Abstract
Minimally invasive transforaminal lumbar interbody fusion (TLIF) offers equivalent postoperative fusion rates compared to posterior lumbar fusion (PLF) and minimizes the amount of iatrogenic injury to the spinal muscles. The objective of this study was to examine the difference in pain perception, stress, mood disturbance, quality of life, and immunological indices throughout the perioperative course among patients undergoing TLIF and PLF. A prospective, nonrandomized descriptive design was used to evaluate these measures among patients undergoing TLIF (n = 17) or PLF (n = 18) at 1 week prior to surgery (T1), the day of surgery (T2), 24 hours postoperatively (T3), and 6 weeks postoperatively (T4). Among TLIF patients, pain, stress, fatigue, and mood disturbance were significantly decreased at the 6-week follow-up visit (T4) compared to patients who underwent PLF. The TLIF group also demonstrated significantly higher levels (near baseline) of CD8 cells atT4 than the PLF group. Interleukin-6 levels were significantly higher in the TLIF group as well, which may be an indicator of ongoing nerve regeneration and healing. Knowledge concerning the effect of pain and the psychological experience on immunity among individuals undergoing spinal fusion can help nurses tailor interventions to improve outcomes, regardless of the approach used.
More than 298,000 lumbar spinal fusion procedures are performed annually in the United States, costing more than $11 billion a year (Katz, 2006). Degenerative processes within the intervertebral disc and lumbar vertebrae from normal aging or repetitive motion can cause segmental instability, recurrent disc herniation, spondylolisthesis, and degenerative disc disease, all of which are indications for lumbar fusion (Slosar, 2002). As the use of minimally invasive fusion techniques has grown, more data on the benefits of these procedures, as opposed to traditional posterior lumbar fusion (PLF), have been accumulating. One of these benefits is the lessened degree of immunologic alterations after the procedure.
The PLF procedure with auto- or allograft requires an extensive, 5- to 7-inch incision that results in iatrogenic injury to the dorsal musculoligamentous, vascular complex. Although the PLF has an excellent rate of fusion (Gill & Blumenthal, 1993), it can often be a source of significant postoperative pain, irritation, and muscle spasm. In contrast, the minimally invasive transforaminal lumbar interbody fusion (TLIF) technique minimizes the amount of operative iatrogenic injury without sacrificing the goals of the traditional open procedure (Khoo, Palmer, Laich, & Fessler, 2002). This technique entails the same degree of decompression and stabilization of the spine and has been used among patients who would have previously required the open PLF approach (Kim, Lee, Chung, & Lee, 2005).
The option to use minimally invasive techniques in spinal surgery has increased as improved patient outcomes and lower hospital costs have been realized. These results are hypothesized to come from the minimized tissue trauma alone. However, it makes sense that these patients also have less tissue inflammation and thus fewer biochemical markers of inflannnation during and after the procedure. Minimizing the amount of inflammation should reduce pain, fatigue, and other symptoms associated with increased pro-inflannnatory cytokine production (Watkins & Maier, 2000; Watkins, Milligan, & Maier, 2003).
Of particular interest is the level of interleukin-6 (IL-6) that has shown an association with the magnitude of surgery. Natural killer cell activity (NKCA) is a strong indicator of innate immune competence and has been used frequently to assess outcomes during the perioperative period (Page, 2005). Although these measures provide a snapshot of immunologic changes within the individual, variations over time may provide important information on the biobehavioral interactions that can work toward improving patient outcomes.
The following study investigated perioperative immune profiles including NKCA, CD4, CD8, CD16, CD56bright, CD56dim, and IL-6 levels as measures of the inflannnatory process in patients undergoing TLIF and PLF to determine differences and associations with outcomes measures of pain, stress, mood disturbance, and quality of life. It was hypothesized that the TLIF group would demonstrate less alteration in immune profiles than the PLF group and that lower levels of IL-6 would be associated with improved pain and quality of life in the postoperative period.
Method
Participants
Thirty-five men and women 18–70 years of age who had been diagnosed with lumbar instability confirmed by magnetic resonance imaging (MRI) and plain films within the past year were recruited for study enrollment. All participants were recruited from the neurological surgery department at a major academic medical center in the Chicago area during their preoperative work-up 1 week prior to surgery. Patients were excluded if they had neoplastic or major immune-based disease; liver, kidney, or heart disease; a past history of drug or alcohol abuse; a mood or mental disorder; or were taking corticosteroids, nonsteroidal antiinflammatories, anxiolytics, or antidepressant drugs. The patients were also excluded if they were incapable of reading or writing English.
