Summary
Cerebral small vessel disease (SVD) is a common accompaniment of ageing. Features seen on neuroimaging include recent small subcortical infarcts, lacunes, white matter hyperintensities, perivascular spaces, microbleeds, and brain atrophy. SVD can present as a stroke or cognitive decline, or can have few or no symptoms. SVD frequently coexists with neurodegenerative disease, and can exacerbate cognitive deficits, physical disabilities, and other symptoms of neurodegeneration. Terminology and definitions for imaging the features of SVD vary widely, which is also true for protocols for image acquisition and image analysis. This lack of consistency hampers progress in identifying the contribution of SVD to the pathophysiology and clinical features of common neurodegenerative diseases. We are an international working group from the Centres of Excellence in Neurodegeneration. We completed a structured process to develop definitions and imaging standards for markers and consequences of SVD. We aimed to achieve the following: first, to provide a common advisory about terms and definitions for features visible on MRI; second, to suggest minimum standards for image acquisition and analysis; third, to agree on standards for scientific reporting of changes related to SVD on neuroimaging; and fourth, to review emerging imaging methods for detection and quantification of preclinical manifestations of SVD. Our findings and recommendations apply to research studies, and can be used in the clinical setting to standardise image interpretation, acquisition, and reporting. This Position Paper summarises the main outcomes of this international effort to provide the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE).
Introduction
Neurodegenerative diseases such as Alzheimer's disease commonly coexist with cerebrovascular disease in older people. Cerebral small vessel disease (SVD) is the most common vascular cause of dementia, a major contributor to mixed dementia, and the cause of about a fifth of all strokes worldwide.1,2 Alzheimer's disease and SVD share risk factors3,4 and both lead to cognitive decline and dementia;5–7 the clinical differentiation of Alzheimer's disease from vascular cognitive impairment or vascular dementia is increasingly recognised to be blurred.8
Signs of SVD on conventional MRI include recent small subcortical infarcts, white matter magnetic resonance (MR) hyperintensities, lacunes, prominent perivascular spaces, cerebral microbleeds, and atrophy.2 However, the terms for and definitions of these lesions have varied substantially between studies.9,10 For example, our systematic review identified 1144 instances of 50 different terms used to describe white matter hyperintensities in 940 papers; in some cases, two different terms were used in the same paper (table 1; appendix). This amount of variation inhibits cross-study comparisons and is a barrier to research on risk factors, pathophysiology, pathological correlations, and clinical consequences of these lesions. Indeed, the same lesions are classified differently across studies—eg, different definitions have resulted in small cavities being classified as perivascular spaces or lacunes.9 Interpretation of data from many reports is hampered by the variable consequences of acute SVD and related lesions and the convergence of lesions with different causes but similar late appearances on MRI (figure 1). Use of more standard terminology, definitions, and methods for image acquisition and analysis across research centres would remove a major barrier to progress. Furthermore, such standardisation could be used in clinical practice to improve diagnosis and better understand the cause of cognitive impairment in elderly patients.
Table 1.
Terms used to describe white matter hyperintensities of presumed vascular origin and frequency of use
| Variants of use of term | Papers that use term in title or abstract*(%) (n=1144) | |
|---|---|---|
| Leukoaraiosis | Ischaemic leukoaraiosis, subcortical leukoaraiosis | 350 (31%) |
| White matter lesions (WML) | MRI white matter lesions, cerebral WML, T2 WML(s), cerebrovascular WML, subcortical WML, WML of Binswanger's disease, cerebral WML of Binswanger's disease, confluent WML, intracranial WML | 275 (24%) |
| White matter hyperintensity (WMH) | Cerebral WMH, age-related WMH, brain WMH, MRI WMH | 217 (19%) |
| White matter changes (WMC) | Age-related cerebral WMC, age-related WMC, cerebral WMC, changes in white matter, age-related changes in white matter | 136 (12%) |
| Leukoencephalopathy | Subcortical ischaemic leukoencephalopathy | 76 (7%) |
| White matter disease (WMD) | Age-related WMD, cerebral WMD, subcortical WMD | 45 (4%) |
| White matter damage | Age-related white matter damage | 5 (0%) |
| Ischaemic white matter disease | Ischaemic subcortical WMD, chronic ischaemic cerebral WMD, subcortical ischaemic WMD | 4 (0%) |
| Others (9) | .. | 17 (1%) |
Data were derived from a structured literature search; for methodology and search strategy and selection criteria, see appendix.
Instances that term was mentioned at least once in abstract or title. WML=white matter lesion. WMH=white matter hyperintensity. WMC=white matter changes. WMD=white matter disease.
Figure 1.
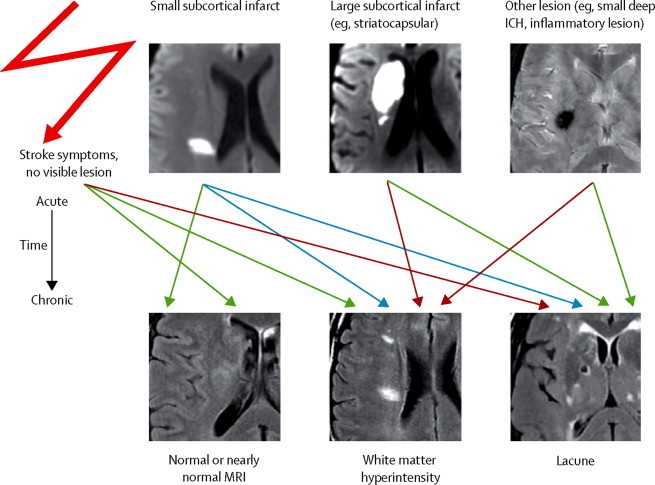
Variable fates of lesions related to small vessel disease and the convergence of acute lesions with different causes but similar late appearances on MRI
Arrows indicate possible late fates of acute MRI findings. Blue arrows indicate common fates of recent small subcortical infarcts, green arrows indicate less common fates, and red lines indicate least common late fates. ICH=intracranial haemorrhage.
Neuroimaging consensus standards for classification of SVD were first proposed by the US National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network as part of the development of standards for research on vascular cognitive impairment.11 Subsequently, a scientific statement from the American Heart Association incorporated neuroimaging evidence for SVD or stroke as part of the criteria for probable vascular mild cognitive impairment and dementia, and included a class 2 recommendation for neuroimaging as part of the clinical investigation of vascular cognitive impairment.12 However, neither of these guidelines provide comprehensive recommendations for the many forms of SVD seen on neuroimaging, and neither include advances in understanding the pathophysiology and measurement of SVD, which are changing rapidly.
Our international effort builds on previous initiatives and aims to provide clear, rigorous, evidence-based, and easy-to-apply definitions and terminology for the structural neuroimaging features of SVD that avoid presumption of mechanisms of pathogenesis. We include examples to help improve the standard use, minimum advisory standards for image acquisition, standards for analysis of imaging data on SVD and related features, and scientific reporting standards to improve clarity of publications on SVD.
Although this Position Paper focuses on the most common manifestations of SVD, other vascular lesions, such as ischaemic or haemorrhagic stroke, subarachnoid haemorrhage, subdural haematoma, and vascular malformations unrelated to SVD, can also contribute to cognitive impairment and dementia, especially after stroke. We provide a description of their features and neuroimaging recommendations in the appendix.
