Abstract
Endometriosis is defined as the presence of functional endometrial tissue outside the uterus, causing diverse progressive symptoms such as infertility, pelvic pain, and dysmenorrhea. Although endometriosis has been described since the 1800s, the mechanisms responsible for its pathogenesis and progression remain poorly understood. It is well established that endometriosis grows and regresses in an estrogen-dependent fashion and the disease can be effectively cured by definitive surgery. However, prolonged medical therapy may be needed in most of the cases since conservative surgery is usually performed especially in young women. This treatment modality is often associated with only partial relief and/or recurrence of the disease. In the present review, up-to-date findings on the treatment of endometriosis will be briefly summarized. The outcomes of surgery in patients with endometriosis will be reviewed in terms of pelvic pain relief as well as infertility treatment largely based on recent Cochrane reviews and clinical reports. The efficacy of newer drugs including aromatase inhibitor, anti-tumor necrosis factor-alpha, and dienogest will be also reviewed based on recent clinical studies.
Keywords: Endometriosis, Pelvic pain, Infertility, Recurrence, Medical treatment
Introduction
Endometriosis is defined as the presence of functional endometrial tissue outside the uterus, causing diverse symptoms including infertility, chronic pelvic pain, and cyclic menstrual pain. The prevalence of endometriosis has been reported ranging from 2% to 18% among women who seek tubal ligations and from 5% to 50% of infertile women [1]. When 10% prevalence rate assumed among reproductive aged women, the annual costs attributed by the disease may be calculated as 22 billion US dollars, suggesting an enormous negative impact on national health systems [2].
Although endometriosis has been described since the 1800s, the mechanisms responsible for its pathogenesis and progression remain poorly understood. It is well established that endometriosis grows and regresses in an estrogen-dependent fashion and the disease can be effectively cured by definitive surgery. However, prolonged medical therapy may be needed in most of the cases since conservative surgery is usually performed especially in young women. This treatment modality is often associated with only partial relief and/or recurrence of the disease.
In the present review, up-to-date findings on the treatment of endometriosis will be briefly summarized largely based on recent Cochrane reviews and clinical reports. Specifically, the outcomes of surgery in patients with endometriosis will be reviewed in terms of pelvic pain relief as well as infertility treatment. The efficacy of newer drugs, such as aromatase inhibitor, anti-tumor necrosis factor-alpha and dienogest, will be also reviewed based on recent clinical studies.
Outcomes of surgery in endometriosis: pelvic pain
1. Results from non-comparative studies
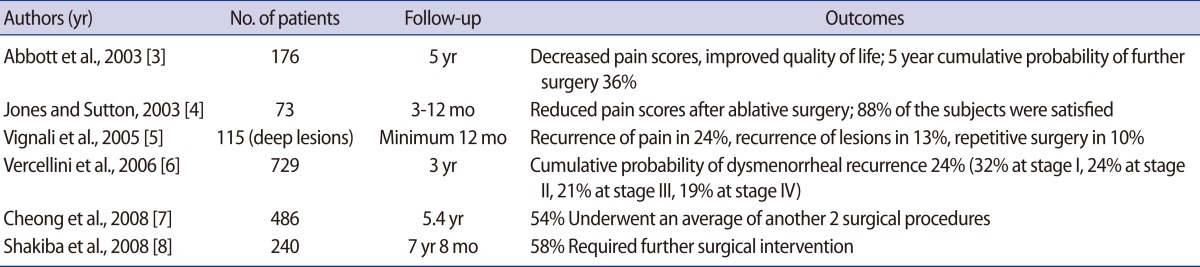
The postoperative outcomes after first-line conservative surgery for symptomatic endometriosis were summarized in Table 1 [3-8]. Most studies reported improvement of dysmenorrhea, non-menstrual pain, dysparenunia, and life quality after surgery. However, pain recurrence or re-operation rate has been reported being from 24% to 54%. The risk of repetitive surgery was remarkably increased in women <30 years old and patients with pelvic pain had a higher re-operation rate compared to subfertile ones [7,8].
Table 1.