Materials
Pain perception
The Short-Form McGill Pain Questionnaire (SF-MPQ) is a reliable, self-report measure of pain perception (Melzack, 1983). It contains 15 verbal descriptors of sensory and affective dimensions of pain, a visual analog scale (VAS), and present pain intensity scale. A total evaluative score can be obtained (ranging from 0 to 70 using a 20-point VAS). The SF-MPQ is scored by adding the numeric value of each pain dimension, with higher scores indicating higher levels of pain perception. Internal consistency of the SF-MPQ is good, and test-retest reliability is reported to be between 0.64 and 0.87 (Graham, Bond, Gerkousch, & Cook, 1988).
Stress
The Perceived Stress Scale (PSS) is the most widely used psychological instrument for measuring the perception of stress. PSS is a brief 10-item scale measuring the degree to which experiences are appraised as uncontrollable (Cohen, 1994). Individuals rate their responses using a 5-point Likert scale. A total score is provided by adding the responses together, with a higher score (0–40) indicating a higher level of perceived stress. Internal consistency of the PSS ranges from .75 to .86, and test-retest reliability is 0.85 (Cohen, Kamarch, & Mermelstein, 1983).
Mood
The Profile of Mood States (POMS) is a well-established self-report measure designed to assess general mood. Respondents are provided with a list of 65 adjectives representative of mood states and are asked to indicate their agreement with the adjective (mood state) using a 5-point Likert scale (ranging from not at all to extremely). A total POMS score and six subscores (tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia, and confusion/bewilderment) are obtained from the scoring algorithm. A higher total score (−24–177) indicates a higher level of mood disturbance. Internal consistency is good (α = .84 and .95), and test-retest coefficients range from 0.65 to 0.74 (McNair, Lorr, & Droppleman, 1992).
Quality of life
The SF-36 Health Survey (a short-form survey with 36 questions) is the most widely used health-status questionnaire in the world. The SF-36 measures eight health concepts: (1) limitations in physical activities because of health problems, (2) limitations in social activities because of physical or emotional problems, (3) limitations in usual role activities because of physical health problems, (4) bodily pain, (5) general mental health (psychological distress and well-being), (6) limitations in usual role activities because of emotional problems, (7) vitality (energy and fatigue), and (8) general health perceptions. A higher score reflects a more favorable health outcome. The SF-36 is scored so that all scales are on the same metric, where 50 is the mean for the U.S. general population and 10 is the standard deviation. Norm-based scoring equates all scores, so scores greater than 50 are better than the general population average for all scales, and scores less than 50 are worse. Test-retest reliability of the SF-36 Version 2 is reported to be high (0.85), and internal consistency is good (α = .88 and .95; Ware, Kosinski, & Dewey, 1992).
Natural killer cell activity (NKCA)
Peripheral blood mononuclear cells (PBMCs) were derived from peripheral blood by Ficoll-Hypaque separation and evaluated for NKCA (Beno, Stover, & Mathews, 1995). Lytic units (LU) were calculated and represent the number of cells per 107 effectors required to achieve 20% lysis of the targets. The K562 tumor cells (American Type Culture Collection) are maintained in our lab in suspension cultures in vitro. Interassay variability ranges did not exceed 7.2%. Measures of NKCA provide a general picture immune system functioning, with higher levels representing healthy immune status (normally more than 125 LU 20% in this age group).
Circulating PEMC number and percentage
We evaluated each blood sample for the number and percentage of circulating PBMCs. Isolated PBMCs were analyzed with specific fluorescently conjugated antibodies to identify CD4, CD8, CD16, CD56bright, and CD56dim subsets of PBMCs using a fluorescent-activated cell sorted (FACS Star Plus) system. These assessments were as, described previously by this laboratory (Beno et al., 1995).
PEMC cytokine production
Unstimulated IL-6 production was assessed as described previously (Witek-Janusek & Mathews, 1999). For cytokine production, PBMCs were cultured with and without phorbol myristate acetate/phytohemagglutinin (PMA/PHA) for 48 hours. Supernatants were stored frozen at −85 °C for later cytokine analysis by enzyme-linked immunosorbant assays using commercially available kits according to the manufacturers’ instructions (R & D Systems, Minneapolis, MN). Sensitivity was 0.7 pg/ml for IL-6. Because limited resources, IL-6 was the only cytokine measured in this study. Measurement of IL-6 provides a general snapshot of inflammation, and levels for healthy adults are usually less than 2.0 pg/ml.