This Position Paper summarises the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE). Our main aim is to recommend standards for research with MRI; however, many of the principles also apply to research with CT, and the standards might also facilitate a more consistent approach to identification of manifestations of SVD on neuroimaging in clinical practice.
Methods
In 2011, the UK Medical Research Council (London, UK), the German Centre for Neurodegenerative Disease (DZNE, Bonn Germany), and the Canadian Institutes of Health Research (Ottawa, ON, Canada) issued a call for proposals under a funding concordat of the Centres of Excellence in Neurodegeneration (COEN), which aimed to accelerate progress in understanding the pathogenesis of neurodegeneration.13 This initiative provided funding for a working group of experts to establish standards for neuroimaging in SVD.
In March, 2012, the core group of experts met in Edinburgh, UK. Three study co-chairs (JMW, MDi, and EES) identified experts from COEN-affiliated centres and supplemented the group with representatives from research groups active in neuroimaging of SVD from non-COEN participant countries. Our group included experts in neurology, neuroradiology, neuroepidemiology, psychiatry, geriatrics, stroke, medical imaging physics, and neuropathology. The working method was based on the Delphi principle with workshops at the beginning and end of the project, and with interim work assignments (appendix). A template helped discussion to focus on achievement of a consensus in key areas: terminology, definitions, image acquisition, image analysis, and reporting standards. We endorsed the principle that terms and definitions should indicate imaging characteristics as descriptively as possible, avoiding presumptions of mechanism or pathological links not well supported by published reports so as to not affect future studies of the pathophysiology of SVD. Working groups developed standards for each of the six key lesion types, using established principles for guideline development published by the Enhancing the QUAlity and Transparency Of health Research (Equator) Network. We did systematic searches to identify relevant published work (appendix).
The group reconvened in Munich, Germany, in November, 2012, to present draft standards from each working group for discussion and revision, with additional review and comment from six new external advisers. We wrote and revised the consensus document with input from all workshop members. All participants reviewed and endorsed the final document.
For the definition of SVD, we discussed size limits to define perforating arteries and arterioles, but they were highly variable in published work, and did not translate well to their appearance on imaging. Therefore, we decided to use the term arteriole to refer to small perforating arteries and arterioles that are affected in SVD. These standards are expected to reliably classify most manifestations of SVD seen on neuroimaging; however, we acknowledge that individual judgment might be needed for classification of ambiguous lesions on the borders between categories (appendix), and that clinical judgment might be needed in some cases when using these standards in clinical practice.
Context, terminology, and definitions of imaging features
Recent small subcortical infarct
Context
Clinically evident recent small subcortical infarcts, commonly called lacunar strokes or lacunar syndrome, cause about 25% of all ischaemic strokes (figure 2). Occasionally, a recent asymptomatic small subcortical infarct is identified by chance on imaging,14,15 and is referred to as a silent cerebral infarct. By contrast, for as yet unknown reasons, in up to 30% of patients, symptomatic lacunar stroke syndromes seem not to be accompanied by visible small subcortical infarcts,16 indicating that MRI is not fully sensitive in the detection of such infarcts. Additionally, some studies have shown that the small subcortical infarcts might have differing fates, evolving into a lacunar cavity or hyperintensity without apparent cavitation on T2-weighted sequences, or might disappear leaving little visible consequence on conventional MRI (figure 1). Estimates of the proportion of recent small subcortical infarcts that cavitate range from 28%17 to 94%.18
Figure 2.
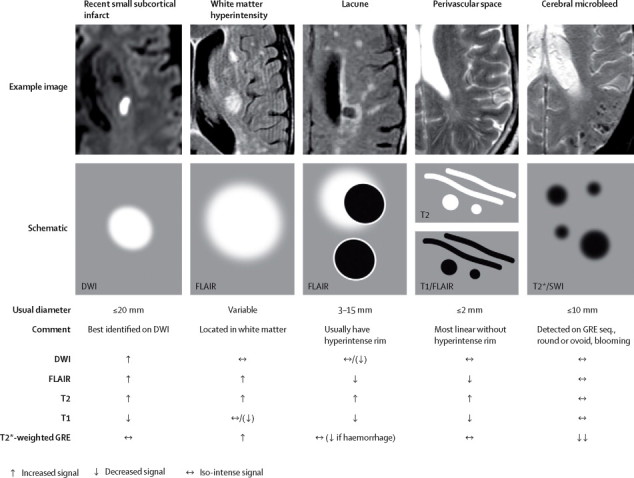
MRI findings for lesions related to small vessel disease
Shows examples (upper) and schematic representation (middle) of MRI features for changes related to small vessel disease, with a summary of imaging characteristics (lower) for individual lesions. DWI=diffusion-weighted imaging. FLAIR=fluid-attenuated inversion recovery. SWI=susceptibility-weighted imaging. GRE=gradient-recalled echo.
Scarce data from pathological correlation studies suggest that small subcortical infarcts are associated with occlusion of small arteries, although the pathogenesis of these infarcts is unclear. Small subcortical infarcts occur in the perfusion territory of a small artery or arteriole penetrating the internal part of the brain. MRI studies suggest that recent small subcortical infarcts can exceed a diameter of 15 mm on axial sections (the usual size limit for lacunes of presumed vascular origin) in the acute phase, and can be up to about 20 mm on axial sections. MRI also shows that small subcortical infarcts and lacunes can be more than 20 mm long when measured in the coronal or sagittal plane (appendix).
Terminology
We sampled 641 abstracts and one previous systematic review9 and identified 159 different terms for recent small subcortical infarcts; the most common terms were lacunar infarcts, lacunar infarctions, and lacunar strokes (appendix). We propose the new consensus term recent small subcortical infarct, removing the word lacunar because of evidence that not all small subcortical infarcts become lacunes (ie, cavities).17,18
Definition
We propose that the term recent small subcortical infarct should refer to neuroimaging evidence of recent infarction in the territory of one perforating arteriole, with imaging features or clinical symptoms consistent with a lesion occurring in the previous few weeks (figure 2, panel 1). Use of the word recent should refer to lesions with symptoms or imaging features that suggest they occurred in the previous few weeks; the word is used instead of acute because it includes the first few weeks of the lesion, and not the hyperacute stage only. Use of the word small indicates a lesion that should be less than 20 mm in its maximum diameter in the axial plane, although some lesions that appear to represent infarction in the territory of one vessel can be somewhat larger in the coronal plane (appendix).19 More investigation is needed to precisely define upper size limits.
Panel 1. Glossary of proposed terms and definitions for neuroimaging features of small vessel disease.
Recent small subcortical infarct
Neuroimaging evidence of recent infarction in the territory of one perforating arteriole, with imaging features or clinical symptoms consistent with a lesion occurring in the previous few weeks.
Lacune of presumed vascular origin
A round or ovoid, subcortical, fluid-filled cavity (signal similar to CSF) of between 3 mm and about 15 mm in diameter, consistent with a previous acute small subcortical infarct or haemorrhage in the territory of one perforating arteriole.
