a & b) consistent with a high circulating plasma E and P levels respectively. E treatment caused pH increase (8.15) while P treatment caused pH reduction (7.35). Administration of glibenclamide, DIDS and ACTZ caused the pH to decrease in the Es and Ps groups as well as following E treatment. Meanwhile, administration of ACTZ and EIPA caused the pH to increase in the Ds group as well as following P treatment. EIPA administration caused a marked increase in pH at metestrus (Ms) and following E+P treatment.
Fig 1.
Changes in uterine fluid pH in (a) rats throughout oestrous cycle phases and (b) steroid treated ovariectomized rats. The pH in the presence of different protein channel/enzyme inhibitors was determined. Higher pH was noted at Es and Ps and following E treatment, while lower pH was noted at Ds and following P treatment. Glibenclamide, DIDS and ACTZ administration caused a decrease in pH under E influence, while ACTZ and EIPA administration caused an increase in pH under P influence. Meanwhile, in E+P group, the pH was not significantly differing from the control. EIPA caused pH increase at Ms and following E+P treatment. E: 0.2µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus, G: glibenclamide, ACTZ: acetazolamide, DIDS: 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate and EIPA: 5-(N-Ethyl-N-isopropyl)-amiloride. † as compared to Es or control, * as compared to the group without inhibitors. (*,† p<0.05), (**,†† p<0.01), (***,††† p<0.001). n= 6 per group. One way ANOVA and Tukey's test were used to analyze the results which were presented as mean ± SEM.
Effects on uterine fluid secretion rate
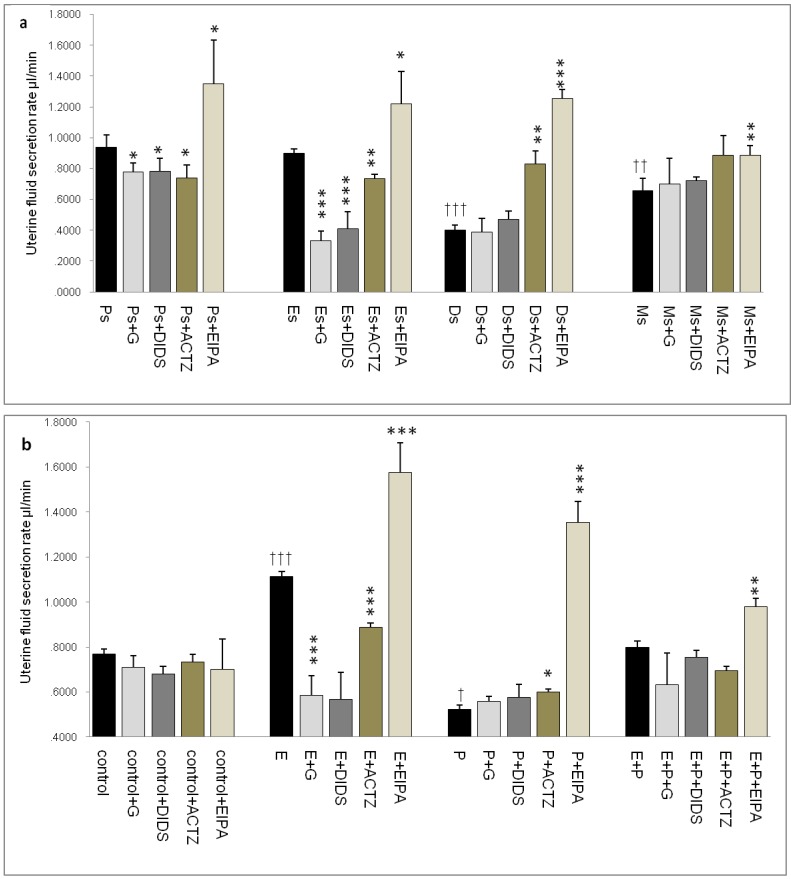
In figure 2a, the rate of uterine fluid secretion was the highest at Ps and Es (0.95 & 0.9μl/min respectively). At Ds and Ms however, the rates were significantly reduced (0.4 & 0.65μl/min respectively). Glibenclamide, DIDS and ACTZ administration caused a decrease in the secretion rate in rats at Ps and Es with glibenclamide and DIDS effects being greater than ACTZ. Meanwhile at Ds, ACTZ and EIPA administration caused an increase in the secretion rate, where the latter caused more than 2 folds increase. At Ms, the rate of secretion was lower than at Es with EIPA administration caused the rate to increase.
Fig 2.
Changes in the rate of uterine fluid secretion in the presence of different protein/enzyme inhibitors in (a) rats at different phases of the oestrous cycle and (b) steroid treated ovariectomized rats. Results indicated that fluid secretion rate was increased under E influence. Glibenclamide, DIDS and ACTZ administration caused reduction in fluid secretion rate at Es, Ps and following E treatment. Interestingly, EIPA caused a marked increase in the secretion rate under E influence. The rate of fluid secretion was decreased under P influence. ACTZ and EIPA administration caused the rate to increase at Ds and following P treatment. E: 0.2 µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus. G: glibenclamide, ACTZ: acetazolamide, DIDS: 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate and EIPA: 5-(N-Ethyl-N-isopropyl)-amiloride † as compared to Es or control, * as compared to the group without inhibitor. (*, † p<0.05), (**,†† p<0.01), (***,††† p<0.001). n= 6 per group. One way ANOVA and Tukey's test were used to analyze the results which were presented as mean ± SEM.
In figure 2b, an increase in fluid secretion rate was observed following E treatment (1.15μl/min) as compared to the control. This increase was significantly inhibited by glibenclamide, DIDS and ACTZ. The inhibitory effects of glibenclamide and DIDS were approximately two times greater than ACTZ. EIPA administration resulted in 1.5 fold increase in the secretion rate in rats receiving E treatment (1.6μl/min). Meanwhile, fluid secretion rate was significantly reduced following P treatment (0.6μl/min) as compared to the control. ACTZ and EIPA administration inhibited this decrease where the latter caused the rate to increase by more than 2.5 folds. Treatment with E+P caused a significant increase in the secretion rate as compared to P treatment alone. Administration of glibenclamide, ACTZ and DIDS caused no significant changes while EIPA caused a significant increase in the rate in this treatment group.