Design and Procedure
This study used a prospective, nonrandomized descriptive comparison design to investigate differences in immune profiles between patients undergoing TLIF (n = 17) and PLF (n = 18). The independent factor was the type of surgery (TLIF versus PLF), and the dependent factors were pain (SF-MPQ), stress (PSS), mood (POMS), quality of life (SF-36 Health Survey), and immune profiles.
After obtaining approval from the institutional review board at the study site, each participant signed a consent form for participation in the study. Participants were already scheduled for their surgical procedure, and the type of surgery was decided upon by the primary surgeon based on the patient’s past surgical history, degree of spondylosis, and anatomy. One surgeon performed all of the surgical procedures in this study. Pain, stress, and mood were measured using self-report instruments 1 week before surgery (T1), the day of surgery (T2), the day after surgery (T3), and 6 weeks after surgery (T4), while quality of life was measured at T1 and T4. Blood (30 ml) was collected for measurement of phenotypic analyses and the stimulated production of cytokine IL-6 at each time point (T1–T4).
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS; Chicago, IL), version 12.0 for Windows, was used for data analyses. Equivalence of groups was established with Levene’s test for equality of variances. Repeated measures analysis of variance was used for each variable, with group (TLIF versus PLF) as the between-subjects measure and time (T1, T2, T3, T4) as the within-subjects measure. A post-hoc least-significant-difference test was performed on signiflcant findings. Correlations were run using Pearson’s correlation coefficient with a probability level of .01.
Results
Demographic data are listed in Table 1. The PLF group had individuals from lower income levels and a lower rate of employment (33% versus 88%) than the TLIF group. The PLF group also had more retired people (39% versus 1%) and disabled people (16% versus 0%). Eight participants in the PLF group and six participants in the TLIF group had a history of prior spinal surgery. Hospitalization data are listed in Table 2. As shown, the PLF group had a higher mean of levels fused (3 versus 2). There were statistically signiflcant differences between groups in relation to blood loss, length of surgery (from flrst incision to closure), and length of stay, with the TLIF group having lower values in all categories. No complications were reported for the study participants throughout the 2-month period.
Table 1.
Demographic Means and Frequencies
| Variable | TLIF (n = 17) | PLF (n = 18) |
|---|---|---|
| Mean age (SD) range |
52 (12) 28–70 |
51 (16) 19–70 |
| Marital status | ||
| Married | 15 | 13 |
| Widowed | 1 | 2 |
| Single | 1 | 3 |
| Employment status | ||
| Employed | 15 | 6 |
| Retired | 1 | 7 |
| Disabled | 0 | 3 |
| Unemployed | 1 | 2 |
| Income level | ||
| >$60,000 | 6 | 2 |
| >$40,000 | 6 | 3 |
| >$20,000 | 4 | 7 |
| <$20,000 | 1 | 6 |
| Onset of symptoms | ||
| >10 years | 3 | 1 |
| >5 years | 3 | 2 |
| >1 year | 9 | 13 |
| <1 year | 2 | 2 |
| Gender | ||
| Male | 10 | 8 |
| Female | 7 | 10 |
| Ethnicity | ||
| Caucasian | 17 | 17 |
| African American | 0 | 1 |
Note. TLIF = transforaminallumbar interbody fusion; PLF = posterior lumbar fusion.
Table 2.
Hospitalization Data
| TLIF (n = 17) | PLF (n = 18) | P | |
|---|---|---|---|
| Number of levels fused | N/A | ||
| Range | 2–3 | 3–4 | |
| Mean | 2 | 3 | |
| Blood loss | |||
| Range | 80–120 ml | 300–500 ml | |
| Mean | 96ml | 427ml | |
| Time of operation | |||
| Range | 70–140 minutes | 120–360 minutes | |
| Mean | 110 minutes | 298 minutes | |
| Length of stay | |||
| Range | 2–5 days | 5–10 days | |
| Mean | 1.8 days | 5 days | |
| Complications | 0 | 0 |
Note. TLIF = transforaminallumbar interbody fusion; PLF = posterior lumbar fusion.
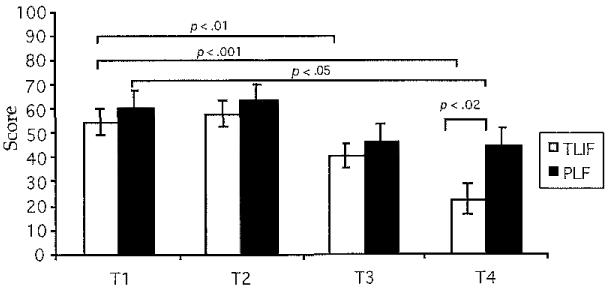
Pain had a significant time interaction (F = 6.9, df = 3, p < .0001) and group by time interaction (F = 261.1, df = 1, p < .0001). As seen in Figure 1, preoperative (T1 and T2) and postoperative (T3) SF-MPQ scores were equivalent between groups. However, the TLIF SF-MPQ score decreased significantly between T1 and T3 (t = 2.94, df = 16, p < .01) and T1 and T4 (t = 2.66, df = 16, p < .001), and the PLF group SF-MPQ score decreased between T1 and T4 (t = 2.14, df = 17, p < .05). At T4, the TLIF group scored significantly lower on the SF-MPQ than the PLF group (t = 2.39, df = 33, p < .02).