White matter hyperintensity of presumed vascular origin
Signal abnormality of variable size in the white matter that shows the following characteristics: hyperintensity on T2-weighted images such as fluid-attenuated inversion recovery, without cavitation (signal different from CSF). Lesions in the subcortical grey matter or brainstem are not included in this category unless explicitly stated. If deep grey matter and brainstem hyperintensities are also included, the collective term should be subcortical hyperintensities.
Perivascular space
Fluid-filled spaces that follow the typical course of a vessel as it goes through grey or white matter. The spaces have signal intensity similar to CSF on all sequences (figure 2). Because they follow the course of penetrating vessels, they appear linear when imaged parallel to the course of the vessel, and round or ovoid, with a diameter generally smaller than 3 mm, when imaged perpendicular to the course of the vessel.
Cerebral microbleed
Small (generally 2–5 mm in diameter, but sometimes up to 10 mm) areas of signal void with associated blooming seen on T2*-weighted MRI or other sequences that are sensitive to susceptibility effects.
Brain atrophy
A lower brain volume that is not related to a specific macroscopic focal injury such as trauma or infarction. Thus, infarction is not included in this measure unless explicitly stated.
Lesions in the basal ganglia and internal capsule that are larger than 20 mm and seem to be due to simultaneous infarction in several penetrating arteries should not be classified as small subcortical infarcts, but rather as striatocapsular infarcts, a subtype of infarct with a distinct cause.20 Similarly, infarcts of the anterior choroidal artery are aetiologically distinct, identifiable by their location (in the caudate nucleus head), and shape (mostly comma shaped), and therefore should not be classified as small subcortical infarcts. Unlike lacunes of presumed vascular origin, no lower size limit is given for small subcortical infarcts because diffusion-weighted imaging allows discrimination of small recent infarcts from perivascular spaces.
Lacune of presumed vascular origin
Context
Regarding pathological changes, Fisher21 wrote: “Historically, the original SVD feature was the lacune (hole), which derived from French for a small fluid-filled cavity that was thought to mark the healed stage of a small deep brain infarct. The term was adopted into English. By a process of medico-linguistic evolution, the precavitary phase became the lacunar infarct, the associated clinical entity became the lacunar stroke and the neurological features became the lacunar syndrome.” Lacunes are frequently seen on imaging in elderly patients with no symptoms and are associated with an increased risk of stroke, gait impairment, and dementia.22–25 The cause of most lacunes is presumed to be small subcortical infarcts, either symptomatic or silent; however, some might result from small deep haemorrhages (figure 1).26
Terminology
Our systematic review identified more than 100 terms that have been used to describe lacunes of presumed vascular origin (appendix). Commonly used terms were lacune, lacunar stroke, and silent brain infarct. We propose the new term lacune of presumed vascular origin, which discriminates between small cavitated lesions of presumed vascular origin and other small brain cavities, and allows for some uncertainty about the ischaemic or haemorrhagic origin of the lesion when no imaging is available in the acute phase, as is commonly the case (panel 1).
Definitions
We define a lacune of presumed vascular origin as a round or ovoid, subcortical, fluid-filled (similar signal as CSF) cavity, of between 3 mm and about 15 mm in diameter, consistent with a previous acute small deep brain infarct or haemorrhage in the territory of one perforating arteriole (panel 1). On fluid-attenuated inversion recovery (FLAIR) images, lacunes of presumed vascular origin generally have a central CSF-like hypointensity with a surrounding rim of hyperintensity; however, the rim is not always present, and a hyperintense rim can also surround perivascular spaces when they pass through an area of white matter hyperintensity (figure 2). In some cases, the central cavity fluid is not suppressed on FLAIR, and the lesion can appear entirely hyperintense, despite MRI having a clear CSF-like intensity on other sequences such as T1-weighted and T2-weighted MRI.18
Lacunes of presumed vascular origin should be distinguished from perivascular spaces. Although pathological studies have not shown an absolute cutoff size, lesions that are less than 3 mm in diameter are more likely to be perivascular spaces than to be lacunes (appendix), and we recommend the use of this size criterion to discriminate between the two lesions, which is consistent with previous studies.27,28 We chose a maximum size of 15 mm for lacunes of presumed vascular origin—which differs from the 20 mm diameter of recent small subcortical infarcts—since old infarcts are generally smaller than recent infarcts because of tissue loss and an ex-vacuo effect in old lesions, and because of swelling in new lesions. However, we recognise that this size boundary is not supported by much objective evidence, and research is needed.
White matter hyperintensity of presumed vascular origin
Context
White matter lesions characterised by bilateral, mostly symmetrical hyperintensities on T2-weighted MRI are common in older individuals. Although strongly associated with cerebrovascular disease and vascular risk factors,29 the pathogenesis of these white matter lesions is not well understood and could be multifactorial.30
White matter hyperintensities are associated with covert neurological and cognitive symptoms and physical difficulties such as gait disturbance.31–35 Hyperintensities can also occur in subcortical grey matter structures, such as the basal ganglia, and have sometimes been analysed alongside white matter hyperintensities. Hyperintensities can also be present in the brainstem. Some investigators have differentiated between hyperintensities of periventricular and deep white matter (appendix), with the suggestion, under debate, that they have differing pathogenesis, risk factors, and clinical consequences. Many investigators have included total white matter hyperintensities in their analyses.
Terminology
Our systematic review of 940 abstracts identified 50 different terms for white matter hyperintensity. The most common terms were leukoaraiosis, white matter lesions, white matter hyperintensities, leukoencephalopathy (usually in the context of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy [CADASIL]), and white matter disease (table 1; appendix).
Leukoaraiosis describes a reduced area of x-ray attenuation on CT,36 and this term was later adopted to denote hyperintensity on T2-weighted and FLAIR MRI, and sometimes also hypointensity on T1-weighted MRI (appendix), a situation in which the radiological description came before the pathological description. We propose the term white matter hyperintensity of presumed vascular origin to exclude white matter lesions from other diseases such as multiple sclerosis or leukodystrophies.
Definitions
White matter hyperintensities of presumed vascular origin are hyperintense on T2-weighted sequences and can appear as isointense or hypointense (although not as hypointense as CSF) on T1-weighted sequences, depending on the sequence parameters and severity of pathological change (figure 2, panel 1; appendix). Members of our group had differing opinions about whether grey matter hyperintensities or brainstem hyperintensities should be routinely classified as white matter hyperintensities, as some previous studies have done. The consensus opinion was that lesions in the subcortical grey matter or brainstem should not be included in the category of white matter hyperintensity of presumed vascular origin unless explicitly stated; we endorsed subcortical hyperintensities as an acceptable alternative collective term for any non-cortical hyperintensities, including those in white matter, deep grey matter, and the brainstem. When using CT, white matter hypoattenuation or white matter hypodensities can be used because of the appearance of the lesions on CT.
