Abstract
Purpose
Previous studies of the impact of renal cell carcinoma histopathology on survival are conflicting and generally limited to institutional analyses. Thus, we determined the role of renal cell carcinoma histopathology on the stage specific survival rate in a large population based cohort.
Materials and Methods
We used the 2000 to 2005 National Cancer Institute SEER (Surveillance, Epidemiology and End Results) database to identify 17,605 patients who underwent surgery for renal cell carcinoma and met study inclusion criteria. Patients were stratified by histological subtype (clear cell, papillary, chromophobe, collecting duct and sarcomatoid differentiation) and pathological stage. We performed Cox proportional hazard modeling and Kaplan-Meier survival analysis to determine overall and cancer specific survival.
Results
Patients with papillary and chromophobe pathology were less likely to present with T3 or greater disease (17.6% and 16.9% ,respectively) while patients with collecting duct and sarcomatoid variants were more likely to present with T3 or greater disease (55.7% and 82.8%, respectively) compared to those with clear cell histology (p <0.001). On multivariate analysis histology was significantly associated with overall and cancer specific survival. Patients with chromophobe pathology had improved survival (HR 0.56, 95% CI 0.40–0.78) while those with collecting duct and sarcomatoid variants had worse survival (HR 2.07, 95% CI 1.44–2.97 and 2.26, 95% CI 1.93–2.64, respectively).
Conclusions
Renal cell carcinoma histological subtype predicts overall and cancer specific survival. Patients with collecting duct and sarcomatoid variants of renal cell carcinoma have poor survival, even those who present with low stage disease. These data suggest inherent differences in renal cell carcinoma biology and may ultimately form the basis of future histologically targeted therapies.
Keywords: kidney, carcinoma, renal cell, SEER program, survival analysis, histology
In 1997 the Heidelberg classification identified 4 distinct histological renal cell neoplasm subtypes, including conventional (clear cell), papillary, chromophobe and collecting duct variants. While sarcomatoid variation is noted in all renal cell subtypes it is not considered a discrete subtype but rather a progression of pathological high grade disease.1 Despite this, sarcomatoid differentiation is a clearly recognized pathological entity with distinct biological activity.2,3
Several groups have addressed the role of renal cell histopathology in survival. Initial studies were largely institutional with a sample size of 186 to 2,466 patients.4–11 On univariate analysis most groups noted that histopathology predicted outcome, specifically citing a survival advantage of the papillary and chromophobe subtypes relative to that of clear cell renal cancer. In a multi-institutional, international study of 4,063 patients Patard et al found that histopathology predicted survival on univariate analysis.12 However, this influence was not statistically significant on multivariate analysis. Only TNM stage, grade and Eastern Cooperative Oncology Group status were independent variables. While this study was the largest at the time, it was limited by the relatively small sample size of certain stage specific histological categories, higher staged cancers from North American sites and no analysis of sarcomatoid or collecting duct pathology.
Recently Capatanio et al used the National Cancer Institute SEER database to evaluate the effect of histology on RCC prognosis.13 They noted in univariate and multivariate models that histopathology was an independent predictor of cause specific mortality. However, accuracy analysis revealed that histopathology contributed only 0.1% to cause specific mortality. While this represents an important finding on the role of histopathology in cause specific mortality, that study was limited by the inclusion of data before the implementation of consensus histological definitions and the absence of collecting duct and sarcomatoid variants.
Thus, we evaluated all renal cell histopathology in a contemporary, population based cohort with an emphasis on the role of histopathology in stage specific, overall and cancer specific survival.
MATERIALS AND METHODS
Subjects
We used the SEER database, a prospective national cancer registry that captured data on approximately 26% of the American population during 1973 to 2005.14 Data before 2000 were excluded from analysis due to the lack of pathological consensus on histopathological subtypes and absent chromophobe histology. We also excluded patients younger than 30 years to omit familial renal cell variants and patients with unknown T stage, grade or histology.
