Abstract
Objective
The aim of this study was to investigate the effect of insulin sensitizing agents on hormonal and metabolic parameters as well as menstrual patterns in women with polycystic ovary syndrome (PCOS).
Methods
One hundred and twenty-three patients with PCOS were included. Metformin was administered to patients at 1,500 mg or 1,700 mg daily for 3 months. If the patients had no improvement of the menstrual cycle or metformin-related adverse effects developed, the patients changed medication to a daily dose of either 15 mg pioglitazone or up to 45 mg. Then resumption of a regular menstrual cycle or recovery of ovulation was evaluated. Hormonal and metabolic profiles were compared between the response and non-response group to insulin sensitizing agents.
Results
One hundred and five patients with PCOS were treated with metformin for 3 months. Forty-eight patients (45.7%) showed improvement of menstrual cycle regularity after 3 months of metformin use, whereas 57 patients (54.3%) had no change. The mean free testosterone measured after 3 months of treatment was significantly lower in metformin responders than in non-responders. The other parameters did not differ between the groups. Of the 23 patients who used pioglitazone for 3 to 6 months, 19 patients (82.6%) showed improvement in their menstrual cycles.
Conclusion
Metformin treatment seems to be effective for the improvement of menstrual cyclicity irrespective of insulin resistance in women with PCOS. When metformin related adverse effect occurred, pioglitazone would be effective for aiding the resumption of the menstrual cycle.
Keywords: Polycystic ovary syndrome, Metformin, Pioglitazone
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting 6% to 10% of reproductive-age women [1], characterized by chronic anovulation and hyperandrogenism. Although the etiology of PCOS remains unclear, insulin resistance has been identified as an important contributor to the pathogenesis of PCOS. Recent studies have indicated that defects in post-binding signaling in insulin-sensitive tissue such as muscle, adipocytes, and ovarian tissue may lead to increased insulin resistance and subsequent hyperinsulinemia in PCOS [2-5].
Insulin sensitizing agents have been found to improve the symptoms of PCOS. Metformin is a biguanide drug for the treatment of type 2 diabetes, which has become the most widely used drug to treat women with insulin resistance and PCOS. Since the first study of metformin in women with PCOS was published in 1994, several studies have demonstrated a positive effect of metformin on both reproductive and metabolic aspects of PCOS [6-11]. Irrespective of the reduction of hepatic glucose production and enhancement of peripheral glucose utilization, the efficacy of metformin in the ovulatory response is probably due to direct action on the ovary, and the ovulatory response to the drug seems to be related to local sensitivity or resistance to the drug [12].
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a nuclear receptor that is associated with obesity, insulin resistance, cardiovascular disease, and adipose tissue formation, and is also a target of insulin sensitizing agents, such as thiazolidinediones (TZDs). TZD-induced effects accelerate the differentiation of adipose cells and improve insulin resistance [13]. By improving insulin resistance and indirectly reducing androgen synthesis in the ovaries, PPAR-γ receptors also affect the reproductive system. The ovaries can also be directly targeted with TZDs to reduce hyperandrogenemia and to stimulate ovulation. Pioglitazone is a new TZD derivative that has been approved for the treatment of type 2 diabetes. Pioglitazone acts after insulin binding by insulin receptors to improve the action of insulin, reduces its resistance to hormones, and inhibits glucose production in the liver. Furthermore, it has both anti-inflammatory and anti-atherosclerotic properties, which may be useful for reducing the mortality of cardiovascular disease. Pioglitazone has been used to treat the insulin resistance, hyperandrogenism, and ovulatory dysfunction that are characteristic of PCOS [14-18]. Therefore, the aim of this study was to evaluate the effects of insulin sensitizing agents on the hormonal and metabolic parameters as well as menstrual patterns in women with PCOS.