Fig 1. Short-Form McGill Pain Questionnaire: Evaluative Overall Intensity of Pain Experience.
Note. Bars represent mean values for the Short-Form McGill Pain Questionnaire scores. Error bars represent standard error of measurement. Independent t tests were used to perform between-group analyses, and paired t tests were used to perform within-group analyses for T1, T2, T3, and T4. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T2 = day of surgery; T3 = day after surgery; T4 == 6 weeks after surgery.
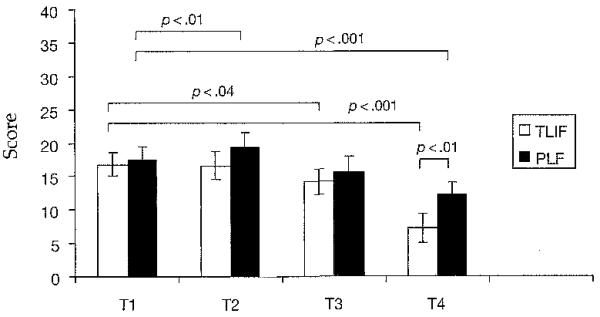
Stress had a significant time interaction (F = 4.29, df = 3, p < .003) and group by time interaction (F = 238.4, df = 1, p < .0001). At T1, stress scores were equivalent between groups. For T2, the PLF group PSS score increased when compared to T1 (t = 3.12, df = 17, p < .01; Fig 2). The TLIF group PSS score decreased between T1 and T3 (t = 2.26, df = 16, p < .04) and T1 and T4 (t = 3.95, df = 16, p < .001), and the PLF group PSS score did not decrease significantly until T4 (t = 3.7, df = 17, p < .001). In addition, the TLIF group had a significantly lower PSS score than the PLF group at T4 (t = 2.11, df = 33, p < .01).
Fig 2. Perceived Stress Scale Scores.
Note. Bars represent mean values for the Perceived Stress Scale scores. Error bars represent standard error of measurement. Independent t tests were used to perform between-group analyses, and paired t tests were used to perform within-group analyses for T1, T2, T3, and T4. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T2 = day of surgery; T3 = day after surgery; T4 = 6 weeks after surgery.
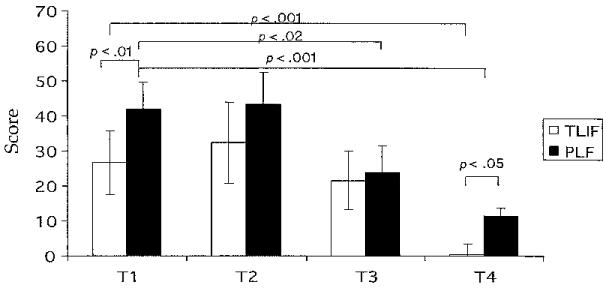
Mood disturbance had a significant time interaction (F = 4.76, df = 3, p < .004) and group by time interaction (F = 35.6, df = 1, p < .0001). As seen in Figure 3, the TLIF group total POMS score was lower than that of the PLF group at T1 (t = 3.3, df = 33, p < .01). The TLIF POMS score decreased between T1 and T4 (t = 4.9, df = 16, p < .001), while the PLF group POMS score decreased between T1 and T3 (t = 2.7, df = 17, p < .02) and T1 and T4 (t = 5.66, df = 17, p < .001). At T4, the TLIF group had a significantly lower POMS score than the PLF group (t = 2.80, df = 33, p < .05).
Fig 3. Profile of Mood States Total Scores.
Note. Bars represent mean values for the Profile of Mood States total scores. Error bars represent standard error of measurement. Independent t tests were used to perform between-group analyses, and paired t tests were used to perform within-group analyses for T1, T2, T3, and T4. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T2 = day of surgery; T3 = day after surgery; T4 = 6 weeks after surgery.