Perivascular space
Context
Perivascular spaces are extensions of the extracerebral fluid space around arteries, arterioles, veins, and venules as they course from the brain surface into and through the brain parenchyma, and they can be followed by sheets of leptomeninges.37 Perivascular spaces are commonly microscopic, and not visible on conventional neuroimaging; however, larger spaces become increasingly apparent with increasing patient age, especially when located at the base of the brain.38 General enlargement of perivascular spaces is associated with other morphological features of SVD such as white matter hyperintensities39 and lacunes,40 but not atrophy.41 Whether the presence of several visible perivascular spaces is clinically significant remains controversial, and the spaces should therefore not be referred to as lesions; however, some studies have associated more prominent perivascular spaces with worse cognitive function.42
Terminology
Synonymous terms for perivascular spaces include Virchow–Robin spaces,40 type 3 lacune,43 or état crible when located predominantly in the basal ganglia.38 Terms used to describe visible perivascular spaces include large, dilated, large dilated, or enlarged Virchow–Robin spaces or perivascular spaces.38,41,42,44–48 We did not consider these terms appropriate because the association between the size of the perivascular spaces and clinical consequences is not well understood, and because the visibility of the spaces depends on MRI sequence characteristics, which vary across studies. Visibility alone cannot be a uniform criterion for pathologically enlarged perivascular spaces. We therefore recommend the consensus term perivascular space.
Definitions
We define perivascular spaces as fluid-filled spaces that follow the typical course of a vessel as it goes through grey or white matter. The spaces have signal intensity similar to that of CSF on all sequences (figure 2); because they follow the course of penetrating vessels, they appear linear when imaged parallel to the course of the vessel, and round or ovoid, with a diameter generally smaller than 3 mm, when imaged perpendicular to the course of the vessel (panel 1, appendix). At high resolution, a central vessel can occasionally be seen in the centre of a perivascular space, which could possibly differentiate the spaces from lacunes. Perivascular spaces are generally most prominent in the inferior basal ganglia, and can also be seen coursing centripetally through the hemispheric white matter and in the midbrain; however, the spaces are rarely seen in the cerebellum. Perivascular spaces can show focal enlargement, and can be especially enlarged (up to 10–20 mm, even with mass effect) in the inferior basal ganglia. Perivascular spaces must be discriminated from small lacunes of presumed vascular origin (appendix). By contrast with lacunes, the diameter of perivascular spaces is not usually more than 3 mm as confirmed pathologically,49,50 and spaces do not have a T2-hyperintense rim around the fluid-filled space on T2-weighted or FLAIR imaging, unless they traverse an area of white matter hyperintensity.51
Cerebral microbleed
Context
Cerebral microbleeds are small hypointense lesions that are visible on paramagnetic-sensitive MR sequences such as T2*-weighted gradient-recalled echo (GRE) or susceptibility-weighted sequences, and are most commonly located in the cortico-subcortical junction, and deep grey or white matter in the cerebral hemispheres, brainstem, and cerebellum (figure 2). Results of some studies30,52,53 suggest that MR-visible lesions correspond to haemosiderin-laden macrophages in perivascular tissue, consistent with vascular leakage of blood cells.52,54–56 Cerebral microbleeds are associated with SVD and Alzheimer's disease.57,58 The presence or absence of strictly lobar microbleeds has been included in research criteria for cerebral amyloid angiopathy.59 Originally thought to be asymptomatic markers of SVD, emerging data shows association between microbleeds and cognitive impairment, although the mechanisms of this association, including whether microbleeds damage the brain and cause dysfunction, are not well understood. Recommendations for imaging and reporting of microbleeds have been published,60 and we endorse the use of these standards. Amyloid-related imaging abnormalities–haemorrhage (ARIA-H) describes microbleeds in the context of Alzheimer's disease or β-amyloid immunotherapies. A consensus group from the US Alzheimer's Association has published standards for assessing microbleeds in this specific clinical context,61 and we also endorse the use of these guidelines.
Terminology
Our systematic review revealed 387 instances of 20 different terms for microbleeds in 370 abstracts (appendix). Microbleed was by far the most common term, followed by cerebral microbleed, then brain microbleed. We propose the consensus term cerebral microbleed.
Definitions
Cerebral microbleeds are visualised as small (generally 2–5 mm in diameter, but up to 10 mm) areas of signal void with associated blooming seen on T2*-weighted MRI or other sequences that are sensitive to susceptibility effects (panel 1),57,60 and are generally not seen on CT, or on FLAIR, T1-weighted, or T2-weighted sequences. When imaged with T2*-weighted GRE sequences, cerebral microbleeds are well defined, of homogeneous low signal, and are either round or oval in shape (figure 2). Because the blooming artifact, and therefore the visualised size, depends on MR field strength and sequence, we do not recommend an absolute criterion for size. When imaged with 1·5 T and 3·0 T GRE sequences, cerebral microbleeds are generally 2–5 mm in diameter, but can be up to 10 mm. Hypointensities of less than 2 mm, which could be attributable to signal loss from only one voxel, should be regarded as questionable on 1·5 T MRI because such small hypointensities could be artifacts.
Susceptibility-weighted imaging can also be used to assess cerebral microbleeds. New quantitative-based methods (ie, quantitative susceptibility mapping) need more investigation, but might improve the assessment of cerebral microbleeds. Several lesions or structures can mimic cerebral microbleeds, such as calcification, normal vessels seen in cross-section, iron deposits from other causes, haemorrhagic metastases (eg, melanoma), and diffuse axonal injury (eg, after head trauma).60 Cerebral microbleeds can be differentiated from an old small deep spontaneous intracerebral haemorrhage because, in general, the intracerebral haemorrhages are larger, irregular with a cystic cavity (figure 1), and will be visible on T1-weighted and T2-weighted or FLAIR sequences.
Other haemorrhagic lesions
We discussed two other haemorrhagic manifestations of SVD: intracerebral haemorrhage and superficial cortical siderosis (appendix). Intracerebral haemorrhage could be a manifestation of SVD62 or secondary to other causes such as vascular malformations. We suggest the use of the consensus term spontaneous intracerebral haemorrhage presumed to be due to SVD, instead of secondary intracerebral haemorrhage from other causes or traumatic intracerebral haemorrhage. Comprehensive guidelines for the diagnosis and management of intracerebral haemorrhage have been published previously.63 In the context of SVD, we encourage researchers to resolve the contribution of different vascular diseases to intracerebral haemorrhage. In the meantime, we recommend that lobar intracerebral haemorrhages, which might be caused by cerebral amyloid angiopathy, should be distinguished from non-lobar intracerebral haemorrhages, which are not thought to be caused by cerebral amyloid angiopathy, and are mostly due to perforating arteriolar vasculopathy.64,65 Results of a small study66 suggested good, but not perfect, inter-rater agreement on the site of origin of intracerebral haemorrhages. The Boston criteria59 for cerebral amyloid angiopathy should be used in assigning the likelihood of underlying cerebral amyloid angiopathy in patients with lobar intracerebral haemorrhages.
We propose the term superficial cortical siderosis for neuroimaging evidence of chronic blood products in the superficial cortex under the pia mater. Superficial cortical siderosis could be a chronic consequence of subarachnoid bleeding or might be the result of very superficial cortical bleeding caused by vascular malformations, cerebral amyloid angiopathy,67 spinal dural defects, or it might also be idiopathic.67,68 T2*-weighted GRE or other blood-sensitive sequences will show superficial cortical siderosis as a linear hypointensity over the cortex, but this disorder might be mimicked by the petechial cortical haemorrhagic transformation of an infarct. Revised research criteria for cerebral amyloid angiopathy include superficial cortical siderosis as an additional haemorrhagic manifestation of cerebral amyloid angiopathy, equivalent to a lobar intracerebral haemorrhage.67 Investigators should describe the location of siderosis (ie, the number of sulci involved67 and in which lobes).