Methods
1. Subjects
We recruited 123 patients with PCOS in the gynecologic clinic at the Seoul National University Hospital. A diagnosis of PCOS was based on the 2003 American Society for Reproduction and Embryology consensus meeting guideline [19]. This guideline proposes a diagnosis of PCOS when a patient meets two of the following three criteria: 1) oligo- and/or anovulation, 2) hyperandrogenism (clinical and/or biochemical), and 3) a polycystic ovary morphology on ultrasonography (either 12 or more follicles measuring 2-9 mm in diameter or increased ovarian volume>10 cm3), also excluding other androgen excess or related disorders. Oligomenorrhea was defined as less than eight periods per year or cycles longer than 35 days, and amenorrhea was defined as the absence of menstruation for more than three months without pregnancy. Clinical hyperandrogenism (HA) was defined by a modified Ferriman and Gallwey score of 6 or greater in our population and biochemical HA was defined as follows: total testosterone >0.68 ng/mL; free testosterone >1.72 pg/mL; and a free androgen index (FAI) >5.36 [20].
All PCOS patients were screened to exclude hyperprolactinemia and thyroid dysfunction. Serum 17-hydroxyprogesterone (OHP) was also measured to exclude non-classical adrenal hyperplasia. If a patient's serum 17-OHP level was greater than 2.0 ng/mL, a repeat test was performed during the early morning follicular phase. The Institutional Review Board for human research of each of the centers approved this project, and written informed consent was obtained from each participant.
2. Study design
The patients with PCOS took metformin at a dose of 500 mg three times a day or 850 mg twice a day for 3 months. We measured the adherence to metformin by patient self-reports. If the patients had no improvement in the menstrual cycle or metformin-related adverse effects developed (nausea, diarrhea, and/or heartburn), those patients changed medication to pioglitazone for 6 months, beginning with a daily dose of 15 mg and increasing if needed to 45 mg after the first 3 months. The outcome parameters of the response to insulin sensitizing drugs included resumption of a regular menstrual cycle or recovery of ovulation. Menstrual cyclicity was defined as subjects having normal menses at 25 to 35 day intervals within the study period. Serum progesterone was measured on cycle day 21 and a progesterone serum level equal to or greater than 3.0 ng/dL was considered a confirmation of ovulation. In addition, hormonal and metabolic profiles were compared between the response and non-response group to insulin sensitizing agents before treatment and after treatment.
3. Clinical and biochemical measurements
Clinical variables such as waist circumference, hip circumference, body weight, height, and blood pressure were assessed, and the body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2).
Basal gonadotropin hormone levels were measured in all PCOS subjects, including serum LH, FSH, and E2. The women with PCOS were evaluated for serum total testosterone, free testosterone, 17-OHP, DHEAS, and sex hormone binding globulin (SHBG) using radioimmunoassay (Siemens, Los Angeles, CA, USA), and plasma insulin levels were measured using a commercial kit (BioSource Europe S.A., Nivelles, Belgium). The FAI was calculated as total testosterone/SHBG×100, and the values for testosterone were converted from ng/mL to nmol/L using the following the index proposed by the manufacturer: 1 ng/mL=3.467 nmol/L. Fasting and postprandial 2-hour glucose and insulin levels were evaluated by a 75-g glucose tolerance test using commercial kits (BioSource Europe S.A.) in order to assess insulin resistance in PCOS patients. The homeostatic model for insulin resistance (HOMA-IR) was calculated by glucose (mg/dL)×insulin (µU/mL)/405.
4. Statistical analysis
All data are presented as mean±SD. Statistical significance was analyzed using the Student's t-test and a p-value less than 0.05 was considered to be statistically significant. All data analyses were done using IBM SPSS ver. 20 (IBM, Armonk, New York, USA).
Results
1. General characteristics of the patients and changes in metformin treatment for 3 months
One hundred and five patients with PCOS were treated with metformin for 3 months. Forty-eight patients (45.7%), considered to be "responders", showed improvement in menstrual cycle regularity and/or recovery of ovulation during 3 months of metformin use, whereas 57 patients (54.3%) had no change, and these patients were considered "non-responders". The baseline characteristics of responders and non-responders with PCOS given metformin were compared to identify any predictors of a clinical response to metformin. Analysis of the baseline features of the two groups did not show any differences (Table 1).
Table 1.