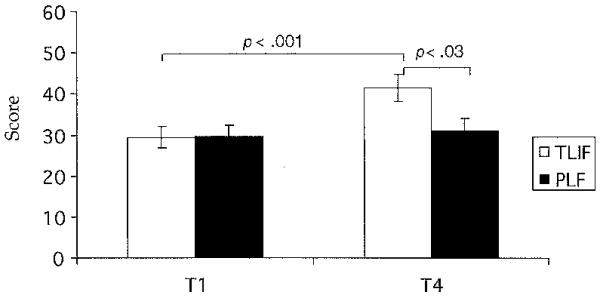
Role physical (RP) scores, a component of the SF-36 that measures the degree in which physical health affects activities of daily living, had a significant time interaction (F = 4.7, df = 1, p < .03) and group-time interaction (F = 5.1, df = 33, p < .01). According to Figure 4, between T1 and T4, the TLIF group RP score increased (t = 3.36, df = 16, p < .001). The TLIF group had a significantly higher RP score than the PLF group at T4 (t = 2.35, df = 33, p < .03). Physical functioning ahd bodily pain (not shown) had significant time interactions (F = 5.17, df = 1, p < .03; F = 11.66, df = 1, p < .002 respectively), such that both groups demonstrated improvements over time: TLIF physical functioning (t = 2.60, df = 16, p < .02; bodily pain t = 2.99, df = 16, p < .01) and PLF physical functioning (t = 4.15, df = 17, p < .001; bodily pain t = 3.87, df = 17, p < .001). No differences between groups were demonstrated for physical functioning or bodily pain. Vitality, another measure of the SF-36 Health Survey, had a significant time interaction (F = 3.2, df = 1, p < .05). The TLIF group vitality score increased between T1 and T4 (t = 2.78, df = 16, p < .01), but no differences between TLIF and PLF were observed at either time point (not shown).
Fig 4. SF-36 Health Survey Role Physical Scores.
Note. Bars represent mean values for the SF-36 Health Survey Role Physical scores. Error bars represent standard error of measurement. Independent t tests were used to perform between-group analyses, and paired t tests were used to perform within-group analyses for T1 and T4. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T4 = 6 weeks after surgery.
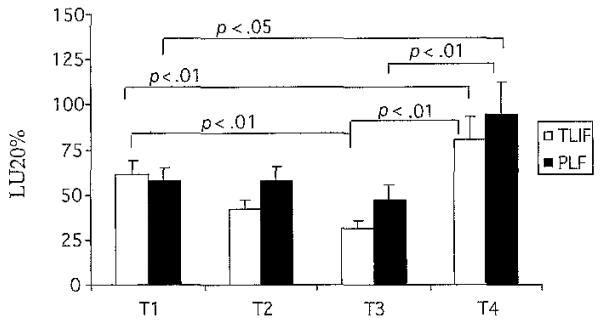
NKCA is a general indicator of overall immune system functioning. NKCA had a significant time interaction (F = 4.28, df = 3, p < .01). As displayed in Figure 5, the TLIF group demonstrated a significant decrease in NKCA between T1 and T3 (t = 3.1, df = 16, p = .01). However, between T1 and T4, the TLIF and PLF group demonstrated a significant. increase in NKCA (t = 3.2, df = 16, p < .01; t = 2.2, df = 17, p < .05 respectively). An interesting finding was observed in the mean NKCA at T1 for all study participants (51.9 ± 30.4): it was significantly below control values of patients who were not undergoing surgery and who did not report any pain (95.2 ± 70.7; p < .02; not shown).
Fig 5. Natural Killer Cell Activity.
Note. Bars represent mean values of Natural Killer Cell Activity. Error bars represent standard error of measurement. Paired t tests were used to perform within-group analysis for immune variables for each group. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T2 = day of surgery; T3 = day after surgery; T4 = 6 weeks after surgery; LU = lytic units.
CD4 (F = 7.5, df = 3, p < .0001), CD8 (F = 19.66, df = 3, p < .0001), CD16 (F = 7.45, df = 3, p < .0001), CD56bright (F = 3.7, df = 3, p < .001), and CD56dim (F = 3.6, df = 3, p < .03) cells had significant time interactions (not shown). However, only the CD8 cells were significantly different between groups. At T4, the TLIF group had a significantly higher CD8 count than the PLF group (t = 3.5; df = 1. p < .05).