Effects on uterine fluid HCO3- concentration
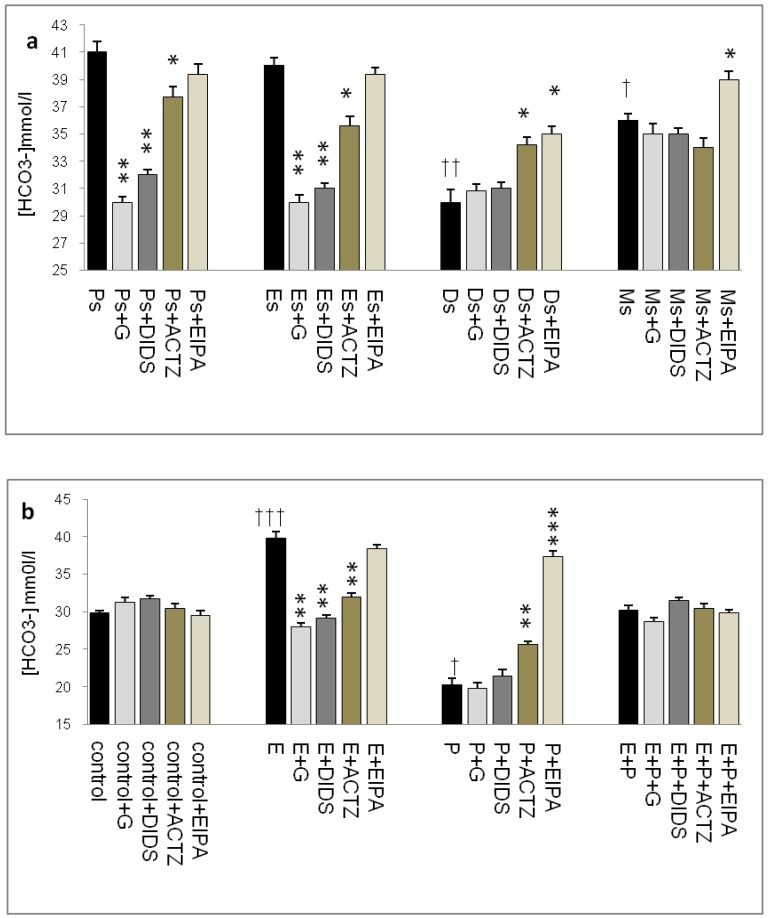
In figure 3a, HCO3- concentration was the highest at Ps (41mmol/l), followed by Es (39mmol/l). Glibenclamide, DIDS and ACTZ administration caused a decrease in its level with glibenclamide effect being the greatest (1.2 fold decrease). Meanwhile, at Ds, HCO3- concentration was the lowest (30mmol/l) with ACTZ and EIPA administration caused a significant increase in its concentration. At Ms, HCO3- concentration was lower than Es but was higher than Ds, with only EIPA caused its level to increase.
Fig 3.
Uterine fluid HCO3- concentration with and without the presence of protein/enzyme inhibitors in (a) rats at different phases of the oestrous cycle and (b) ovariectomized rats receiving steroid replacement. Uterine fluid HCO3- concentration was increased under E influence. Glibenclamide, DIDS and ACTZ administration caused a decrease in HCO3- concentration at Es, Ps and following E treatment. HCO3- concentration was reduced under P influence. ACTZ and EIPA administration caused an increase in HCO3- concentration at Ds and following P treatment. E: 0.2 µg 17β-oestradiol, P: progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus. G: glibenclamide, ACTZ: acetazolamide DIDS: 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate and EIPA: 5-(N-Ethyl-N-isopropyl)-amiloride. † as compared to Es or control, * as compared to the group without inhibitor.(*, † :p<0.05), (**,†† :p<0.01), (***,††† p<0.001). n= 6 per group. One way ANOVA and Tukey's test were used to analyze the results which were presented as mean ± SEM.
In figure 3b, a significant increase in HCO3- concentration (40mmol/l) was observed following E treatment with glibenclamide, DIDS and ACTZ administration inhibiting this increase. Glibenclamide effect was greater than DIDS and ACTZ. Meanwhile, a significant decrease in HCO3- concentration (20mmol/l) was observed following P treatment. ACTZ and EIPA administration caused its level to increase with the latter caused approximately two fold increase. Meanwhile, in the E+P group, no significant changes in HCO3- concentration were noted following administration of these inhibitors.
The highest HCO3- concentration (~40mmol/l) as observed at Es and following E treatment was consistent with its reported increase during the follicular phase of the reproductive cycle (35mmol/l). HCO3- concentration was reported to peak to approximately 90 mM at around the time of ovulation 24. This study reported for the first time uterine fluid pH and HCO3- concentration at different phases of the oestrous cycle. Besides uterus, E-dependent HCO3-secretion has also been reported in the duodenum 25, 26.