Brain atrophy
Context
Brain atrophy can be general or focal (affecting only particular lobes or specific brain regions—eg, the hippocampus), symmetrical or asymmetrical, or tissue selective (affecting a certain tissue class—eg, white matter), and occurs in many disorders. The pathological changes of atrophy are heterogeneous and not necessarily indicative of neuronal loss.69–71 Brain atrophy occurs with the usual ageing process, but the extent varies between individuals. In the context of vascular disease and dementia, neuropathological substrates of atrophy include neuronal loss,72 cortical thinning, subcortical vascular pathology with white matter rarefaction and shrinkage, arteriolosclerosis, venous collagenosis, and secondary neurodegenerative changes.73,74 Many imaging studies report an association between the presence and severity of SVD and brain atrophy, including global atrophy, corpus callosum atrophy, central atrophy (increased ventricular size and atrophy of the basal ganglia), mesencephalic atrophy, and hippocampal atrophy, and focal cortical thinning in brain regions connected to subcortical infarcts (figure 3).75,76 Therefore, vascular lesions should be included in studies of atrophy; conversely, atrophy is an important measure in imaging studies that are done to assess the burden of vascular damage in the brain, and atrophy is thought to mediate, at least partially, the effects of vascular lesions on cognition.77–79
Figure 3.
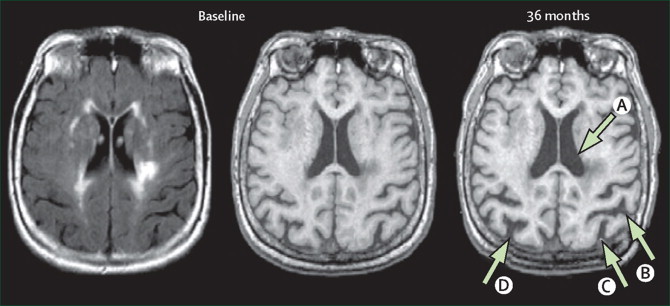
Secondary brain atrophy in a 55-year-old patient with documented small vessel disease
Baseline (middle). The follow-up scan (T1-weighted MRI; right) shows clear sulcal widening (arrow B, C, and D), particularly in occipital regions, and ventricular enlargement (arrow A) without new infarctions during the observational period. Fluid-attenuated inversion recovery image (left) shows substantial white matter hyperintensity.
Terminology
Our systematic review of studies on SVD and atrophy revealed the use of many synonymous terms, including atrophy, brain volume, volume loss, and others. We propose the consensus term brain atrophy. We also propose that studies should use unambiguous terms about whether the atrophy assessment was cross-sectional or longitudinal, and should avoid terms such as accelerated atrophy or brain volume loss in cross-sectional studies in favour of more atrophy or lower brain volume, respectively. When discussing atrophy, investigators should be clear about the specific brain volumes they have measured and should include the brain subregion in their description—eg, hippocampal atrophy.
Definitions
We define brain atrophy in the context of SVD on imaging as a lower brain volume that is not related to a specific macroscopic focal injury such as trauma or infarction (panel 1). Tissue loss is assumed from the enlargement of peripheral (sulcal) and central (ventricular) CSF spaces in relation to intracranial volume and other measures (figure 3). However, ideally, such assumptions should be formally confirmed with longitudinal observation. Tissue loss from discrete focal lesions, such as cortical infarcts, is easier to see on cross-sectional imaging, and should not be confused with generalised global or regional brain atrophy that is probably secondary to a diffuse process.
Other vascular lesions
These consensus standards focus on SVD. However, other vascular lesions can also be seen on imaging, particularly in elderly individuals and in those with cognitive impairment. These include ischaemic lesions unrelated to SVD, intracerebral haemorrhage from other causes, subarachnoid haemorrhage, subdural haematoma, vascular malformations, and large artery ischaemic disease. These features are discussed in detail in the appendix.
Advised minimum standards and parameters for imaging of SVD
Image acquisition
If no contraindications are known, MRI, rather than CT, is preferred for research and routine clinical use because it has higher sensitivity and specificity for detecting most manifestations of SVD (table 2). A field strength of 3·0 T might be preferred, but images from modern 1·5 T MRI scanners are often of a similarly high definition,80 and are therefore acceptable; 1·5 T systems are also more widely available than 3·0 T systems, an important consideration for multicentre studies. Imaging sequences should be optimised for field strength and scanner configuration. If CT is used to image SVD, investigators should obtain a thin-section volume sequence, without contrast, covering the whole brain, and using a brain parenchymal algorithm and axial 5 mm reconstructions. However, CT is no longer recommended, except possibly in large-scale epidemiological studies, or if MRI is not available or is too expensive.
Table 2.