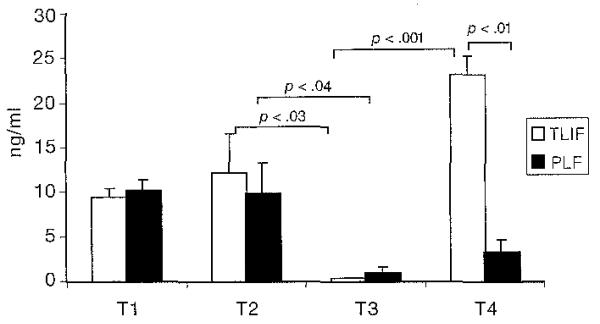
IL-6 production has a significant time interaction (F = 4.17, df = 3, p < .05) and group-time interaction (F = 9.2, df = 1, p < .005). As displayed in Figure 6, the TLIF group (t = 3.4, df = 9. p < .03) and PLF group (t = 3.1, df = 14, p < .04) IL-6 production decreased between T2 and T3. However, the TLIF group IL-6 production increased between T3 and T4 (t = 4.1, df = 9, p < .00l).At T4, the TLIF IL-6 production was significantly higher than the PLF group (F = 3.9, df = 24, p < .01).
Fig 6. Peripheral Interleukin-6 Production.
Note. Bars represent mean values of interleukin-6 production. Error bars represent standard error of measurement. Paired t tests were used to perform within-group analysis, and paired t tests were used to perform between-group analysis for immune variables for each group. TLIF = transforaminal lumbar interbody fusion, n = 17; PLF = posterior lumbar fusion, n = 18; T1 = 1 week before surgery; T2 = day of surgery; T3 = day after surgery; T4 = 6 weeks after surgery.
Pain was highly correlated with stress (r = .76, p < .005), mood disturbance (r = .78, p < .008), and IL-6 production (r = .674, p < .04) during the preoperative and immediate postoperative periods (T1–T3) for both groups, but not at T4.
Discussion
This study identified important psychological and immunological differences among patients that received a minimally invasive fusion (TLIF) compared to patients that received the open approach (PLF). Among TLIF patients, pain, stress, mood disturbance, and several components of quality of life were significantly improved at T4 compared to the PLF group. Although physical functioning and bodily pain improved significantly among both groups. the TLIF group had additional improvements in vitality and role physical scores. These findings are congruent with recent studies evaluating outcomes of minimally invasive spine procedures (Hee, Castro, Majd, Holt, & Myers. 2001; Potter et al., 2005; Schwender, Holly, Rouben, & Foley, 2005).
In regard to immunological profiles, the TLIF group demonstrated significantly higher levels of CD8 cells at T4. Both groups experienced a universal decrease in immune variables at T3, which has been described in other studies and is attributed to the effect of anesthesia (Angele & Faist, 2002; Volk et al., 2004). The TLIF group had a significantly higher production of IL-6 than the PLF group at T4. However, there is evidence to support the theory that this increase may be protective or part of the nerve regeneration process.
For instance, sensory axonal regeneration is attenuated in IL-6 knockout mice (Zhong & Heumann, 1995). and neuronal loss in the dorsal root ganglion after nerve injury was higher than in wild-type mice. meaning that the deficit of IL-6 was a cause of neuron death and dysfunction (Murphy et al., 2000). These and other studies indicate a positive effect of IL-6 on neuronal survival and nerve regeneration in vivo (Efron & Moldawer, 2004; Lin, Kondo, Ishida, Takayasu, & Mukaida, 2003). This result may indicate that the increase in IL-6 at T4 was a sign of neuronal regeneration (as opposed to acute inflammation) in the TLIF group. Further investigation on the postoperative recovery of spinal patients and peripheral cytokine production is needed to verifY this finding.
The results have several nursing implications for patients undergoing spine surgery. It is theorized that surgical patients who are exposed to infectious agents or who have an underlying immune-based rlisease process (i.e., cancer, multiple sclerosis, lupus) are highly vulnerable during the postoperative period because of the characteristic immunodepression (Page, 2005). Postoperative pain is widely accepted as a contributing factor to immune dysfunction because of known interactions between the central nervous system and the immune system (Watkins et al., 2003). In addition, past studies have eloquently demonstrated that preoperative stress and anxiety do indeed affect postoperative recovery (Glaser, Rice, Speicher, Stout, & Kiecolt-Glaser, 1986; Johnston, 1986; Kiecolt-Glaser, Page, Marucha, MacCallum, & Glaser, 1998), as well as immune profiles (Maes et al., 1998).
Nursing interventions that decrease pain, stress, and mood disturbance, and are known to improve immune status—such as massage (Goodfellow, 2003; Hernandez-Reif et al., 2004), relaxation therapy, and biofeedback (McGrady et al., 1994)—may be beneficial during this vulnerable period. Kain and colleagues (2000) demonstrated that presurgical administration of midazolam not only reduced the postsurgical levels of pain and anxiety but also reduced the incidence of surgical wound infections. As this study found, surgical patients are especially susceptible to perioperative stress and subsequent immune dysfunction, which may allow similar interventions a significant opportunity to modify infection rates and other outcomes in this population.