Effects on uterine fluid Cl- concentration
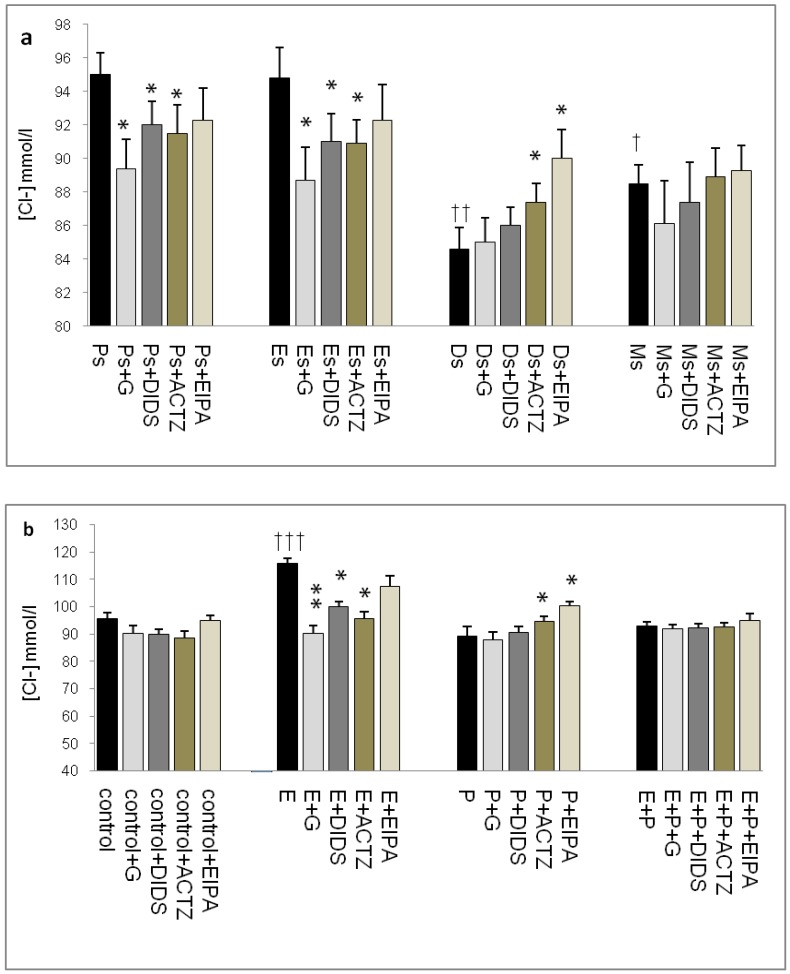
In figure 4a, Cl- concentration was the highest at Ps (95mmol/l) and Es (95mmol/l) Glibenclamide, DIDS and ACTZ administration caused a significant decrease in Cl- concentration in these groups. The lowest concentration was noted at Ds (85mmol/l) with ACTZ and EIPA administration caused its level to increase. Meanwhile, Cl- concentration was slightly high at Ms (88mmol/l). In figure 4b, an increase in Cl- concentration was observed following E treatment (116mmol/l). This increase was inhibited by glibenclamide, DIDS and ACTZ. P treatment caused decrease in Cl- concentration (88.5mmol/l) as compared to control (95.6mmol/l). ACTZ and EIPA inhibited this decrease. Meanwhile no significant change in Cl- concentration was observed following E+P treatment (93.5mmol/l) as compared to control and in the presence of these inhibitors.
Fig 4.
Uterine fluid Cl- concentrations with and without the presence of protein/enzyme inhibitors in (a) rats at different stages of the oestrous cycle and (b) ovariectomized rats receiving steroid replacement. The highest Cl- level was observed under E influence. Glibenclamide and ACTZ administration caused a decrease in Cl- concentration in rats at Es, Ps and following E treatment. Meanwhile, Cl- concentration was reduced under P influence. ACTZ and EIPA administration caused an increase in Cl- concentration at Ds and following P treatment. E: 0.2µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus. G: glibenclamide, ACTZ: acetazolamide, DIDS: 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate and EIPA: 5-(N-Ethyl-N-isopropyl)-amiloride † as compared to Es or control, * as compared with the group without inhibitor. (*:p<0.05), (**:p<0.01), (***p<0.001). n= 6 per group. One way ANOVA and Tukey's test were used to analyze the results which were presented as mean ± SEM.
Effects on uterine fluid Na+ concentration
In figure 5a, Na+ concentration was the highest at Ps (152mmol/l) followed by Es (150mmol/l) with glibenclamide, DIDS and ACTZ administration caused its level to decrease. At Ds, Na+ concentration was noted to be the lowest (130mmol/l) with ACTZ and EIPA administration caused its level to increase, where the latter effect was greater than the former. A slightly high concentration was noted at Ms (137 mmol/l) which was increase further following EIPA administration. In figure 5b, an increase in Na+ concentration (150mmol/l) was observed following E treatment which was inhibited by glibenclamide, DIDS and ACTZ. Meanwhile, P treatment caused Na+ concentration to decrease (132mmol/l) which was significantly inhibited by ACTZ and EIPA, where the latter effect was greater than the former. Meanwhile, no significant change in Na+ concentration was observed following E+P treatment as compared to control with only EIPA administration caused its level to increase.
Fig 5.
Uterine fluid Na+ concentration with and without the presence of protein/enzyme inhibitors in (a) rats at different stages of the oestrous cycle and (b) steroid replaced ovariectomized rats. Na+ concentration was high under E influence, however was low under P influence. Glibenclamide and DIDS administration caused a decrease in Na+ concentration at Es, Ps and following E treatment. Meanwhile, ACTZ and EIPA administration caused an increase in Na+ concentration under P influence. E: 0.2µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus. G: glibenclamide, ACTZ: acetazolamide, DIDS: 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate and EIPA: 5-(N-Ethyl-N-isopropyl)-amiloride. †as compared to control and Es, * as compared to the group without inhibitor (*,† :p<0.05), (**,†† p<0.01), (***,††† p<0.001). n= 6 per group. One way ANOVA and Tukey's test were used to analyze the results, which were presented as mean ± SEM.