The outcomes after first-line conservative surgery for symptomatic endometriosis: non-comparative studies
2. Results from controlled studies
A double-blind study on 63 women with minimal-to-moderate endometriosis compared pain symptoms between laser destruction and expectant management [9,10]. After 6 months and 1 year follow-up, 63% and 56% of women in the laser group reported improved symptom profiles, respectively, while 23% and 23% of women reported improvement in the expectant management group, respectively. Abbott et al. [11] performed a small, blinded randomized clinical trial on 39 women with minimal-to-severe endometriosis: patients allocated either to excision or expectancy. At 6 months follow-up, 80% of excision group had symptomatic improvement compared to 32% in the expectancy group. Jarrell et al. [12,13] allocated 29 women with minimal-to-moderate endometriosis and severe symptoms either to laparoscopic excision or observational laparoscopy. Based on pain diaries reported by a small number of patients, no significant difference was observed in visual analogue scale pain score reduction at short-term follow-up [12]. According to the long-term follow-up data, overall rate of repeated surgical operation at 12 to 14 years was 52% in the excision group and 48% in the observational laparoscopy group, which was not statistically different [13].
3. Efficacy in the treatment of pelvic pain: recent Cochrane review
A recent Cochrane review assessed the efficacy of laparoscopic surgery in the treatment of pelvic pain associated with endometriosis [14]. Only randomized controlled trials were selected for comparison of effectiveness of laparoscopic surgery, other treatment modalities, or diagnostic laparoscopy only. Five studies were included in the meta-analysis (three full papers and two conference reports). All trials except one compared different laparoscopic surgical techniques with diagnostic laparoscopy only. The meta-analysis demonstrated an advantage of laparoscopic surgery in terms of pain symptoms at 6 months when compared to diagnostic laparoscopy only (odds ratio [OR], 5.72; 95% confidence interval [CI], 3.09 to 10.60; 171 participants). A single study compared pain scores at 12 months after the procedure and also demonstrated an advantage of laparoscopic surgery than diagnostic laparoscopy only (OR, 7.72; 95% CI, 2.97 to 20.06; 33 participants).
4. Which one is better for pelvic pain and recurrence in ovarian endometrioma, excisional surgery versus ablative surgery? recent Cochrane review
A recent Cochrane review evaluated the most effective technique for treating an ovarian endometrioma, either excision of the cyst capsule or drainage followed by electrocoagulation of the cyst wall, measuring the primary outcome as pain symptom improvement [15]. Two randomized studies of the laparoscopic management of ovarian endometrioma, greater than 3 cm were included. Laparoscopic excision of the cyst wall of the endometrioma was associated with a reduced recurrence rate of dysmenorrhea (OR, 0.15; 95% CI, 0.06 to 0.38), dyspareunia (OR, 0.08; 95% CI, 0.01 to 0.51) and non-menstrual pelvic pain (OR, 0.10; 95% CI, 0.02 to 0.56). For the secondary outcome measures, laparoscopic excision of the cyst wall was associated with a reduced rate of recurrence of the endometrioma (OR, 0.41; 95% CI, 0.18 to 0.93) and with a reduced requirement for further surgery (OR, 0.21; 95% CI, 0.05 to 0.79) compared with ablative surgery.
Outcomes of laparoscopic surgery in endometriosis: infertility
1. Efficacy of laparoscopic surgery for subfertility in minimal-to-mild endometriosis: recent Cochrane review
A recent Cochrane review compared the reproductive outcomes between laparoscopic surgical interventions and diagnostic laparoscopy in patients with minimal-to-mild endometriosis [16]. Two studies were eligible for inclusion within the review and both studies compared laparoscopic surgical treatment of minimal-to-mild endometriosis with diagnostic laparoscopy only. Combining live birth rate and ongoing pregnancy after 20 weeks, the meta-analysis demonstrated an advantage of laparoscopic surgery compared to diagnostic laparoscopy only (OR, 1.64; 95% CI, 1.05 to 2.57). The meta-analysis also showed an advantage of laparoscopic surgery regarding clinical pregnancy rate (OR, 1.66; 95% CI, 1.09 to 2.51) but not demonstrating an effect of laparoscopic surgery on fetal loss.
2. Which one is better for fertility in ovarian endometrioma, excisional surgery versus ablative surgery?: recent Cochrane review
A recent Cochrane review compared fertility outcomes between excisional and ablative surgery in women with ovarian endometrioma [15]. In that review, excision of the cyst wall was associated with a higher spontaneous pregnancy rate (OR, 5.21; 95% CI, 2.04 to 13.29) compared to women who underwent laparoscopic ablation. However, there was insufficient evidence supporting excisional surgery over ablative surgery in terms of pregnancy chance after ovarian stimulation and intra-uterine insemination.