Baseline characteristics of the subjects on metformin treatment in women with PCOS
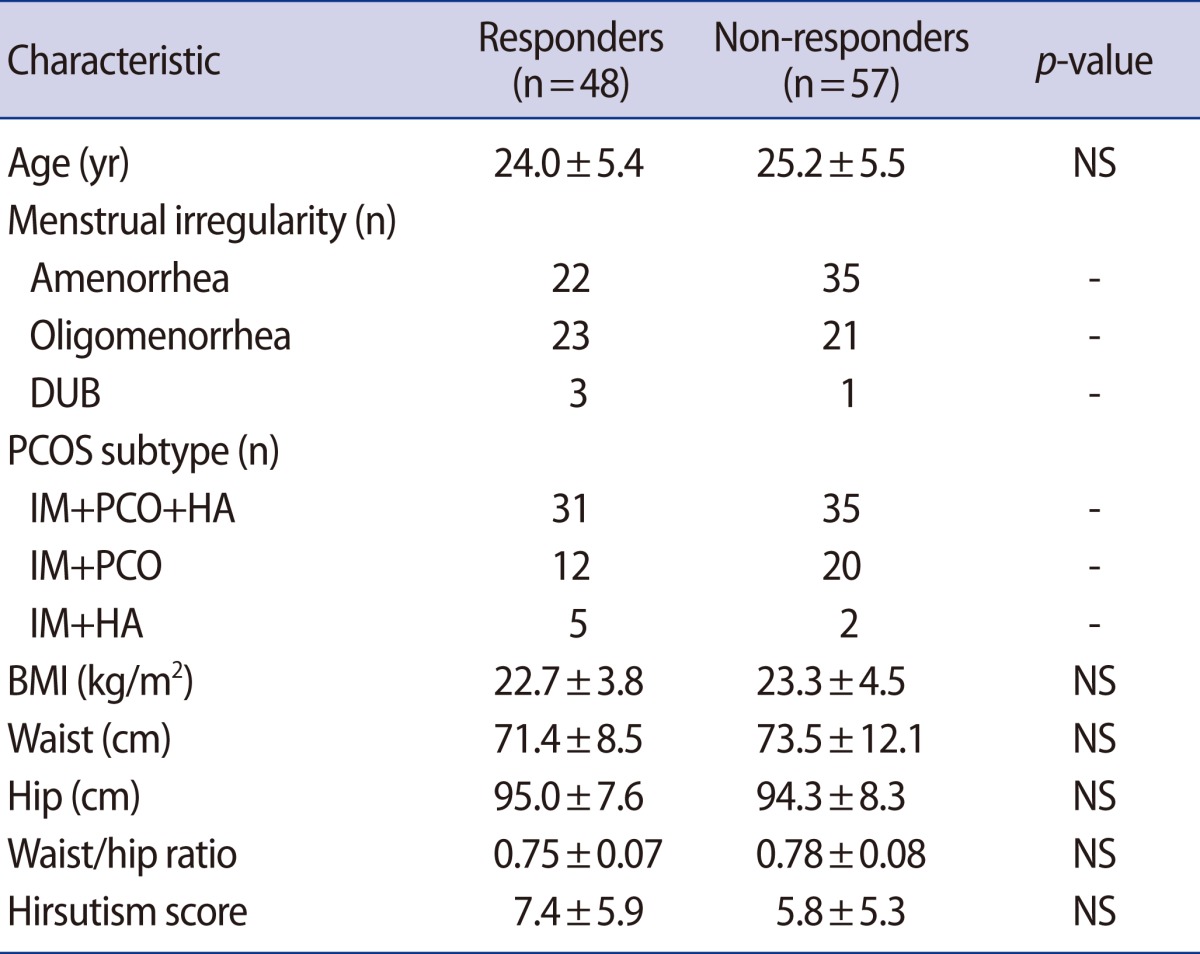
Values are shown as mean±SD.
PCOS, polycystic ovary syndrome; NS, not significant; DUB, dysfunctional uterine bleeding; IM, irregular menstruation; PCO, polycystic ovary; HA, hyperandrogenism; BMI, body mass index.
After 3 months of metformin treatment, there was no significant change in fasting glucose, postprandial 2-hour glucose, fasting insulin, postprandial 2-hour insulin, or HOMA-IR in either the responder or the non-responder group. However, the free testosterone level was significantly lower in the responders than non-responders (Table 2).