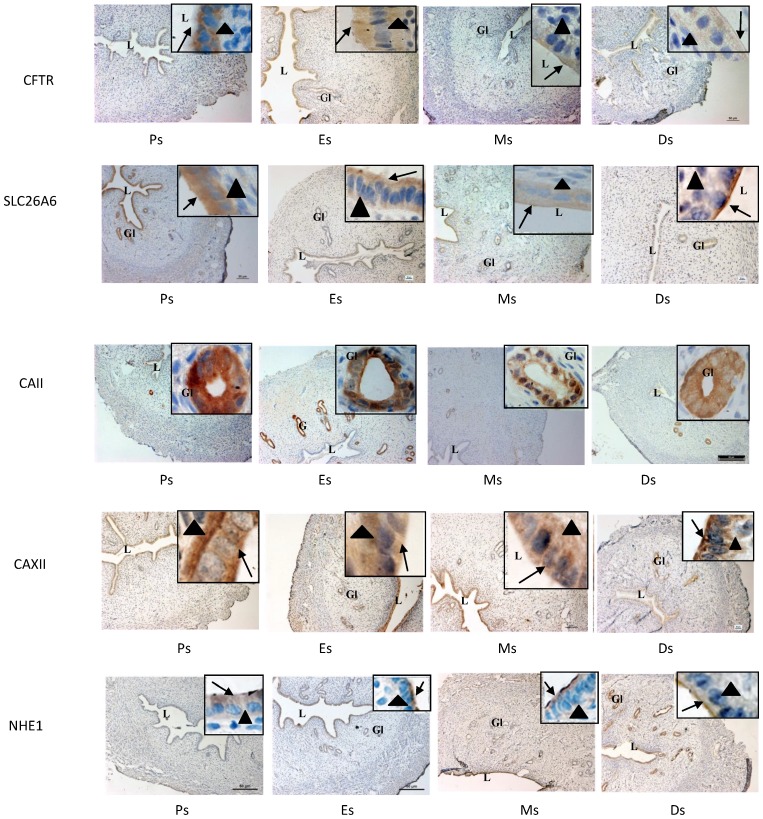
Expression of CFTR, SLC26A6, CAII & XII and NHE-1 at oestrous cycle phases
In figure 6 and table 1, high CFTR expression was observed at the apical membrane of the luminal and glandular epithelia at Es while low expression was seen in the stroma. Meanwhile, lower CFTR expression was observed at Ds compared to Es. High SLC26A6 expression was noted at the apical membrane of the luminal and glandular epithelia at Es and Ps while lower expression was observed at Ds. CAII was highly expressed at all stages of the oestrous cycle mainly in the glandular epithelia. CA XII expression was the highest at the basolateral membrane of the luminal epithelia at Es and Ps, while at Ds, the expression was high at the apical membrane. Glandular expression of CAXII was observed at all stages of the oestrous cycle. High NHE-1 expression was observed at the apical membrane of the luminal epithelia at Ds, however its low expression was observed at Es and Ps. No staining was observed following incubation with non-immune normal rabbit serum which acts as a negative control.
Fig 6.
Immunolocalization of CFTR, SLC26A6, CAII, CAXII and NHE1 in the uterus at different stages of the oestrous cycle. High CFTR and SLC26A6 expression were noted at Es and Ps. CAII was expressed mainly in the glands at all stages of the oestrous cycle, while CA XII was expressed predominantly in the glands and at the apical membrane of the luminal epithelia at Ds. At Es and Ps however, high CAXII expression was observed at the basolateral membrane. Objectives 10X and 100X were used to observe the sections under light microscope. No stainings was observed in the section pre-incubated with non-immune normal serum which acts as a negative control. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus. Arrow: apical membrane, arrowhead: basolateral membrane L: lumen, Gl: gland
Table 1.
Semi-quantitative assessment on the intensity of CFTR, SLC26A6, CAII, CAXII and NHE1 immunostainings at different phases of the oestrous cycle and following different sex-steroid treatment. The staining intensity was graded as: 0- negative; 1- faint; 2- moderate; 3- strong; and 4- very intense, as previously described 44. The intensity was confirmed by 3 independent observers. C: control E: 0.2 µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone. Ps: proestrus, Es: estrus, Ms: metestrus, Ds: diestrus.
| Groups | CFTR | SLC26A6 | CAXII | NHE1 | CAII | ||||
|---|---|---|---|---|---|---|---|---|---|
| apical | basal | apical | basal | apical | basal | apical | basal | ||
| C | 2 | 0 | 2 | 1 | 2 | 2 | 1 | 0 | 1 |
| E | 3 | 0 | 3 | 1 | 2 | 3 | 2 | 1 | 3 |
| P | 2 | 0 | 2 | 1 | 3 | 1 | 3 | 1 | 3 |
| E+P | 2 | 0 | 2 | 1 | 1 | 3 | 2 | 1 | 3 |
| Ps | 3 | 1 | 3 | 2 | 2 | 4 | 2 | 1 | 4 |
| Es | 4 | 2 | 3 | 1 | 2 | 3 | 2 | 0 | 3 |
| Ms | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 2 |
| Ds | 2 | 0 | 2 | 0 | 2 | 1 | 3 | 0 | 3 |
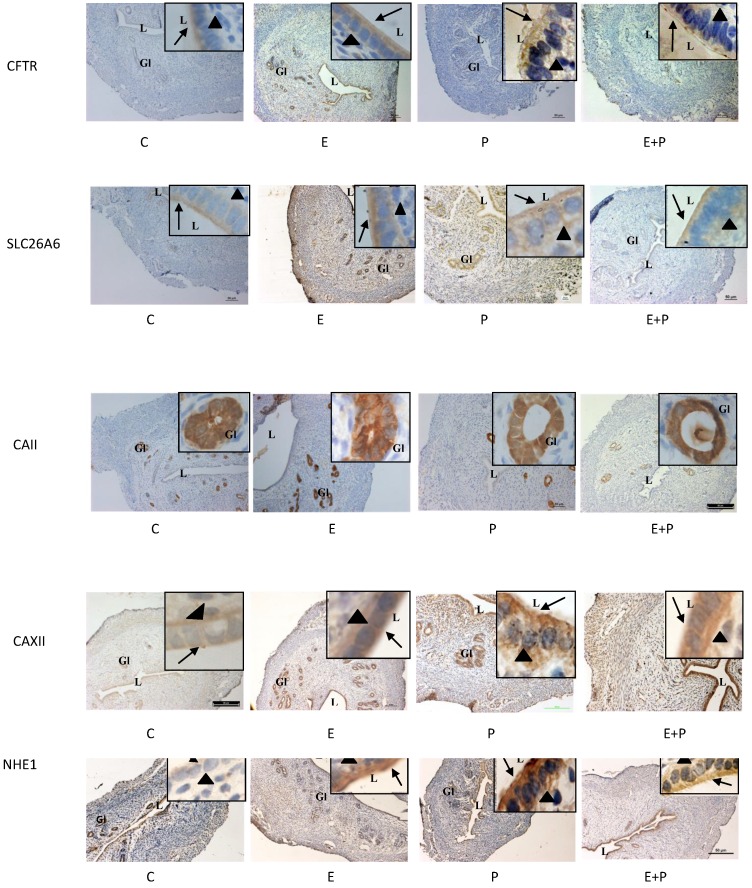
Expression of CFTR, SLC26A6, CAII & XII and NHE-1 in steroid-replaced ovariectomized rats
In figure 7 and table 1, high CFTR expression was observed following E treatment mainly at the apical membrane of the luminal and glandular epithelia. Low expression of this protein was observed following P and E+P treatments. No staining was seen in the stroma and myometrium. SLC26A6 expression was high at the apical membrane of the luminal and glandular epithelia and in the stroma following E treatment. Its expression however was low following P and E+P treatments. CA II was expressed mainly in the glandular epithelia in all treatment groups. Meanwhile, CAXII was expressed at the basolateral membrane following E and E+P treatments. P treatment resulted in high expression of this isoenzyme mainly at the apical membrane of the luminal epithelia. Moderate expression of CAXII was noted in the glandular epithelia following E or P treatments. High NHE-1 expression was observed following P treatment mainly at the apical membrane of the luminal epithelia. No staining was observed following incubation with non-immune normal rabbit serum which acts as a negative control.
Fig 7.