3. Efficacy of interventions for women with endometrioma prior to assisted reproductive technology (ART): recent Cochrane review
A recent Cochrane review assessed the effectiveness of surgery for improving reproductive outcomes among women with endometrioma prior to undergoing ART cycles [17]. Randomized controlled trials of surgical or expectant management for endometrioma prior to ART were analyzed. Four trials with 312 participants were included. Comparing the clinical pregnancy rate between the surgery (aspiration or cystectomy) and the expectant management groups, there was no difference between the two groups. Although cystectomy was associated with a decreased ovarian response to ovarian stimulation, there was no difference in the number of mature oocytes retrieved.
4. Interventions for women with endometrioma prior to ART: European Society of Human Reproduction and Embryology (ESHRE) guideline
A guideline suggested that laparoscopic ovarian cystectomy is recommended if an ovarian endometrioma ≥4 cm in diameter is present to confirm the diagnosis histologically, reduce the risk of infection and improve access to follicles and possibly improve ovarian response [18]. It is also recommended that woman should be counseled regarding the risks of reduced ovarian function after surgery and the possibility of oophorectomy thus the decision should be reconsidered if she underwent previous ovarian surgery.
The efficacy of new drugs
1. Aromatase inhibitors (AIs)
Based on the findings of increased expression of aromatase P450 in the endometriotic tissue, some investigators used AIs to treat pain symptoms in patients with endometriosis. A recent systemic review assessed the efficacy of AIs on treatment of pain caused by endometriosis [19]. The review was composed of the results of 10 publications including a total of 251 women. All of the observational studies showed that AIs combined with either progestogens or oral contraceptive pill reduce the severity of pain symptoms and improve quality of life. One randomized clinical trial demonstrated that combining letrozole with norethisterone acetate results in a lower incidence of adverse effects and lower discontinuation rate than combination of letrozole and triptorelin. Two randomized clinical trials demonstrated that postoperative AI treatment combined with GnRH analogue for 6 months reduces the risk of endometriosis recurrence when compared with GnRH analogue alone. Based on these findings, they concluded that AIs effectively reduce the severity of endometriosis-related pain symptoms. However, other researcher proposed that AIs need to be investigated further in well-designed studies to confirm the hypothetical impact on endometriotic lesions, since there have been no strong evidences favoring the efficacy or benefit of AIs compared to other hormonal drugs in currently available clinical trials [20].
2. Anti-tumor necrosis factor-alpha (anti-TNF-α)
Since mounting evidence showed that altered immune function plays a crucial role in the pathogenesis and pathophysiology of endometriosis, several investigators suggested that modulating the inflammation could be an alternative approach for treatment of endometriosis. A Cochrane review evaluated the effectiveness and safety of anti-TNF-α drugs in the management of pelvic pain associated with endometriosis [21]. Only one trial involving 21 participants was included in this review. Results showed no evidence of improvement of pain score or reduced use of pain killers after treating by infliximab, one of the known anti-TNF-α drugs. Although there was no evidence of an increase in adverse events in the infliximab group compared with placebo, clinical benefits of infliximab was not found with regard to endometriotic lesions, dysmenorrhea, dyspareunia or pelvic tenderness.
3. Dienogest
Dienogest (Visanne, Bayer HealthCare, Berlin, Germany) is a synthetic oral progestin possessing strong progestational and moderate antigonadotrophic effects, but no androgenic, glucocorticoid or mineralocorticoid activity. A dosage of 2 mg/day only moderately suppresses estradiol levels, and has high oral bioavailability and a half-life suitable for once-daily administration. A Randomized clinical trial showed that oral dienogest is more effective than placebo in reducing pelvic pain in patients with confirmed endometriosis [22]. Clinical trials comparing oral dienogest for 16 or 24 weeks with GnRH analogues in patients with endometriosis-dienogest had similar effect on reducing pelvic pain, improvement of combined symptoms/signs scores and revised American Fertility Society staging and scores compared with GnRH analogues [23]. According to another randomized clinical trial, women in the dienogest group had less hypoestrogenic side effects and little changes in bone markers and bone mineral density, thus offer advantages in safety and tolerability [24]. However, dienogest was associated with a higher incidence of abnormal menstrual bleeding patterns, although this was generally well tolerated by patients.