Table 2.
Changes in women with PCOS on metformin treatment for 3 months
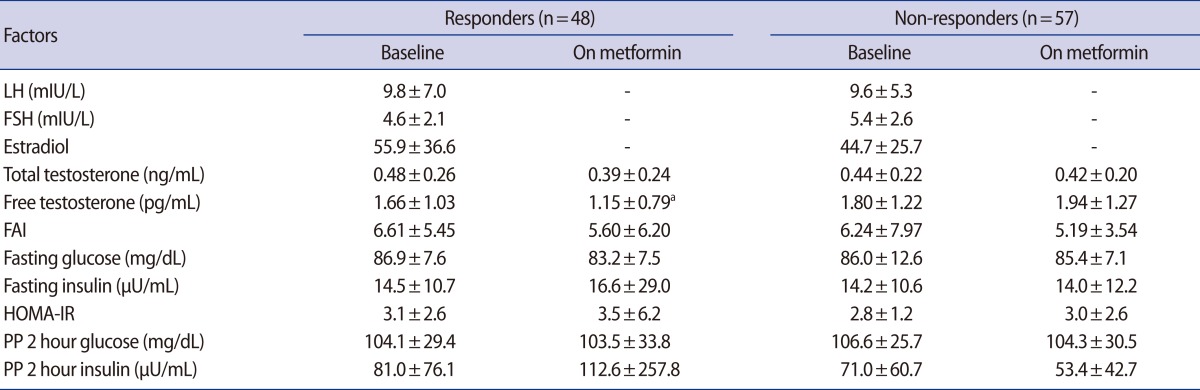
Values are shown as mean±SD.
FAI, free androgen index; HOMA-IR, homeostasis model assessment for insulin resistance; PP, postprandial.
ap<0.01 by paired t-test used for baseline versus on metformin in responders.
2. Comparison of reproductive hormones and metabolic status between responders and non-responders in pioglitazone treatment
We administered one 15 mg tablet of pioglitazone to 42 patients who consisted of patients from the non-response group of metformin or those who did not take metformin because of the adverse effects. Eleven out of 42 patients who were administered one 15 mg tablet of pioglitazone were not able to visit the center for tracing. After 3 months, 8 patients showed regular menstruation recovery accompanied by dysmenorrhea or breast tenderness and 23 patients did not show recovery of regular menstruation or ovulation. Of the 23 non-responders to 15 mg of pioglitazone, 15 patients finished the study on 45 mg of pioglitazone for the final 3 months. Among them, 11 patients showed recovery of menstruation or ovulation and 4 patients still did not in the end. The comparison of baseline reproductive hormone and metabolic parameters between the responders and the non-responders to pioglitazone treatment is shown in Table 3.
Table 3.
Comparison of reproductive hormones and metabolic status between responders and non-responders to 6 months of pioglitazone treatment
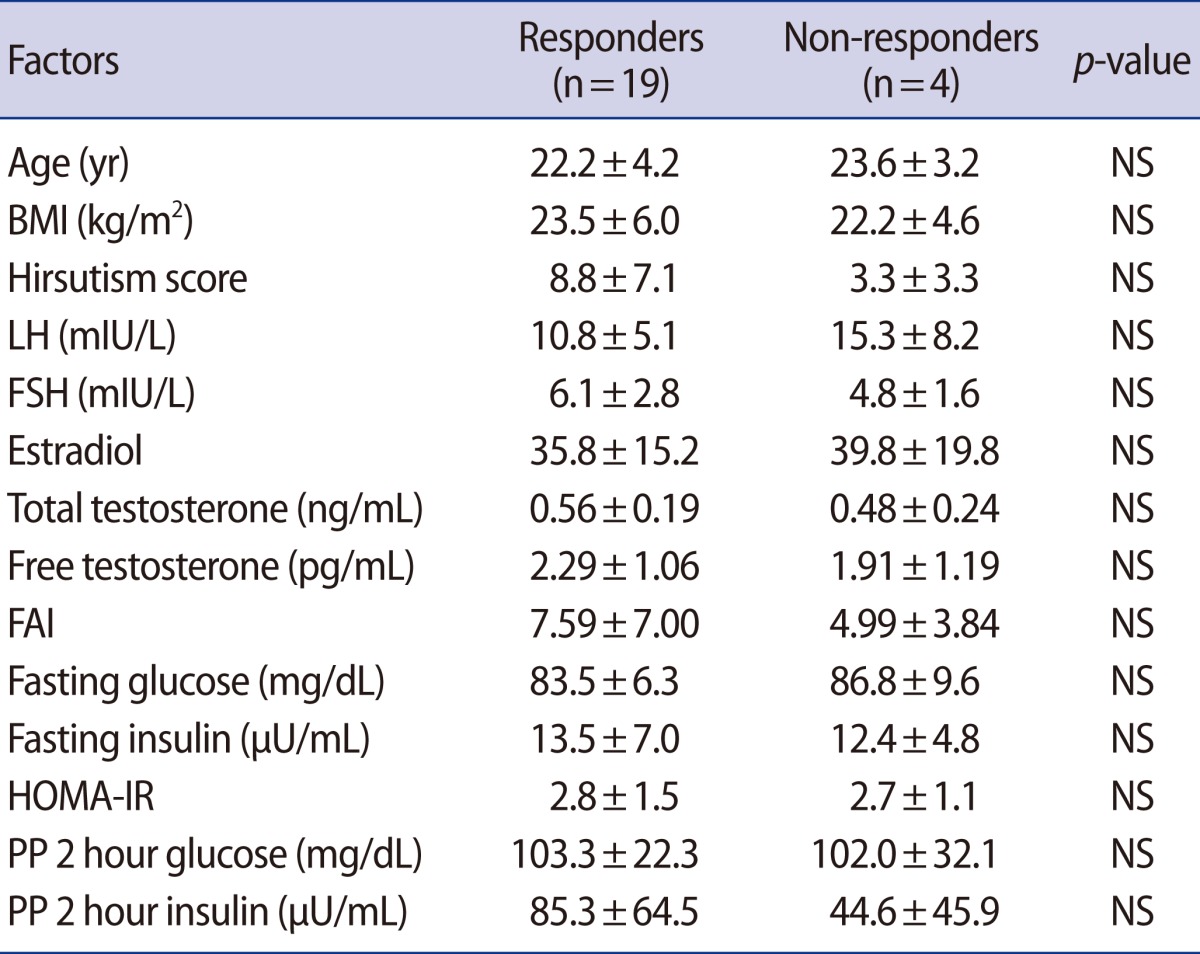
Values are shown as mean±SD. p-value was evaluated by Mann-Whitney test.