Immunolocalization of CFTR, SLC26A6, CAII, CAXII and NHE1 in steroid-replaced ovariectomized rats. CFTR and SLC26A6 were localized mainly at the apical membrane under E influence. Meanwhile, CAII was expressed predominantly in the glandular epithelia under both E and P influence. CAXII was expressed at the apical and basolateral membranes of the luminal epithelia under P and E influences respectively. Meanwhile, NHE1 expression was high under P influence. Objectives 10X and 100X were used to observe the uterine sections under light microscope. No staining was observed in the section pre-incubated with non-immune normal serum which acts as a negative control. E: 0.2µg 17β-oestradiol, P: 4mg progesterone, E+P: 0.2 µg 17β-oestradiol + 4mg progesterone Arrow: apical membrane, arrowhead: basolateral membrane L: lumen, Gl: gland.
Discussion
To the best of our knowledge, this study reported for the first time concomitant changes in volume, pH and electrolyte concentration of the uterine fluid under different sex-steroid influence. Using in-vivo perfusion system, we have shown that changes in these parameters were mediated via common transporter proteins and enzyme i.e. CFTR, SLC26A6, NHE and CA isoenzymes that were differentially expressed under different sex-steroid influence. Our findings indicated that CFTR, SLC26A6 and CA isoenzymes were involved in mediating changes at Es, Ps and following E treatment based on the observed effects of glibenclamide, DIDS and ACTZ while NHE and CA isoenzymes were involved in mediating changes at Ds and following P treatment, based on the observed effects of EIPA and ACTZ. The functional involvement of these transporters and enzyme were supported by their high level of expression under the related conditions. Our findings therefore provide greater understanding on their role in regulating changes in these uterine fluid parameters. In-vivo perfusion confers advantage over in-vitro study as it maintains continuous tissue perfusion; therefore changes that occur in the uterus can by accurately detected.
In this study, the inhibitory effect of glibenclamide indicated that CFTR was involved in the increase in volume, pH, Na+, Cl- and HCO3- concentrations under E influence. Increased CFTR activity will cause increase in HCO3- secretion, therefore increasing the pH. High CFTR expression was observed at the apical membrane of the luminal epithelia which supported the observed increase in its function. CFTR involvement in Cl- and HCO3- secretion has been reported in several tissues 27. In the uterus, CFTR mediated increase in Cl- and HCO3- secretion was reported in in-vitro studies in the cultured endometrial epithelial cells 15, 16, 28, 29. An inhibition on surface pH increase in forskolin-stimulated endometrial tissue pre-exposed to E was also reported following CFTR antagonist administration in mice 3. Apart from these in-vitro observations, no in-vivo studies have yet being performed to investigate CFTR involvement in mediating changes in these uterine fluid parameters. Besides Cl- and HCO3-, our finding indicated that CFTR was also involved in mediating uterine fluid secretion. Abnormal increase in CFTR expression has been reported in disease related to excess uterine fluid accumulation 2. The mechanism underlying CFTR-mediated uterine fluid secretion was unknown; however this may occur secondary to Cl- secretion. CFTR-mediated fluid secretion has been reported in the airway 30 and kidney 31 epithelial cells. Apart from CFTR, E-induced fluid secretion was also reported to occur via a 'leaky' tight junction 32. Meanwhile, the increase in Na+ secretion was likely driven by CFTR-mediated increase in Cl- secretion via other membrane transporter which was up-regulated by E 33.
The observed effects of DIDS on these uterine fluid parameters indicated the involvement of SLC26A6 in mediating these changes. Under E influence, SLC26A6 might participate in HCO3- secretion which would subsequently lead to pH increase. The observed effect of DIDS was lesser than glibenclamide, suggesting greater role of CFTR in mediating pH increase under E dominance. Limited in-vitro observation indicated that SLC26A6 was involved in mediating the surface pH increase in endometrial tissue pre-exposed to E 3. Apart from uterus, SLC26A6 was reported to mediate HCO3- secretion in the pancreatic duct 34. In addition to mediating pH and HCO3- concentration changes, SLC26A6 could also mediate the increase in volume, Na+ and Cl- concentrations at Es and following E treatment. These effects may indirectly involve CFTR. Interaction between SLC26A6 and CFTR has been reported 35, 36 where SLC26A6 inhibition will cause decrease in CFTR activity 37. High SLC26A6 expression at the apical membrane of the endometrial epithelia under E influence was consistent with the observed increase in its function. This was further supported by a reported increase in its protein and mRNA expression under similar hormonal condition 3, 8.