Conclusion
Based on the recent Cochrane reviews and clinical reports, treatment of endometriosis can be summarized as follows: 1) laparoscopic surgery is effective in reducing pain symptoms compared to diagnostic laparoscopy alone; 2) laparoscopic surgery is also effective in improving pregnancy rate compared to diagnostic laparoscopy alone in women with minimal-to-mild endometriosis; 3) regarding management of endometrioma prior to ART, ESHRE guideline suggested that laparoscopic ovarian cystectomy is recommended if an ovarian endometrioma is 4 cm or more in diameter, although randomized controlled trials failed to prove any advantage of ovarian cystectomy in improving pregnancy rate; 4) AIs and dienogest might be effective newer drugs for medical treatment of endometriosis, but long-term follow-up studies are necessary to estimate the cost-effectiveness of those treatments.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 2.Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 3.Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2-5 year follow-up. Hum Reprod. 2003;18:1922–1927. doi: 10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- 4.Jones KD, Sutton C. Patient satisfaction and changes in pain scores after ablative laparoscopic surgery for stage III-IV endometriosis and endometriotic cysts. Fertil Steril. 2003;79:1086–1090. doi: 10.1016/s0015-0282(02)04957-9. [DOI] [PubMed] [Google Scholar]
- 5.Vignali M, Bianchi S, Candiani M, Spadaccini G, Oggioni G, Busacca M. Surgical treatment of deep endometriosis and risk of recurrence. J Minim Invasive Gynecol. 2005;12:508–513. doi: 10.1016/j.jmig.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Fedele L, Aimi G, De Giorgi O, Consonni D, Crosignani PG. Reproductive performance, pain recurrence and disease relapse after conservative surgical treatment for endometriosis: the predictive value of the current classification system. Hum Reprod. 2006;21:2679–2685. doi: 10.1093/humrep/del230. [DOI] [PubMed] [Google Scholar]
- 7.Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28:82–85. doi: 10.1080/01443610701811761. [DOI] [PubMed] [Google Scholar]
- 8.Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111:1285–1292. doi: 10.1097/AOG.0b013e3181758ec6. [DOI] [PubMed] [Google Scholar]
- 9.Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 10.Sutton CJ, Pooley AS, Ewen SP, Haines P. Follow-up report on a randomized controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal to moderate endometriosis. Fertil Steril. 1997;68:1070–1074. doi: 10.1016/s0015-0282(97)00403-2. [DOI] [PubMed] [Google Scholar]
- 11.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82:878–884. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Jarrell J, Mohindra R, Ross S, Taenzer P, Brant R. Laparoscopy and reported pain among patients with endometriosis. J Obstet Gynaecol Can. 2005;27:477–485. doi: 10.1016/s1701-2163(16)30531-x. [DOI] [PubMed] [Google Scholar]
- 13.Jarrell J, Brant R, Leung W, Taenzer P. Women's pain experience predicts future surgery for pain associated with endometriosis. J Obstet Gynaecol Can. 2007;29:988–991. doi: 10.1016/S1701-2163(16)32689-5. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson TZ, Duffy JM, Barlow D, Koninckx PR, Garry R. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2009;(4):CD001300. doi: 10.1002/14651858.CD001300.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992. doi: 10.1002/14651858.CD004992.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson TZ, Duffy JM, Barlow D, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2010;(1):CD001398. doi: 10.1002/14651858.CD001398.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Benschop L, Farquhar C, van der Poel N, Heineman MJ. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst Rev. 2010;(11):CD008571. doi: 10.1002/14651858.CD008571.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 19.Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol. 2011;9:89. doi: 10.1186/1477-7827-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colette S, Donnez J. Are aromatase inhibitors effective in endometriosis treatment? Expert Opin Investig Drugs. 2011;20:917–931. doi: 10.1517/13543784.2011.581226. [DOI] [PubMed] [Google Scholar]
- 21.Lv D, Song H, Shi G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2010;(3):CD008088. doi: 10.1002/14651858.CD008088.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Strowitzki T, Faustmann T, Gerlinger C, Seitz C. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151:193–198. doi: 10.1016/j.ejogrb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 23.McCormack PL. Dienogest: a review of its use in the treatment of endometriosis. Drugs. 2010;70:2073–2088. doi: 10.2165/11206320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25:633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]