NS, not significant; BMI, body mass index; FAI, free androgen index; HOMA-IR, homeostasis model assessment for insulin resistance; PP, postprandial.
Discussion
PCOS is the most common endocrine disorder of women of reproductive age. Given the well-documented association between PCOS and insulin resistance, metformin and other insulin sensitizing drugs have been used in the management of PCOS. Nevertheless, the mechanisms underlying the beneficial effects of insulin sensitizing agents in the treatment of PCOS remain incompletely understood. Since not all PCOS patients are obese or insulin resistant, it has thus far remained unclear whether PCOS patients without evidence of insulin resistance would also benefit from insulin sensitizing agents.
In our study, 105 patients took 500 mg of metformin three times a day or 850 mg twice a day for 3 months. Although improvements in insulin sensitivity in these women were not marked, 45.7% of these subjects, most with severe oligomenorrhea at baseline, had their cycles normalized. Conversely, almost half of the women given metformin had no improvement in their menstrual abnormalities. The remarkable differences in clinical response to metformin among PCOS subjects are difficult to explain; this phenomenon might reflect the heterogeneity of PCOS, and it emphasizes the importance of testing treatments on clearly defined subgroups of PCOS. These findings are consistent with the results of our preliminary study [21]. Even though the assessment of adherence to metformin medication was not done directly, we measured the medication adherence to metformin by patient self-reports, and we were able to distinguish between metformin non-responders and those patients who stopped metformin treatment due to adverse effects.