An interesting finding was observed following EIPA administration. At Ps, Es and following E treatment, EIPA caused a significant increase in the uterine fluid volume. This effect could be mediated via NHE inhibition, which resulted in increased CFTR activity 38. Interaction between NHE and CFTR has been reported in tissues such as skeletal muscle 39 and intestine 38. Parallel to the increase in fluid volume, Na+ and Cl- concentrations decrease due to dilution. At Ds and following P treatment, EIPA effects on the uterine fluid pH, volume and electrolyte concentration suggested NHE involvement. High NHE-1 expression at the apical membrane of the endometrial epithelia further supported the observed increase in its function. Meanwhile, low CFTR and SLC26A6 expression was also observed under P influence, consistent with the reported decrease in these proteins and mRNA expression under this condition 8. Increased NHE-1 and decreased CFTR and SLC26A6 expressions and functional activities will cause a net decrease in the pH due to increased H+ and decreased HCO3- secretions respectively. Reduced HCO3- concentration was also due to buffering action of excess H+ secretion. Besides regulating the pH, NHE was also involved in fluid volume reduction at Ds and following P treatment. NHE-mediated reduction in fluid volume has been reported in the epididymis, which was associated with fluid acidification 40. We speculated that this mechanism is responsible for uterine fluid loss under P influence. Under this condition, Na+ reabsorption via NHE will drive Cl- influx, creating osmotic gradient necessary for H2O reabsorption 41. At the same time, paracellular fluid movement was prevented via the formation of a “tight” tight junction 32.
In this study, ACTZ effects on uterine fluid volume, pH, Na+, Cl- and HCO3- concentrations indicate CA involvement. CA-mediated increase in endometrial surface pH was documented in-vitro in uterine tissue pre-exposed to E 3. We speculated that under E influence, CAII catalyzes intracellular HCO3- generation, which will then be excreted into the lumen. Meanwhile, concomitant H+ secretion occur via the basolateral membrane transporter to maintain intracellular pH homeostasis. High basolateral CAXII expression under E influence will convert the expelled H+ with plasma-derived HCO3- into CO2, which will then diffuse into the cell for further H+ and HCO3- generation. These mechanisms sustain continuous apical HCO3- secretion under the E influence 12. In addition to mediating pH changes, CA isoenzymes also involve in uterine fluid volume and electrolyte concentration changes which may indirectly occur via other membrane transporters, however the actual mechanisms are unknown. In this study, high CAII expression in the glands and luminal epithelia and high CAXII expression at the basolateral membrane of the luminal epithelia supported their observed increase in functional activities under E influence. Meanwhile, increased expression of these isoenzymes proteins and mRNA has also been reported in the uterus pre-exposed to E 3.
At Ds and following P treatment, CA might play opposite role in causing changes to these uterine fluid parameters. Under these conditions, CAII catalyzes intracellular generation of H+ and HCO3-, with H+ being excreted into the lumen via the apical NHE-1 4. In this study, high CAXII expression at the apical membrane under P influence might be involved in intraluminal CO2 generation. CO2 will then diffused into the cell for further H+ and HCO3- synthesis. Excess H+ will buffer the HCO3-, resulting in a net decrease in luminal fluid pH. Meanwhile, the mechanisms underlying CA-mediated decrease in the volume, Na+ and Cl- concentrations were unknown; although these may likely involve other membrane transporters.
The pH changes as observed in this study were due to interaction between multiple transporters/enzyme. CAII was reported to form a transport metabolon with NHE-1 42, therefore this may likely enhance luminal H+ secretion under P influence. CAII has also been reported to interact with SLC26A6 43, which could further increase luminal HCO3- secretion under E influence. So far, changes that were described in steroid-replaced ovariectomised rats were clearly observed following E or P treatments. In the situation where the uterus was exposed to E and P (E+P) as to mimic the hormonal profile throughout the reproductive cycle, no significant changes in these parameters could be seen in the presence of different inhibitors. There was however evidence that NHE was involved in mediating the changes in pH and electrolyte concentration under this condition. Meanwhile, changes that occur at Ms may reflect the transition from Es to Ds.
In conclusion, concomitant changes in pH, volume and electrolytes under different sex-steroid create optimum uterine fluid environment necessary for different reproductive processes to occur. While most studies with regard to these changes were conducted in-vitro, this study has provided the first in-vivo evidence on the involvement of multiple proteins and enzymes in mediating these changes that are essential for successful reproduction.
Acknowledgments
This study was funded by UMRG (RG404/12HTM), PPP (PV067/2011B) and High Impact Research Grants (UM/MoHE/HIRGrant E000046-20001), University of Malaya, Kuala Lumpur, Malaysia. We would like to thank Dr Junedah Sanusi from the Department of Anatomy, Faculty of Medicine, University of Malaya for supporting this project.
Abbreviations
- CFTR
Cystic fibrosis transmembrane conductance regulator
- NHE
sodium-hydrogen exchanger
- CA
Carbonic anhydrase
- ENaC
Epithelial sodium channel
- E
17β estradiol
- P
progesterone
- Ps
proestrus
- Es
estrus
- Ms
metestrus
- Ds
diestrus
- E+P
17β estradiol+ progesterone, IHC: immunohistochemistry
- ACTZ
acetazolamide
- DIDS
4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate
- EIPA
5-(N-Ethyl-N-isopropyl)-amiloride.