Population
All renal cancer cases (ICD-9 code 189.0) between the 2000 and 2005 were identified. Patients were included in study if they underwent partial or radical nephrectomy and final histology on pathological evaluation was clear cell, papillary, chromophobe, collecting duct or sarcomatoid differentiation. Four cases of medullary renal cell carcinoma were categorized as collecting duct renal cell carcinoma.
Variables
For each identified patient we abstracted data on age, race, gender, treatment, lymph node status, cause of death, time from diagnosis to death or last followup, and tumor histopathology, grade, size, extension and stage. Tumor size, extension, lymph node status and stage were used to construct a final stage scheme (T1N0M0, T2N0M0, T3N0M0, T4N0M0, N+M0, M+). For analysis we used the revised 1997 American Joint Committee on Cancer TNM staging system. We defined treatment as partial or radical nephrectomy.
Statistical Analysis
The primary outcome measures were the cancer specific and overall survival rates based on histopathological subtype at nephrectomy. Univariate and multivariate Cox proportional hazard modeling and Kaplan-Meier survival analysis were used to evaluate survival based on histopathology. Covariates included in the adjusted models were age, gender, race, grade and stage. The Chi-squared test was used to determine the association between histopathology and each covariate. Statistical analysis was done using R, version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 17,605 patients with RCC who underwent radical or partial nephrectomy between January 2000 and December 2005, and met our study inclusion criteria (table 1). Of these patients 78.6%, 12.9%, 5.4%, 2.5% and 0.6% had clear cell, papillary, chromophobe, sarcomatoid differentiation and collecting duct pathology, respectively. Median overall survival was 19 months (range 0 to 71). A total of 1,699 patients in our cohort died of RCC. Median survival in those who died of RCC was 10 months (range 0 to 66). Median survival in patients with sarcomatoid and collecting duct RCC was 6 (range 0 to 68) and 13 months (range 0 to 59), respectively, which was significantly less than for the clear cell (19 months, range 0 to 71), papillary (18, range 0 to 71) and chromophobe (19, range 0 to 71) subtypes (Pearson chi-square test p <0.001).