There was no significant reduction in the fasting glucose:insulin ratio (G:I ratio) or HOMA-IR in the metformin responders or in the non-responders. These findings may be caused by the short three-month duration of administration of metformin. The standard administration period for metformin has not been established; therefore, more related studies should be performed. In addition, considering that 74% (78/105) the subjects of our study were non-obese patients with PCOS, it is difficult to exclude the possibility that the recovery of insulin resistance is relatively slow, unlike that in obese patients. It is still under debate whether non-obese women with PCOS are insulin resistant. Moreover, isolated insulin resistance cannot be reliably diagnosed with surrogate markers. Fasting insulin levels reflect insulin secretion and clearance as well as insulin resistance and are not sufficiently predictive of euglycemic clamp measures of insulin action to be used for the diagnosis of insulin resistance in individual patients. Other fasting measures, such as the G:I ratio, HOMA-IR, and the quantitative insulin sensitivity check index are all based on fasting glucose and insulin levels and essentially provide identical information. As discussed above, even when insulin resistance is assessed using the euglycemic glucose clamp, it is clear that some women with PCOS have normal insulin sensitivity [22-24].
In the present study, there was a significant reduction in serum levels of free testosterone after metformin treatment in the responders group. Presumably, the decline of free testosterone represents a real decline in androgen bioavailability in our patients during metformin treatment. Moreover, the effect of metformin on abnormal ovarian androgen metabolism in women with chronic anovulation and hyperandrogenism may include a direct ovarian sensitizing action without effecting a change on circulating insulin concentrations [25]. Therefore, it can be concluded that metformin therapy is effective for androgen excess, and free testosterone may be a predictor of response.
The result of administering pioglitazone to the metformin non-responders and to those patients who stopped metformin treatment due to adverse effects such as digestive complications revealed that, in the end, 19 of 23 patients (82.6%) showed recovery of regular menstruation or ovulation at 6 months. With pioglitazone treatment, no significant difference was seen on reproductive hormones or metabolic parameters between the responders and the non-responders group. Although the number of subjects is small, the result shows that pioglitazone could possibly be considered an effective drug for patients with PCOS. According to Brettenthaler et al. [14], the rate of induction of ovulation with pioglitazone was increased from 5.6% to 41.2% (p<0.02) compared with a placebo control group, and free androgen and LH levels were significantly decreased. Moreover, Koo et al. [26] found that 45.5% of 28 women with PCOS showed clinical improvement in their menstrual cycles during treatment with 15 mg of pioglitazone and these patients showed a different response pattern according to their baseline free testosterone levels. In a recent systematic review and meta-analysis to assess the comparative efficacy of pioglitazone and metformin, pioglitazone was more suitable for treating hyperinsulinemia and insulin resistance among PCOS patients, while metformin was more effective in reducing body weight [27]. Although the action of pioglitazone is obviously different from metformin, the comparative efficacy of pioglitazone versus metformin in the treatment of PCOS remains controversial.
Our results failed to demonstrate that metformin had any effect on the parameters studied, except for free testosterone. The severity of insulin resistance, hyperandrogenism, obesity, and menstrual abnormalities have been proposed as predictors of clinical response to metformin [7,8,27,28], although metformin has also been shown to improve metabolic and reproductive parameters in women with PCOS independently of pretreatment insulin resistance or BMI [11]. However, there remain a number of unanswered questions concerning the use of metformin in women with PCOS, including which parameters may best predict a response and the appropriate dose for a given body mass.
In conclusion, metformin treatment seems to be effective for the improvement of menstrual cyclicity irrespective of insulin resistance expressed as the glucose-to-insulin ratio and HOMA. When metformin-related adverse effects occur, pioglitazone could be effective in aiding the resumption of the menstrual cycle. Further studies with large-scale, randomized, and long-term data are needed to clarify the role of insulin-sensitizing agents in the treatment of clinical and biochemical PCOS characteristics.