Proposed image acquisition standards for neuroimaging of small vessel disease
| Purpose* | Orientation | Target-slice thickness and in-plane resolution | Comment | |
|---|---|---|---|---|
| Minimum essential sequences—eg, for clinical or large-scale epidemiological studies, available on most MRI scanners | ||||
| T1-weighted | Important for discriminating lacunes from dilated perivascular spaces; for discriminating grey from white matter, and for studying brain atrophy | 2D axial, sagittal, or coronal | 3–5 mm, and 1 mm × 1 mm | At least one sequence in sagittal or coronal plane is helpful to visualise full extent and orientation of lesions |
| DWI | The most sensitive sequences for acute ischaemic lesions; positive for up to several weeks after cerebrovascular event | 2D axial | 3–5 mm, and 2 mm × 2 mm | Reduced signal on apparent diffusion coefficient map helps to discriminate recent lesions from old lesions |
| T2-weighted | To characterise brain structure; to differentiate lacunes from white matter hyperintensities and perivascular spaces; to identify old infarcts | 2D axial | 3–5 mm, and 1 mm × 1 mm | .. |
| FLAIR | To identify white matter hyperintensities and established cortical or large subcortical infarcts; to differentiate white matter lesions from perivascular spaces and lacunes | 2D axial | 3–5 mm, and 1 mm × 1 mm | .. |
| T2*-weighted GRE | To detect haemorrhage, cerebral microbleeds, siderosis; for measurement of intracranial volume | 2D axial | 3–5 mm, and 1 mm × 1 mm | Only reliable routine sequence for detection of haemorrhage |
| Other routine sequences, available on most MR scanners | ||||
| Proton density-weighted | To detect white matter hyperintensities, infarcts, perivascular spaces (with T2-weighted dual echo), or other pathologies | 2D axial | 3–5 mm, and 2 mm × 2 mm | Mostly replaced by FLAIR |
| MRA | To detect stenosis of vertebral, basilar, internal carotid, middle cerebral, anterior cerebral, or posterior cerebral artery, or other pathologies | Post-contrast or 3D time-of-flight for intracranial arteries | 3D, axial, coronal, sagittal reconstruction; 1 mm isotropic voxels | Only large vessels visible at 1·5 T or 3·0 T; see below for perforating arterioles |
| Sequences commonly available on commercial clinical MR scanners; at present, used more for research studies, but some techniques are increasingly used in clinical protocols | ||||
| DTI with six-gradient direction diffusion encoding | To diagnose recent infarct; measurement of mean diffusivity and fractional anisotropy | 2D axial | 3–5 mm, and 2 mm × 2 mm | More detailed characterisation than with DWI; acquisition time is double that for DWI |
| SWI or equivalent | Very sensitive to haemosiderin, measurement of intracranial volume | 2D or 3D axial | 2D: 3–5 mm, and 2 mm × 2 mm; 3D: 1 mm isotropic voxels | Enables visualisation of more cerebral microbleeds than T2*-weighted GRE imaging and is more sensitive to artifacts including motion |
| Research-only sequences; require research expertise | ||||
| Isotropic volumetric T2-weighted | To display fine detail of perivascular spaces | 3D axial | 1 mm isotropic voxels | Allows post-acquisition reformatting; could potentially replace 2D T2-weighted imaging if signal-to-noise ratio is adequate |
| Isotropic volumetric 3D T1-weighted (eg, MP-RAGE) | Provides improved global and regional volumetric brain measurements | 3D axial | 1 mm isotropic voxels | Allows post-acquisition reformatting; could potentially replace 2D T1-weighted imaging if signal-to-noise ratio is adequate |
| Isotropic volumetric FLAIR | Enables identification of white matter hyperintensities; used for imaging cortical or subcortical infarcts | 3D axial | 1 mm isotropic voxels | Allows post-acquisition reformatting; could potentially replace 2D FLAIR imaging if signal-to-noise ratio is adequate; more homogeneous CSF suppression |
| Advanced DTI with more than six-direction diffusion encoding (eg, 32 or more diffusion-encoding directions) | Provides refined and superior quantitative measurements of microscopic tissue changes | 2D axial | 3–5 mm, and 2 mm × 2 mm | Allows for tractography, connectome mapping, and more accurate measurements of mean diffusivity and fractional anisotropy |
| MTR | To detect demyelination and axonal loss | 2D axial | 3–5 mm, and 1 mm × 1 mm | Experience in acquisition and interpretation needed; involves two measurements (with and without magnetisation transfer-pulse) |
| T1 mapping | To measure water content of tissue | Axial | 3–5 mm, and 2 mm × 2 mm | Experience in acquisition and interpretation needed |
| Permeability imaging | To estimate permeability of the blood–brain barrier | Axial; sequential before and after contrast | 3–5 mm, and 2 mm × 2 mm | Intravenous contrast injection needed; involves complex image processing; methods improving rapidly |
| ASL perfusion imaging | To measure tissue perfusion; quantitative, with assumptions | 2D axial | 3–5 mm, and 2 mm × 2 mm | Complex to set up and run accurately; needs post-processing; optimum processing strategies not yet confirmed; contrast injection not needed |
| Perfusion imaging (DCE or DSC) | To semiquantitatively measure blood perfusion in tissue | 2D axial | 3–5 mm, and 2 mm × 2 mm | Needs intravenous injection of contrast agent and post-processing; optimum acquisition and processing not yet confirmed for T1 (DCE) or T2*-weighted (DSC) approaches |
| fMRI | To measure brain function in response to tasks or stimuli, or at rest for default mode networks | 2D axial | 3–5 mm, and 2 mm × 2 mm | Complex set-up, acquisition, and processing |
| QSM | To provide quantitative measures of susceptibility changes, independent of scanner or acquisition variables | 2D or 3D axial | 2D: 3–5 mm, and 2 mm × 2 mm; 3D: 1 mm isotropic voxels | Uses an SWI-like acquisition, but needs very complex post-processing methods; post-processing strategies currently under investigation |
| Microatheroma and arteriolar imaging | To visualise perforating arteriolar anatomy and atheroma | Uncertain, emerging method | Uncertain, emerging method | Promising experimental approach that needs a scanner that is more than 3·0 T |
DWI=diffusion-weighted imaging. FLAIR=fluid-attenuated inversion recovery. GRE= gradient-recalled echo. MRA=magnetic resonance angiography. DTI=diffusion tensor imaging. SWI=susceptibility-weighted imaging. MP-RAGE=magnetisation-prepared rapid acquisition with gradient echo. MTR=magnetisation transfer ratio. ASL=arterial spin labelling. DCE=dynamic contrast-enhancement. DSC=dynamic susceptibility contrast. fMRI=functional MRI. QSM=quantitative susceptibility mapping.
MRI at 3·0 T is preferred to 1·5 T. However, these standards are listed as minimum and essential to research-only applications. These categories are not absolute; purposes are variable, and will vary with investigators' interest, expertise, and available technology.
For MRI, the minimum acceptable examination should include axial diffusion-weighted imaging (and apparent diffusion coefficient map), FLAIR, T2-weighted, and T2*-weighted GRE or susceptibility-weighted imaging, and T1-weighted imaging. The diffusion-weighted imaging sequence is very important for identification of recent infarcts—eg, when used in patients with symptoms. MRI with diffusion-weighted imaging should be considered the reference standard for recent small subcortical infarcts, although results might be falsely negative in some cases.16 The combination of clinical syndrome plus MRI results but without diffusion-weighted imaging, or clinical syndrome plus CT results, is not optimal; clinical syndrome alone is the least reliable method of assessment.81 The use of one sequence (usually T1-weighted MRI) in the coronal or sagittal plane instead of the axial plane helps to visualise the correct lesion dimensions (appendix). Slice thickness should be 5 mm or less, ideally with no gap; in-plane resolution should be 1 mm by 1 mm or better. Whole-brain coverage is recommended for all sequences to achieve optimum image analysis. Three-dimensional T1-weighted thin-section isotropic sequences are now widely available and quick and can be reformatted on thicker slices for ease of viewing in multiple planes; this sequence also allows anatomical coregistration and quantification of brain volume. The Alzheimer's Disease Neuroimaging Initiative has validated three-dimensional magnetisation prepared T1-weighted sequence protocols for the major scanner types at 1·5 T and 3·0 T; sequence protocols are freely available.
For a comprehensive research MRI protocol for SVD, investigators should consider use of higher-resolution MRI than the sequences specified above, and use volume acquisitions to allow for volumetric whole-brain imaging with near-isotropic resolution. These three-dimensional acquisitions are now possible on most scanners for T1-weighted, T2-weighted, GRE, and FLAIR techniques. Other high-resolution sequences sensitive to paramagnetic content, such as susceptibility-weighted imaging and equivalents, should be considered for three-dimensional image acquisition, although these sequences increase the presence of artifacts. Diffusion tensor imaging builds on findings with diffusion-weighted imaging, and should be used in research protocols. Diffusion tensor imaging is useful in the assessment of surrogates for microarchitectural integrity in normal-appearing and lesional white matter, in the determination of structural connectivity of white matter, and in tractography. For quantitative volumetric assessments of atrophy, we advise a high-resolution (1·0 mm to 1·5 mm isotropic voxels) T1-weighted sequence with good grey–white matter differentiation. Other MR measurements show promise for imaging of SVD in research, including measurements of perfusion, magnetisation transfer, blood–brain barrier permeability, vascular reactivity, metabolites, and microatheroma in perforating arterioles.