References
- 1.Chan H, Chen H, Ruan Y, Sun T. Physiology and pathophysiology of the epithelial barrier of the female reproductive tract: role of ion channels. Adv Exp Med Biol. 2012;763:193–217. doi: 10.1007/978-1-4614-4711-5_10. [DOI] [PubMed] [Google Scholar]
- 2.He Q, Tsang LL, Ajonuma LC, Chan HC. Abnormally up-regulated cystic fibrosis transmembrane conductance regulator expression and uterine fluid accumulation contribute to Chlamydia trachomatis-induced female infertility. Fertil Steril. 2010;93:2608–14. doi: 10.1016/j.fertnstert.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 3.He Q, Chen H, Wong CHY, Tsang LL, Chan HC. Regulatory mechanism underlying cyclic changes in mouse uterine bicarbonate secretion: role of estrogen. Reproduction. 2010;140:903–10. doi: 10.1530/REP-10-0178. doi:10.1530/rep-10-0178. [DOI] [PubMed] [Google Scholar]
- 4.Salleh N, Ahmad V, Kasim N, Amri S& Onn Y. The effect of progesterone on uterine fluid ph &endometrial nhe-1 protein expression in rats. Health. 2011;3:66–72. [Google Scholar]
- 5.Yang JZ, Jiang X, Dong J, Guo J, Chen H, Tsang LL. et al. Abnormally enhanced cystic fibrosis transmembrane conductance regulator-mediated apoptosis in endometrial cells contributes to impaired embryo implantation in controlled ovarian hyperstimulation. Fertil steril. 2011;95:2100–6.e2. doi: 10.1016/j.fertnstert.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Salleh N, Baines DL, Naftalin RJ & Milligan SR. The Hormonal Control of Uterine Luminal Fluid Secretion and Absorption. J Membr Biol. 2005;206:17–28. doi: 10.1007/s00232-005-0770-7. [DOI] [PubMed] [Google Scholar]
- 7.Zheng XY, Chen GA, Wang HY. Expression of cystic fibrosis transmembrane conductance regulator in human endometrium. Hum Reprod. 2004;19:2933–41. doi: 10.1093/humrep/deh507. doi:10.1093/humrep/deh507. [DOI] [PubMed] [Google Scholar]
- 8.Gholami K, Muniandy S, Salleh N. Progesterone downregulates oestrogen-induced expression of CFTR and SLC26A6 proteins and mRNA in rats' uteri. J Biomed Biotechnol. 2012:596084. doi: 10.1155/2012/596084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunham PB, Kelley SJ, Logue PJ. Extracellular Na+ inhibits Na+/H+ exchange: cell shrinkage reduces the inhibition. Am J Phys - Cell Physiology. 2004;287:C336–C44. doi: 10.1152/ajpcell.00582.2003. doi:10.1152/ajpcell.00582.2003. [DOI] [PubMed] [Google Scholar]
- 10.Wang XF, Yu MK, Lam SY, Leung KM, Jiang JL, Leung PS, Ko W H, Leung PY, Chew SBC, Liu C Q, Tse CM & Chan HC. Expression, Immunolocalization, and Functional Activity of Na+/H+ Exchanger Isoforms in Mouse Endometrial Epithelium. Biol Reprod. 2003;68:302–8. doi: 10.1095/biolreprod.102.005645. [DOI] [PubMed] [Google Scholar]
- 11.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3--related species. BBA - Proteins and Proteomics. 2010;1804:410–21. doi: 10.1016/j.bbapap.2009.10.021. doi: http://dx.doi.org/10.1016/j.bbapap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karhumaa P, Kaunisto K, Parkkila S, Waheed A, Pastoreková S, Pastorek J. et al. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol Hum Reprod. 2001;7:611–6. doi: 10.1093/molehr/7.7.611. doi:10.1093/molehr/7.7.611. [DOI] [PubMed] [Google Scholar]
- 13.Ge ZH, Spicer SS. Immunocytochemistry of ion transport mediators in the genital tract of female rodents. Biol Reprod. 1988;38:439–52. doi: 10.1095/biolreprod38.2.439. [DOI] [PubMed] [Google Scholar]
- 14.Hynninen P, Hamaleinen J, Pastorekova S, Pastorek J, Waheed A, Sly W, Tomas E, Kirkinen P, Parkkila S. Transmembrane carbonic anhydrase isozymes IX and XII in the female mouse reproductive organs. Reprod Biol Endocrin. 2004;2:73. doi: 10.1186/1477-7827-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan LN, Wang XF, Tsang LL, Liu CQ, Chan HC. Suppression of CFTR-Mediated Cl- Secretion by Enhanced Expression of Epithelial Na+ Channels in Mouse Endometrial Epithelium. Biochem Bioph Res Co. 2000;276:40–4. doi: 10.1006/bbrc.2000.3426. doi: http://dx.doi.org/10.1006/bbrc.2000.3426. [DOI] [PubMed] [Google Scholar]
- 16.Chan LN, Chung YW, Leung PS, Liu CQ, Chan HC. Activation of an Adenosine 3′,5′-Cyclic Monophosphate-Dependent Cl- Conductance in Response to Neurohormonal Stimuli in Mouse Endometrial Epithelial Cells: The Role of Cystic Fibrosis Transmembrane Conductance Regulator. Biol Reprod. 1999;60:374–80. doi: 10.1095/biolreprod60.2.374. doi:10.1095/biolreprod60.2.374. [DOI] [PubMed] [Google Scholar]
- 17.Naftalin R, Thiagarajah JR, Pedley K, Pocock V, Milligan S. Progesterone stimulation of fluid absorption by the rat uterine gland. Reproduction. 2002;123:633–8. doi: 10.1530/rep.0.1230633. doi:10.1530/rep.0.1230633. [DOI] [PubMed] [Google Scholar]
- 18.Marcondes FK, Bianchi FJ & Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 19.Breton S, Hammar K, Smith PJS & Brown D. Proton secretion in the male reproductive tract: involvement of Cl-independent HCO3- transport. Am J Physiol Cell Physiol. 1998;275:C1134–42. doi: 10.1152/ajpcell.1998.275.4.C1134. [DOI] [PubMed] [Google Scholar]
- 20.Masereel B, Pochet L. & Laeckmann. An overview of inhibitors of Na /H exchanger.EUR J Med Chem. 2003;38:547–54. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 21.Dale B, Menezo Y, Cohen J, Dimatteo L & Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13:964–70. doi: 10.1093/humrep/13.4.964. [DOI] [PubMed] [Google Scholar]