Future investigations might incorporate biobehavioral methods specifically designed to decrease stress, anxiety, and mood disturbance while tracking clinical outcomes, such as immunity and infection rates. In addition, peripheral proinflammatory cytokines other than IL-6 can be investigated to elucidate the relationship between the magnitude of surgery and biochemical markers.
Limitations
This study had several notable weaknesses, the first being the small sample size. Second, although all study participants were diagnosed with lumbar spondylosis with instability, they were preselected by the neurosurgeon to receive either TLIF or PLF. There could have been additional pathology that was not controlled for in the inclusion/exclusion criteria, such as the degree of spondylosis, number of levels fused, or other comorbidities that may have had an influence on results. Third, peripheral IL-6 production did not correlate with the magnitude of surgery in this study. Biomarkers of skeletal turnover may be a better indicator of spinal pathology and treatment (Poole, 2006). In addition, other influences on IL-6 production, such as body-mass index, anesthesia, and sleep disturbances, were not accounted for in this study. Other researchers have shown that higher levels of IL-6 are associated with greater body fat and sleep disturbances (Vgontzas et al., 1997; Vgontzas et al., 1999).
Summary
This study demonstrated that patients undergoing spinal surgery regardless of the approach, experience extensive pain, stress, and mood disturbance prior to surgery. However, substantial improvements in these measures were observed by the 6-week follow-up visit. Comparatively, the TLIF participants had statistically significant improvements in pain, stress, mood disturbance, and several quality-of-life indicators at T4 compared to the PLF group. Immune profiles demonstrated that the TLIF group had a quicker postoperative recovery in CD8 cells and that IL-6 levels increased substantially, possibly indicating more pronounced neuronal regeneration and healing. These findings, along with the documented benefits of less blood loss, less operative time, and shorter length of stay, make the TLIF an appealing procedure compared to the open approach.
Acknowledgments
We would like to thank the American Association Spinal Cord Injury Nurses, Neuroscience Nursing Foundation, Sigma Theta Tau Alpha Beta Chapter, and the Illinois Emergency Nurses Association for their support in conducting this research.
Contributor Information
Angela R. Starkweather, Intercollegiate College of Nursing at Washington State University, Spokane, WA.
Linda Witek-Janusek, Niehoff School of Nursing at Loyola University Chicago, Chicago, IL.
Russ P. Nockels, Department of Neurological Surgery at Loyola University Medical Center, Maywood, IL.
Jonna Peterson, Department of Microbiology and Immunology at Loyola University Medical Center, Maywood, IL.
Herb L. Mathews, Department of Microbiology and Immunology at Loyola University Chicago, Chicago, IL.
References
- Angele MK, Faist E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Critical Care. 2002;6:298–305. doi: 10.1186/cc1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno DW, Stover AG, Mathews H. Growth inhibition of candida albicans hyphae by CD8+ lymphocytes. Journal of Immunology. 1995;154:5273–5281. [PubMed] [Google Scholar]
- Cohen S. Perceived stress scale. Mind Garden; Palo Alto, CA: 1994. [Google Scholar]
- Cohen S, Kamarch T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Efron PA, Moldawer LL. Cytokines and wound healing: The role of cytokine and anticytokine therapy in the repair response. Journal of Burn Care Rehabilitation. 2004;25:149–160. doi: 10.1097/01.bcr.0000111766.97335.34. [DOI] [PubMed] [Google Scholar]
- Gill K, Blumenthal SL. Posterior lumbar interbody fusion: A 2-year follow-up of 238 patients. Acta Orthopedica Scandinavica. 1993;64:108–110. doi: 10.3109/17453679309160137. [DOI] [PubMed] [Google Scholar]
- Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with decrease in natural killer cell activity. Behavioral Neuroscience. 1986;100:675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- Goodfellow LM. The effects of therapeutic back massage on psychophysiologic variables and immune function in spouses of patients with cancer. Nursing Research. 2003;52:318–328. doi: 10.1097/00006199-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Graham C, Bond SS, Gerkousch MM, Cook MR. Use of the McGill Pain Questionnaire in the assessment of cancer pain: Replicability and consistency. Pain. 1988;8:377–387. doi: 10.1016/0304-3959(80)90081-0. [DOI] [PubMed] [Google Scholar]
- Hee HT, Castro FP, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: Analysis of complications and predictive factors. Journal of Spinal Disorders. 2001;14:533–540. doi: 10.1097/00002517-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Ironson G, Field T, Hurley J, Katz G, Diego M, et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. Journal of Psychosomatic Research. 2004;57:45–52. doi: 10.1016/S0022-3999(03)00500-2. [DOI] [PubMed] [Google Scholar]