Image analysis
Qualitative (visual rating) and quantitative (computational analysis) methods are established or are emerging for all lesion types. For some of the lesions, qualitative and quantitative approaches are closely related,82,83 and both have advantages and disadvantages that should be considered in the design of any study of SVD (appendix).
For all lesion types, observers should be trained in neuroimaging interpretation, and specifically how to recognise lesions of SVD. If possible, particularly in trials or large observational studies, images should be rated centrally by one or a few experienced raters. In studies with more than one rater, inter-rater reliability should be assessed. Observers should have good knowledge of normal neuroanatomy to measure global and regional atrophy. Observers should train on established training sets, and should aim for high observer agreement before taking actual measurements. Regular recalibration against standard examples is useful to maintain consistency when rating large numbers of scans or when rating images from different sessions.
When investigators use automated computational methods for analysis, we emphasise that the accuracy of each individual's results should be visually confirmed by a trained observer. This validation step is crucial for studies of SVD because automated methods have not been developed for the disorder, and there is a high risk of misclassification of lesions, especially when unfamiliar pulse sequences are used or scanners are upgraded. Known examples to avoid are the misclassification of white matter hyperintensities as normal grey matter, of lacunes as ventricle or white matter hyperintensity, or of small subcortical infarcts as white matter hyperintensities.84 For several of these misclassifications, manual correction will be needed.78,85
For small numbers of discrete lesions, such as recent small subcortical infarcts, lacunes, and microbleeds, we recommend that the location of each lesion and the total number of lesions are recorded. At minimum, we recommend recording of the number of lesions in each of the following brain subregions, on both the right and left sides: cerebral cortex (divided by lobe), corona radiata and centrum semiovale, putamen, globus pallidus, thalamus, internal capsule, external capsule, brainstem, and cerebellum (table 3, appendix).
Table 3.
Proposed analysis standards for neuroimaging features of small vessel disease
| Measures of interest | Qualitative analysis standards | Quantitative analysis standards | Study design | Accuracy, reliability, feasibility | General comment | |
|---|---|---|---|---|---|---|
| Recent small subcortical infarct | Number (multiplicity might indicate other causes);85,86 size (maximum diameter); volume; location (anatomical region, vascular territory); shape (round, ovoid, tubular); swelling (indicates recent, not old) | Various coding schemes available for location: anatomical (eg, centrum semiovale, corona radiata, basal ganglia, thalamus, internal capsule, external capsule, optic radiation, cerebellum, and brainstem), and the vascular territory (eg, middle cerebral artery, posterior cerebral artery, internal carotid artery, and basilar artery) | Possible, but impractical for size and volume | Cross-sectional and longitudinal: recent small subcortical infarcts are typically detected in the setting of an acute clinical event, but can also be an incidental finding | Easy to identify on DWI, reliability depends on time between infarct and imaging; more difficult when using other sequences or CT without longitudinal data | Mimics include acute inflammatory multiple sclerosis plaques; acute lesions generally have increased signal on DWI and reduced signal on apparent diffusion coefficient images |
| Lacune of presumed vascular origin | Number (one or many); size (maximum diameter); shape (round, ovoid, tubular, other); location (anatomical region); evidence of previous haemorrhage; ex-vacuo effect | Various coding schemes available for shape and location: anatomical (eg, lentiform nucleus, thalamus, internal capsule, centrum semiovale, brainstem); prominent ex-vacuo effect indicates lesion was originally larger (eg, striatocapsular infarct)20 | Protocols for quantitative measurement available, need manual correction | Cross-sectional and longitudinal: particular care is needed to differentiate lacunes from perivascular spaces; longitudinal: difference in imaging helps to identify incident lacunes | Differentiation from perivascular spaces can be difficult; high observer agreement should be achieved before undertaking actual ratings | Hypointense rim on T2*-weighted imaging suggests previous small deep haemorrhage |
| White matter hyperintensity | Volume; location (anatomical region); number | Various coding schemes available for anatomical location (eg, periventricular, deep, subcortical, brainstem; or centrum semiovale, corona radiata, internal capsule, external capsule, optic radiation, brainstem; or frontal, temporal, parietal, occipital) | Various visual rating scores87–92 and protocols for quantitative measurement93,94–97 are available; the two approaches are complementary;82,83 outputs should be visually reviewed by an experienced rater for mimics, artifacts, focal infarcts, and mislabelling | Cross-sectional and longitudinal: consider masking recent small subcortical lesions, lacunes, and perivascular spaces when measuring volume of white matter hyperintensity to avoid inflating the volume; longitudinal: difference imaging might help to identify new white matter lesions | Inter-rater and intra-rater reliability for both qualitative and quantitative analysis of white matter hyperintensity is high if done by trained raters, with intraclass correlation coefficients generally above 0·90; visual rating scores might have ceiling or floor effect so performance can differ with extent of disease | Careful visual checking is needed at all stages of computational analysis to avoid difficulties from excess lesion distortion by, for example, bias field correction; regular recalibration against standard examples is needed in rating large numbers of scans |
| Perivascular space | Number (multiplicity); location (anatomical region); size (maximum diameter); shape | Anatomical: midbrain, hippocampus, basal ganglia, centrum semiovale | Visual scores used to rate number of lesions in basal ganglia, centrum semiovale, midbrain;42,45,46 various threshold-based methods are in development | Cross-sectional: consider masking perivascular spaces when measuring volume of white matter hyperintensity, although this might be difficult; longitudinal: little experience | Difficult to determine, especially when numerous, and in the presence of white matter hyperintensities | Can be difficult to distinguish from lacunes; giant perivascular spaces can be greater than 2 cm, and are most commonly located below the putamen |
| Cerebral microbleed | Number (few or multiple); location (lobar, deep, or infratentorial; anatomical region); size | Some semi-automated approaches segment cerebral microbleeds as an extra tissue class or radial symmetry and mask areas of mineralisation,98,99 but these are experimental at present, and need validation | Several visual scores are available;60,100,101 no methods available for automated detection | Cross-sectional and longitudinal: consider use of visual scores; longitudinal: no specific scores available for longitudinal studies | Inter-rater agreement for the presence or absence of one or two microbleeds varies, but agreement (ie, 0·8) between the numbers of microbleeds is reasonable; reliability can be improved through the use of standardised scales60,100,101 | Lobar and deep cerebral microbleeds might have different risk factors and causes (eg, lobar cerebral microbleeds are associated with cerebral amyloid angiopathy) |
| Brain atrophy | Whole brain should be adjusted for intracranial volume; regional (hippocampus, specific gyri, lobes should be adjusted for whole-brain volume); cortical or subcortical; superficial or deep (sulcal or ventricular enlargement; whole-brain volume adjustment needed) | If scans are not suitable for volumetric techniques or if such techniques are not available, qualitative rating scales could provide an alternative102,103 | Automated or semi-automated quantitative methods are preferred but visual checking and manual editing are commonly needed to avoid including the orbits and excluding the brainstem from the whole-brain volume;69,93,104 regional or subregional brain volume computational methods are in development, but their reliability, especially in individuals with disease, is still to be determined78,105 | Cross-sectional: brain atrophy can be estimated by comparison with the inner-skull volume (an estimate of maximum brain size in patients at around age 20 years); all intracranial contents must be included in the intracranial volume, including veins and meninges, which expand into space left by shrinking brain;76 longitudinal: serial brain volumes can be measured; a registration-based approach is preferred, although the discipline is advancing rapidly106 | Computational approaches have high reliability; visual rating is more varied but can be improved with reference to a standard visual template;103 subcortical and cortical vascular lesions affect the reliability of automated volumetric techniques,79 particularly in subjects with a high lesion load | Consider masking recent small subcortical lesions, lacunes, and perivascular space when measuring brain volume;78,105 specific standards are emerging for hippocampal volume measurement107 |
DWI=diffusion-weighted imaging.