Table 1.
Demographics of 17,605 patients with surgically treated RCC stratified by histopathology (p <0.001)
| Variables | No. Clear Cell (%) | No. Papillary (%) | No. Chromophobe (%) | No. Sarcomatoid (%) | No. Collecting Duct (%) | Total No. (%) |
|---|---|---|---|---|---|---|
| Age group: p value | ||||||
| 30–40 | 658 (4.8) | 89 (3.9) | 78 (8.2) | 20 (4.5) | 5 (5.2) | 850 (4.8) |
| 40–50 | 2,064 (14.9) | 305 (13.4) | 169 (17.8) | 61 (13.9) | 13 (13.4) | 2,612 (14.8) |
| 50–60 | 3,651 (26.4) | 613 (26.9) | 213 (22.4) | 121 (27.5) | 34 (35.1) | 4,632 (26.3) |
| 60–70 | 3,768 (27.2) | 636 (27.9) | 228 (24.0) | 118 (26.8) | 20 (20.6) | 4,770 (27.1) |
| 70–80 | 2,923 (21.1) | 500 (21.9) | 187 (19.7) | 93 (21.1) | 16 (16.5) | 3,719 (21.1) |
| 80+ | 777 (5.6) | 135 (5.9) | 74 (7.8) | 27 (6.1) | 9 (9.3) | 1,022 (5.8) |
| Gender: p value | ||||||
| M | 8,501 (61.4) | 1,740 (76.4) | 568 (59.9) | 300 (68.2) | 68 (70.1) | 11,177 (63.5) |
| F | 5,340 (38.6) | 538 (23.6) | 381 (40.1) | 140 (31.8) | 29 (29.9) | 6,428 (36.5) |
| Race: p value | ||||||
| White | 12,022 (86.9) | 1,728 (75.9) | 760 (80.1) | 374 (85.0) | 69 (71.1) | 14,953 (84.9) |
| Black | 853 (6.2) | 482 (21.2) | 137 (14.4) | 37 (8.4) | 20 (20.6) | 1,529 (8.7) |
| Other | 966 (7.0) | 68 (3.0) | 52 (5.5) | 29 (6.6) | 8 (8.2) | 1,123 (6.4) |
| Stage: p value | ||||||
| T1N0M0 | 8,375 (60.5) | 1,592 (69.9) | 577 (60.8) | 36 (8.2) | 35 (36.1) | 10,615 (60.3) |
| T2N0M0 | 1,579 (11.1) | 285 (12.5) | 212 (22.3) | 40 (9.1) | 8 (8.2) | 2,124 (12.1) |
| T3N0M0 | 2,412 (17.4) | 228 (10.0) | 107 (11.3) | 82 (18.6) | 19 (19.6) | 2,848 (16.2) |
| T4N0M0 | 84 (0.6) | 8 (0.4) | 6 (0.6) | 39 (8.9) | 2 (2.1) | 139 (0.8) |
| N+M0 | 172 (1.2) | 67 (2.9) | 11 (1.2) | 46 (10.5) | 7 (7.2) | 303 (1.7) |