- Johnston M. Pre-operative emotional states and post-operative recovery. Advances in Psychosomatic Medicine. 1986;15:1–22. doi: 10.1159/000411845. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Sevarino F, Pincus S, Alexander GM, Wang SM, Ayoub C, et al. Attenuation of the preoperative stress response with midazolam: Effects on postoperative outcomes. Anesthesiology. 2000;93:141–147. doi: 10.1097/00000542-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. Journal of Bone and Joint Surgery. 2006;88-A:S21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- Khoo LT, Pahner S, Laich DT, Fessler RG. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51:S2166–S2181. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Page G, Marucha P, MacCallum R, Glaser R. Psychological influences on surgical recovery. American Psychology. 1998;53:1209–1218. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee S, Chung SK, Lee H. Comparison of multifidus muscle atrophy and trunk extension muscle strength: Percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. [PubMed] [Google Scholar]
- Lin ZQ, Kondo T, Ishida Y, Takayasu T, Multaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of Leukocyte Biology. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- McGrady A, Conran P, Dickey D, Garman D, Farris E, Schumann-Brzezinski C. The effects of biofeedback-assisted relaxation on cell-mediated immunity, cortisol, and white blood cell count in healthy adult subjects. Journal of Behavioral Medicine. 1994;15:343–354. doi: 10.1007/BF00844727. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman L. The manual for the Profile of Mood States. Educational and Industrial Testing Services; San Diego, CA: 1992. [Google Scholar]
- Melzack R. The McGill Pain Questionnaire. In: Melzack R, editor. Pain measurement and assessment. Raven Press; New York: 1983. pp. 41–47. [Google Scholar]
- Murphy PG, Borthwick LA, Altares M, Gauldie J, Kaplan D, Richardson PM, et al. Reciprocal actions of interleukin-6 and brain derived neurotrophic factor on rat and mouse primary sensory neurons. European Journal of Neuroscience. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- Page G. Surgery-induced immunosuppression and postoperative pain management. AACN Clinical Issues. 2005;16:302–309. doi: 10.1097/00044067-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Poole AR. Biological markers and disc degeneration. Journal of Bone and Joint Surgery. 2006;88-A:845–847. doi: 10.2106/JBJS.E.01326. [DOI] [PubMed] [Google Scholar]
- Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW, Kukla TR. Transforaminal lumbar interbody fusion: Clinical and radiographic results and complications in 100 consecutive patients. Journal of Spinal Disorders. 2005;18:337–346. doi: 10.1097/01.bsd.0000166642.69189.45. [DOI] [PubMed] [Google Scholar]
- Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. Journal of Spinal Disorders. 2005;18:Sl–S6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- Slosar PJ. Indications and outcomes of reconstructive surgery in chronic pain of spinal origin. Spine. 2002;27:2555–2562. doi: 10.1097/00007632-200211150-00031. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. Journal of Clinical Endocrinology and Metabolism. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. Journal of Clinical Endocrinology and Metabolism. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Volk T, Schenk M, Voigt K, Tohtz S, Putzier M, Kox W. Postoperative epidural anesthesia preserves lymphocyte, but not monocyte, immune function after major spine surgery. Anesthesia and Analgesia. 2004;98:1086–1092. doi: 10.1213/01.ANE.0000104586.12700.3A. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36 Health Survey. Medical Outcomes Trust and Quality Metric Incorporated; Lincoln, RI: 1992. [Google Scholar]
- Watkins LR, Maier SF. The pain of being sick: Implications of immune-to-brain communication for understanding pain. Annual Review of Psychology. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: Implications for clinical pain. In: Machelska H, Stein C, editors. Immune mechanisms of pain and analgesia. Landes Biosciences; New York: 2003. pp. 1–21. [PubMed] [Google Scholar]
- Witek-Janusek L, Mathews HL. Differential effects of glucocorticoids on colony stimulating factors produced by neonatal mononuclear cells. Pediatric Research. 1999;45:224–229. doi: 10.1203/00006450-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Zhong J, Heumann R. Lesion-induced interleukin-6 mRNA expression in rat sciatic nerve. Annals of the New York Academy of Science. 1995;762:488–490. doi: 10.1111/j.1749-6632.1995.tb32377.x. [DOI] [PubMed] [Google Scholar]