- 22.Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G & Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Cln Endocr Metab. 1994;78:216–24. doi: 10.1210/jcem.78.1.8288707. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Zou S, Li J, Chen Y, Wang D, Gao Z. Temporospatial expression of vascular endothelial growth factor and basic fibroblast growth factor during mandibular distraction osteogenesis. J Cranio Maxill Surg. 2003;31:238–43. doi: 10.1016/s1010-5182(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 24.Maas DH, Storey BT & Mastroianni L. Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil Steril. 1977;28:981–5. [PubMed] [Google Scholar]
- 25.Smith A, Contreras C, Ko K H, Chow J, Dong X, Tuo B, Zhang HH, Chen D B & Dong H. Gender-Specific Protection of Estrogen against Gastric Acid-Induced Duodenal Injury: Stimulation of Duodenal Mucosal Bicarbonate Secretion. Endocrinology. 2008;149:4554–456. doi: 10.1210/en.2007-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuo B, Wen G, Wei J, Liu X, Wang X, Zhang Y, Wu H, Dong X, Chow JYC, Vallon V & Dong H. Estrogen Regulation of Duodenal Bicarbonate Secretion and Sex-Specific Protection of Human Duodenum. Gastroenterology. 2011;141:854–63. doi: 10.1053/j.gastro.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hug MJ, Tamada T, Bridges RJ. CFTR and Bicarbonate Secretion to Epithelial Cells. Physiology. 2003;18:38–42. doi: 10.1152/nips.01412.2002. doi:10.1152/nips.01412.2002. [DOI] [PubMed] [Google Scholar]
- 28.Chan HC, Shi QX, Zhou CX, Wang XF, Xu WM, Chen WY. et al. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol Cell Endocrinol. 2006;250:106–13. doi: 10.1016/j.mce.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Chan HC, Ruan YC, He Q. et al. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol. 2009;587:2187–95. doi: 10.1113/jphysiol.2008.164970. doi:10.1113/jphysiol.2008.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan J, Liao J, Huang J. et al. Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J Physiol. 2012;590:5273–97. doi: 10.1113/jphysiol.2012.236893. doi:10.1113/jphysiol.2012.236893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int. 1996;50:1327–36. doi: 10.1038/ki.1996.445. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay LA, Murphy C. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: a study with light and electron microscopy. Reproduction. 2006;131:369–78. doi: 10.1530/rep.1.00914. [DOI] [PubMed] [Google Scholar]
- 33.Crowell MD. Lubiprostone: trials and tribulations. Nat Rev Gastroenterol Hepatol. 2009;6:259–60. doi: 10.1038/nrgastro.2009.62. [DOI] [PubMed] [Google Scholar]
- 34.Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J. et al. Effect of Slc26a6 deletion on apical Cl-/HCO3- exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol- Gastr L. 2007;292:G447–G55. doi: 10.1152/ajpgi.00286.2006. doi:10.1152/ajpgi.00286.2006. [DOI] [PubMed] [Google Scholar]
- 35.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U. et al. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol - Cell Ph. 2003;284:C769–C79. doi: 10.1152/ajpcell.00270.2002. doi:10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR. et al. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–57. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko SBH, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L. et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–50. doi: 10.1038/ncb1115. http://www.nature.com/ncb/journal/v6/n4/suppinfo/ncb1115_S1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa O, Bi LC, Guth PH, Engel E, Hirokawa M, Kaunitz JD. NHE3 inhibition activates duodenal bicarbonate secretion in the rat. Am J Physiol- Gastr L. 2004;286:G102–G9. doi: 10.1152/ajpgi.00092.2003. doi:10.1152/ajpgi.00092.2003. [DOI] [PubMed] [Google Scholar]
- 39.Tu J, Lu L, Cai W, Ballard HJ. cAMP/Protein Kinase A Activates Cystic Fibrosis Transmembrane Conductance Regulator for ATP Release from Rat Skeletal Muscle during Low pH or Contractions. PLoS ONE. 2012;7:e50157.. doi: 10.1371/journal.pone.0050157. doi:10.1371/journal.pone.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung GPH, Tse CM, Cheng Chew SB, Wong PYD. Expression of Multiple Na+/H+ Exchanger Isoforms in Cultured Epithelial Cells from Rat Efferent Duct and Cauda Epididymidis. Biol Reprod. 2001;64:482–90. doi: 10.1095/biolreprod64.2.482. doi:10.1095/biolreprod64.2.482. [DOI] [PubMed] [Google Scholar]
- 41.Naftalin RJ, Zammit PS, Pedley KC. Regional differences in rat large intestinal crypt function in relation to dehydrating capacity in vivo. J Physiol. 1999;514:201–10. doi: 10.1111/j.1469-7793.1999.201af.x. doi:10.1111/j.1469-7793.1999.201af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purkerson J, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71:103–15. doi: 10.1038/sj.ki.5002020. [DOI] [PubMed] [Google Scholar]
- 43.Vince J, Reithmeier RAF. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl-/HCO3- exchanger. J Biol Chem. 1998;23(273):28430–7. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 44.Meduri G, Bausero P, Perrot-Applanat M. Expression of vascular endothelial growth factor receptors in the human endometrium: modulation during the menstrual cycle. Biol Reprod. 2000;62:439–47. doi: 10.1095/biolreprod62.2.439. [DOI] [PubMed] [Google Scholar]