| M+ | 1,219 (8.8) | 98 (4.3) | 36 (3.8) | 197 (44.8) | 26 (26.8) | 1,576 (9.0) |
| Grade: p value | ||||||
| 1 | 2,487 (18.0) | 371 (16.3) | 81 (8.5) | 7 (1.6) | 10 (10.3) | 2,956 (16.8) |
| 2 | 7,410 (53.5) | 1,251 (54.9) | 558 (58.8) | 16 (3.6) | 20 (20.6) | 9,255 (52.6) |
| 3 | 3,247 (23.5) | 577 (25.3) | 263 (27.7) | 163 (37.0) | 48 (49.5) | 4,298 (24.4) |
| 4 | 697 (5.0) |
79 (3.5) |
47 (5.0) |
254 (57.7) |
19 (19.6) |
1,096 (6.2) |
| Totals | 13,841 (78.6) | 2,278 (12.9) | 949 (5.4) | 440 (2.5) | 97 (0.6) | 17,605 (100) |
Tumor stage was significantly associated with histopathology (p <0.001). Compared to patients with clear cell histology, of whom 28% had T3, T4, N+ or M+ disease, those with sarcomatoid and collecting duct variants were more likely to present with advanced disease (82.8% and 55.7% ,respectively) while patients with papillary and chromophobe variants were less likely to present with advanced disease (17.6% and 16.9%, respectively). Similarly a significant association was found between grade and histopathology with 94.7% of sarcomatoid and 69.1% of collecting duct cases presenting with grade 3 or 4 tumors compared with only 28.5%, 28.8% and 32.7% of clear cell, papillary and chromophobe cases, respectively (p <0.001).
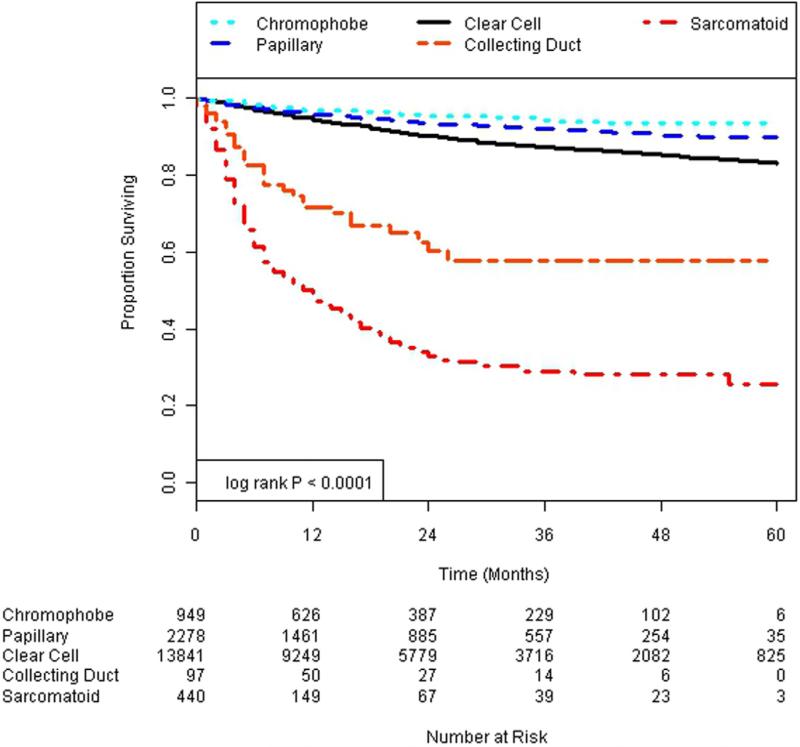
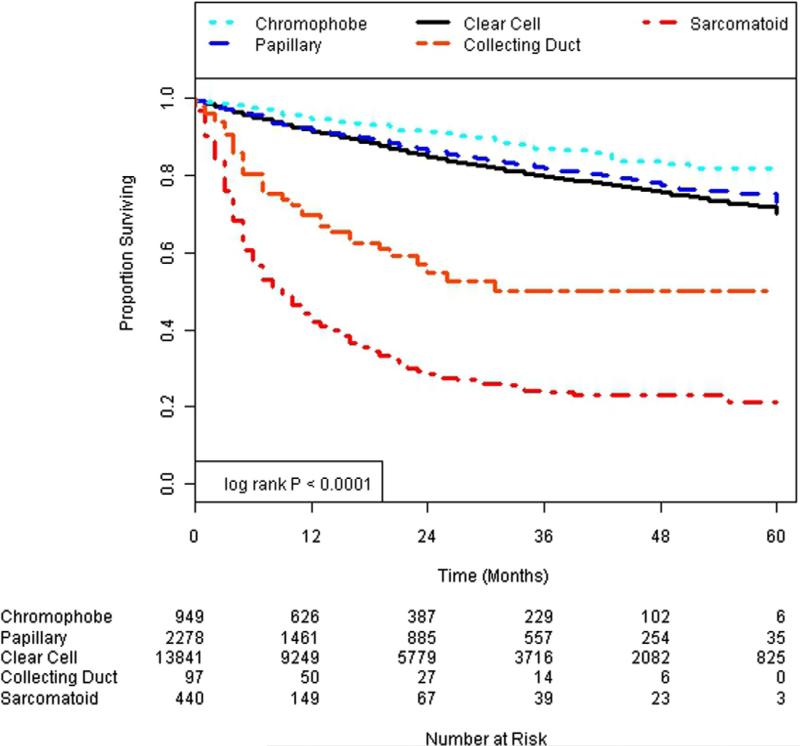
Figures 1 and 2 show Kaplan-Meier survival plots of the impact of histological subtype on cancer specific and overall survival. Univariate Cox proportional hazard models revealed that all variants of histopathology were statistically significant compared with the referent clear cell pathology for cancer specific and overall survival (table 2). Specifically papillary and chromophobe variants were associated with a significantly lower hazard of cancer specific and overall mortality compared with clear cell histology. In contrast, collecting duct and sarcomatoid histologies were associated with a significantly higher hazard of overall and cancer specific survival.
Figure 1.
Cancer specific survival.
Figure 2.
Overall survival.
Table 2.