Footnotes
This research was supported by a grant from the Seoul National University College of Medicine Research Fund 2009.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96:801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Youngren JF, Dunaif A, Goldfine ID, Maddux BA, Zhang BB, et al. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 2002;87:4088–4093. doi: 10.1210/jc.2002-020363. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13–17. doi: 10.1385/ENDO:30:1:13. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43:647–654. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 7.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 8.Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab. 2002;87:569–574. doi: 10.1210/jcem.87.2.8261. [DOI] [PubMed] [Google Scholar]
- 9.Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1–13. doi: 10.1016/s0015-0282(02)04554-5. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402, e1–e10. doi: 10.1016/j.ajog.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157:669–676. doi: 10.1530/EJE-07-0294. [DOI] [PubMed] [Google Scholar]
- 12.Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25:1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 13.Koenen TB, Tack CJ, Kroese JM, Hermus AR, Sweep FC, van der Laak J, et al. Pioglitazone treatment enlarges subcutaneous adipocytes in insulin-resistant patients. J Clin Endocrinol Metab. 2009;94:4453–4457. doi: 10.1210/jc.2009-0517. [DOI] [PubMed] [Google Scholar]
- 14.Brettenthaler N, De Geyter C, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835–3840. doi: 10.1210/jc.2003-031737. [DOI] [PubMed] [Google Scholar]
- 15.Romualdi D, Guido M, Ciampelli M, Giuliani M, Leoni F, Perri C, et al. Selective effects of pioglitazone on insulin and androgen abnormalities in normo- and hyperinsulinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18:1210–1218. doi: 10.1093/humrep/deg264. [DOI] [PubMed] [Google Scholar]
- 16.Ortega-Gonzalez C, Luna S, Hernandez L, Crespo G, Aguayo P, Arteaga-Troncoso G, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1360–1365. doi: 10.1210/jc.2004-1965. [DOI] [PubMed] [Google Scholar]
- 17.Aroda VR, Ciaraldi TP, Burke P, Mudaliar S, Clopton P, Phillips S, et al. Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2009;94:469–476. doi: 10.1210/jc.2008-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glueck CJ, Moreira A, Goldenberg N, Sieve L, Wang P. Pioglitazone and metformin in obese women with polycystic ovary syndrome not optimally responsive to metformin. Hum Reprod. 2003;18:1618–1625. doi: 10.1093/humrep/deg343. [DOI] [PubMed] [Google Scholar]
- 19.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Chae SJ, Kim JJ, Choi YM, Hwang KR, Jee BC, Ku SY, et al. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod. 2008;23:1924–1931. doi: 10.1093/humrep/den239. [DOI] [PubMed] [Google Scholar]
- 21.Hwang KR, Choi YM, Choi DS, Baek KH, Jeon HW, Bae KB, et al. Effect of metformin treatment and insulin resistance in patients with polycystic ovary syndrome. Korean J Obstet Gynecol. 2004;47:1949–1953. [Google Scholar]
- 22.Ferrannini E, Balkau B. Insulin: in search of a syndrome. Diabet Med. 2002;19:724–729. doi: 10.1046/j.1464-5491.2002.00794.x. [DOI] [PubMed] [Google Scholar]
- 23.Hücking K, Watanabe RM, Stefanovski D, Bergman RN. OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity (Silver Spring) 2008;16:1938–1945. doi: 10.1038/oby.2008.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall JC, Dunaif A. Should all women with PCOS be treated for insulin resistance? Fertil Steril. 2012;97:18–22. doi: 10.1016/j.fertnstert.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirwany IR, Yates RW, Cameron IT, Fleming R. Effects of the insulin sensitizing drug metformin on ovarian function, follicular growth and ovulation rate in obese women with oligomenorrhoea. Hum Reprod. 1999;14:2963–2968. doi: 10.1093/humrep/14.12.2963. [DOI] [PubMed] [Google Scholar]
- 26.Koo YA, Shin SY, Yoon BK, Choi D. Pioglitazone for treating polycystic ovary syndrome in non-obese women of reproductive age with different clinical presentations. Gynecol Endocrinol. 2007;23:461–467. doi: 10.1080/09513590701492689. [DOI] [PubMed] [Google Scholar]
- 27.Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13:527–537. doi: 10.1093/humupd/dmm026. [DOI] [PubMed] [Google Scholar]
- 28.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Ovulatory response to treatment of polycystic ovary syndrome is associated with a polymorphism in the STK11 gene. J Clin Endocrinol Metab. 2008;93:792–800. doi: 10.1210/jc.2007-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]