Univariate and multivariate Cox proportional hazards models of cancer specific and overall survival
| Survival HR (95% CI) |
||
|---|---|---|
| Histopathology | Ca Specific | Overall |
| Univariate | ||
| Clear cell | Referent | Referent |
| Chromophobe | 0.44 (0.32–0.61) | 0.60 (0.48–0.75) |
| Papillary | 0.65 (0.54–0.78) | 0.90 (0.80–1.02) |
| Collecting duct | 4.49 (3.14–6.41) | 3.29 (2.38–4.55) |
| Sarcomatoid | 10.63 (9.26–12.22) | 7.52 (6.63–8.53) |
| Multivariate | ||
| Histopathology: | ||
| Clear cell | Referent | Referent |
| Chromophobe | 0.56 (0.40, 0.78) | 0.67 (0.53, 0.83) |
| Papillary | 0.85 (0.70, 1.02) | 0.98 (0.87, 1.12) |
| Collecting duct | 2.07 (1.44, 2.97) | 1.81 (1.31, 2.52) |
| Sarcomatoid | 2.26 (1.93, 2.64) | 2.21 (1.92, 2.55) |
| Age group: | ||
| 30–40 | Referent | Referent |
| 40–50 | 1.42 (1.02, 1.97) | 1.32 (1.01, 1.74) |
| 50–60 | 1.47 (1.07, 2.01) | 1.57 (1.21, 2.03) |
| 60–70 | 1.75 (1.28, 2.40) | 2.14 (1.66, 2.77) |
| 70–80 | 2.06 (1.50, 2.83) | 2.78 (2.15, 3.61) |
| 80+ | 2.44 (1.71, 3.48) | 4.41 (3.35, 5.80) |
| Gender: | ||
| M | Referent | Referent |
| F | 1.04 (0.94, 1.16) | 0.96 (0.89, 1.04) |
| Race: | ||
| White | Referent | Referent |
| Black | 1.29 (1.08, 1.54) | 1.49 (1.31, 1.69) |
| Other | 0.72 (0.58, 0.88) | 0.73 (0.62, 0.86) |
| Stage: | ||
| T1N0M0 | Referent | Referent |
| T2N0M0 | 2.71 (2.17, 3.39) | 1.41 (1.23, 1.63) |
| T3N0M0 | 5.20 (4.36, 6.21) | 2.10 (1.88, 2.35) |
| T4N0M0 | 16.88 (12.40, 22.98) | 5.57 (4.30, 7.23) |
| N+M0 | 16.33 (12.89, 20.73) | 5.50 (4.56, 6.63) |
| M+ | 33.23 (28.18, 39.18) | 10.74 (9.69, 11.92) |
| Grade: | ||
| 1 | Referent | Referent |
| 2 | 1.16 (0.94, 1.42) | 0.99 (0.88, 1.13) |
| 3 | 1.97 (1.60, 2.43) | 1.44 (1.26, 1.64) |
| 4 | 2.62 (2.08, 3.31) | 1.93 (1.64, 2.27) |
The effects of histopathology on cancer specific and overall survival were then explored using a multivariate Cox proportional hazards regression model adjusted for the covariates patient age, gender, race, grade and stage. Except for patient gender each covariate remained a statistically significant predictor of survival in the multivariate model (table 2). The effects of histopathology were decreased substantially after accounting for the covariates, although the general direction of the relationships was unchanged. Compared with clear cell tumors a lower hazard of cancer specific mortality was identified in patients with chromophobe variants (HR 0.56, 95% CI 0.40–0.78) while a higher hazard of mortality was found in those with sarcomatoid and collecting duct pathology (HR 2.26, 95% CI 1.93–2.64 and 2.07, 95% CI 1.44–2.97, respectively). It was not statistically significant on multivariate analysis but there was a trend toward a lower hazard of cancer specific mortality in patients with papillary histology (HR 0.85, 95% 0.70–1.02). Notably there was little difference in overall survival between papillary and clear cell pathologies.
DISCUSSION
These data reveal several important findings regarding the proportion of specific renal cell subtypes and the demographic distribution of renal cell histopathology. Of note, this Importantly, this secondary data analysis reflects a population based cohort after the incorporation of consensus pathology definitions and during a contemporary era of increased detection of incidental renal masses. As expected, clear cell and papillary variants are the 2 most common RCC pathologies and their proportions were approximately similar to those in previous series.4–6,8 All RCCs show a male predominance, although chromophobe tumors are relatively more common in women, a finding noted but not elaborated upon in other series.11,15,16 Congruent with previous reports11,15,16 chromophobe variants present at greater size than clear cell variants (22.3% vs 11.1% were T2). Patients with chromophobe RCC remain at lower risk for cancer specific and overall mortality despite this tendency toward higher stage at presentation. Lastly, chromophobe tumors have an increased tendency to present at age extremes relative to other RCC pathologies.
Demographics aside, prior data are conflicting regarding the impact of RCC histopathology on survival. On univariate analysis initial studies showed that histopathology predicts survival. Some groups discovered a survival advantage in patients with papillary and chromophobe variants.4–6,8 Consistent with these studies our data reveal that patients with chromophobe and papillary RCC had improved cancer specific survival on univariate analysis relative to those with clear cell RCC. This trend persisted in multivariate analysis, although it was not statistically significant. Our data demonstrate that papillary histology is disproportionately represented in black Americans. Furthermore, papillary RCC is associated with end stage and acquired cystic renal disease.17 While speculative, the increased comorbidities, lower socioeconomic status and health care disparities associated with these groups18,19 may explain the discrepancy between papillary cancer specific and overall survival.
In contrast to chromophobe and papillary RCC, the collecting duct and sarcomatoid variants have a universally poor outcome relative to that of clear cell RCC. For sarcomatoid differentiation, poor outcomes are statistically significant despite adjusting for stage and grade. Also, sarcomatoid and collecting duct tumors present with a high percent of metastatic disease (55.3% and 34.0%, respectively, p <0.001), showing higher metastatic potential than clear cell RCC (10.0%).
Our data suggest that the most powerful predictor of outcome is tumor stage, as borne out by previous series. Notably, this study was sufficiently powered to detect significant differences in survival by each histological subtype. The conflicting results of variable outcomes by histology in the literature may be due to a substantial co-linear relationship of histopathology with stage and grade.
In the last 25 years there has been an increase in the incidence of renal cell cancer with subsequent stage migration.20,21 Paradoxically, the increased detection and treatment of RCC is associated with a rise in cancer specific mortality from 1.5 deaths per 100,000 population in 1983 to 6.5/100,000 in 2002.22 The implications of this are twofold. This not only bolsters the claim that observation may be warranted for a proportion of small renal masses since surgery for these masses has not improved survival but also indicates that the biological behavior of renal masses is disparate. While some tumors are indolent, others are destined to progress. Furthermore, aggressive tumors, such as sarcomatoid or collecting duct lesions, show diverse gene mutations23–25 as well as a variable response to chemotherapy and immunotherapy for metastatic disease.26,27 In sum these data point to inherent differences in RCC tumor biology and call for improved pretreatment diagnostic techniques, ie biopsy and molecular markers, since exploiting these biological differences would likely form the basis of future treatment decision making and targeted therapy.
While this analysis is robust, it is limited in several respects. Our data show that high tumor grade is an independent predictor of cancer specific and overall survival but considerable debate exists on the validity and accuracy of grading for nonclear cell renal cancers.28–30 Also, SEER data are retrospective in nature with inherent limitations, such as omitted variable bias. Furthermore, the likelihood of undergoing surgery favors patients with resectable disease. Thus, there exists a potential selection bias against patients who present with high stage disease. Moreover, unlinked SEER registry data lack coding for comorbidity, immunotherapy, and as well as performance status and symptom data. The latter parameters were significant determinants of outcome in key institutional series.6,11 Further limitations that are particularly germane to this analysis are the limitations of SEER data due to the lack of centralized pathological review and the inability to differentiate types I and II papillary RCC. These are shortcomings of many administrative data sets in terms of interrater variability, lack of consistency in grading schema and uniform pathological interpretation.28
Despite limitations our findings are notable. To our knowledge this report represents the largest study to date to evaluate the role of all renal cell histopathology on patient survival in the computerized tomography era.
CONCLUSIONS
Histological subtype in patients with RCC predicts overall and cancer specific survival. Despite adjustment for stage, patients with collecting duct and sarcomatoid RCC variants have poor survival. These findings may inform the role of percutaneous renal biopsy and other pretreatment diagnostic techniques since differences in histology reflect intrinsic biological variance and may ultimately form the basis of future targeted therapy.
Acknowledgments
Supported by the National Institutes of Health K12 Paul Calabresi Career Development Award for Clinical Oncology CA-90625 (KAK) and Ruth L. Kirschstein National Research Service Award Extramural, 1 F32 CA144461-01 (K.C.); American Cancer Society, 117496-PF-09-147-01-CPHPS (KC); Jonsson Comprehensive Cancer Center Seed Grant (KC).
Abbreviations and Acronyms
- RCC
renal cell carcinoma
REFERENCES
- 1.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ro J, Ayala A, Sella A, et al. Sarcomatoid renal cell carcinoma: clinicopathologic study of 42 cases. Cancer. 1987;59:516. doi: 10.1002/1097-0142(19870201)59:3<516::aid-cncr2820590327>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Alamdari FI, Stenling R, et al. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol. 1999;36:565. doi: 10.1159/000020049. [DOI] [PubMed] [Google Scholar]
- 5.Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histological classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000;89:604. [PubMed] [Google Scholar]
- 6.Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 9.Dall'Oglio MF, Antunes AA, Pompeo AC, et al. Prognostic relevance of the histological subtype of renal cell carcinoma. Int Braz J Urol. 2008;34:3. doi: 10.1590/s1677-55382008000100002. [DOI] [PubMed] [Google Scholar]
- 10.Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol. 2009;182:2132. doi: 10.1016/j.juro.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibovitch BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Patard JJ, Leray E, Rioux-LeClerq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 13.Capitanio U, Cloutier V, Zini L, et al. A critical assessment of the prognostic value of clear cell, papillary, and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int. 2008;103:1496. doi: 10.1111/j.1464-410X.2008.08259.x. [DOI] [PubMed] [Google Scholar]
- 14.SEER Cancer Statistics, 1973–2005. Surveillance, Epidemiology and End Results Limited-Use Data (1973–2005) National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; Bethesda: [March 2009]. released April 2008, based on the November 2007 submission. Available at http://seer.cancer.gov. [Google Scholar]
- 15.Rothman J, Egelston B, Wong YN, et al. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181:29. doi: 10.1016/j.juro.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpe A, Novara G, Antonelli A, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. doi: 10.1111/j.1464-410X.2011.10690.x. Epub ahead of print November 1, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa I, Kovacs G. High incidence of papillary renal cell tumours in patients on chronic haemodialysis. Histopathology. 1993;22:135. doi: 10.1111/j.1365-2559.1993.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 18.Berndt SI, Carter HB, Schoenberg MP, et al. Disparities in treatment and outcome for renal cell cancer among older black and white patients. J Clin Oncol. 2007;25:3589. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- 19.Hellenthal NJ, Bermejo CE. The role of socioeconomic status in renal cell carcinoma. Urol Oncol. 2012;30:89. doi: 10.1016/j.urolonc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 21.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki K, Sakamoto M, Ohta T, et al. Overexpression of KIT in chromophobe renal cell carcinoma. Oncogene. 2003;22:847. doi: 10.1038/sj.onc.1206153. [DOI] [PubMed] [Google Scholar]
- 24.Jones TD, Eble JN, Wang M, et al. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer. 2005;104:1195. doi: 10.1002/cncr.21288. [DOI] [PubMed] [Google Scholar]
- 25.Salvi A, Marchina E, Benetti A, et al. Germline and somatic c-met mutations in multifocal/bilateral and sporadic papillary renal carcinomas of selected patients. Int J Oncol. 2008;33:271. [PubMed] [Google Scholar]
- 26.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 27.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 28.Delahunt B. Advances and controversies in grading and staging of renal cell carcinoma. Mod Pathol, suppl. 2009;22:S24. doi: 10.1038/modpathol.2008.183. [DOI] [PubMed] [Google Scholar]
- 29.Finley DS, Shuch B, Said JW, et al. The chromophobe tumor grading system is the preferred grading scheme for chromophobe renal cell carcinoma. J Urol. 2011;186:2168. doi: 10.1016/j.juro.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 30.Sukov WR, Lohse CM, Leibovich BC, et al. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol. 2012;187:54. doi: 10.1016/j.juro.2011.09.053. [DOI] [PubMed] [Google Scholar]