Reporting standards for vascular findings on neuroimaging
Vascular findings from neuroimaging should be reported according to standards established by the Equator Network for observational studies, epidemiological studies, diagnostic test assessments, or randomised clinical trials. Additionally, we suggest specific points that will help in the progression and standardisation of future research with imaging (panel 2). We encourage investigators to use the terms and definitions proposed in future studies. Where possible, studies with visual rating scales should use established and widespread rating scales so that studies are comparable and meta-analyses can be done.
Panel 2. Proposed reporting standards for neuroimaging studies of small vessel disease.
Demographic details of the research participants and reference population
-
•
Proportions of individuals with vascular risk factors and how these were measured.
-
•
Proportions of individuals with stroke and its subtypes, as well as other vascular disease.
-
•
Time from disease presentation to imaging and clinical assessments (if relevant).
-
•
Any clinical or imaging observational period with time intervals.
-
•
For studies on cognition or specific physical functions: details of test versions used, who administered them, and their training.
-
•
For cognitive studies: assessment of premorbid cognitive ability and depression.
Image acquisition
-
•
Scanner characteristics (type and manufacturer, field strength, coils, high-order shim and use of shimming routines, quality assurance protocol for scanner and frequency of quality assurance assessment).
-
•
Use of several scanners.
-
•
Change of scanners or change to scanner system during study.
-
•
MRI sequences, acquisition parameters (including as appropriate: repetition time, echo time, inversion time, echo train length), acquisition and reconstruction matrices, field-of-view, slice thickness including gaps and scanning plane, details of selected options (tailored excitation pulses, parallel imaging, flow compensation, preparation pulses, etc), and total acquisition time. If a work-in-progress package is used, provide as much information as possible.
Image analysis and postprocessing
-
•
Use and qualification of a central analysis facility, or training procedure across several analysis centres.
-
•
Whether analyses were done blinded to initial presentation or to other data (should be specified) that might affect interpretation.
-
•
Details of qualitative visual rating and quantitative computational methods, including the URL if available for download or an appendix describing the method in detail.
-
•
For visual rating scales: whether images were rated centrally by one or a small number of readers; the raters' background (eg, neurology, psychiatry, neuroradiology, or radiology) and experience; rater reliability (intra-rater and inter-rater).
-
•
For studies using computational image analysis programmes: training of the analysts, any expert supervision, and the background of the expert; repeatability.
-
•
Statistical methods used in data analysis.
-
•
Ideally: sample size estimation.
Small vessel disease-specific aspects
-
•
For recent small subcortical infarcts: specify whether infarcts are symptomatic or not; state the location, size, shape, and number; specify the delay from stroke to imaging; state the proportion with visible acute lesion on diffusion-weighted imaging and fluid-attenuated inversion recovery, plus T2-weighted imaging.
-
•
For lacunes of presumed vascular origin: specify location, size, shape, and number; distinguish haemorrhagic lesions from lacunes; for volumetric methods, state whether lacunes are counted as part of CSF volume, as part of the white matter hyperintensity volume, or as separate lacune volume.
-
•
For white matter hyperintensities of presumed vascular origin: specify whether deep grey matter and brainstem hyperintensities are included (and if so, refer to all hyperintensities collectively); state the rating scale or volume measurement software used and observer reliability; specify whether the white matter hyperintensity volume was adjusted for intracranial or brain volume and how this was done; state whether lacunes were included in white matter hyperintensities or measured separately and whether acute lesions were masked.
-
•
For perivascular spaces: separate perivascular spaces of the basal ganglia and white matter; describe how qualitative aspects (number, location, size, etc) are defined; state the observer reliability of the rating scale.
-
•
For cerebral microbleeds: specify number and distribution divided into lobar, deep, and infratentorial (brainstem and cerebellum); provide full details of image acquisition parameters; specify application of standardised rating scales.
-
•
For atrophy: specify the rating scale or method of volume measurement, whether corrected for intracranial volume, and method used to do this.
Future developments and challenges
Advances in the technology for imaging, as well as in novel protocols for image acquisition and post-processing, have improved imaging of the various manifestations of SVD. These advances have the potential to help explain the role of SVD in neurodegenerative disease and to identify new mechanisms of disease. Examples of promising technology are advances in small-artery imaging with ultra-high-field strength MRI (>3·0 T), diffusion tensor imaging of detailed structural connectivity, magnetisation transfer assessment of white matter myelination, imaging of blood–brain barrier permeability and of vascular reactivity, perfusion imaging, imaging of retinal vessels, quantitative susceptibility imaging, and others. The advantages of multimodal imaging should be used to advance understanding of pathophysiology. For example, combining diffusion tensor imaging and atrophy allows the study of the effects of vascular lesions on distantly connected brain regions.108
Interest is increasing in the clinical relevance of microinfarcts, another small infarct subtype. Microinfarcts are very small ischaemic lesions recorded mostly in the cortex during autopsy in older people, but are occasionally seen on high-field MRI.109,110 Their true vascular pathological relation to SVD is still unknown. Although yet to be confirmed, some very small cortical acute lesions seen on diffusion-weighted imaging might actually be microinfarcts.109 Microinfarcts seem very clinically relevant, so neuroimaging methods to measure them or a validated close surrogate are needed. Larger cortical microinfarcts have been visualised on 7·0 T MRI, and these lesions were sometimes visible on conventional 3·0 T MRI.111
We now need comprehensive studies that account for both vascular and neurodegenerative pathological changes in patients observed for longer and from a younger age than have been done so far (panel 3). These studies should incorporate some of the following: multimodal MRI, amyloid imaging with PET, detailed clinical testing, and biomarkers from serum and CSF to elucidate the crosstalk between vascular and neurodegenerative pathological changes, and how these interact to cause clinical symptoms, particularly cognitive decline, gait impairment, physical disability, and depression. Researchers should also investigate the connection between imaging and pathological changes.30,52 Additionally, carefully conducted imaging studies with short-term (eg, monthly) observational periods are needed to study the progression of imaging features of SVD and their effects on brain tissue loss.
Panel 3. Questions to be addressed in the future study of imaging small vessel disease.
Recent small subcortical infarct
-
•
How can causally distinct subtypes (eg, embolic, parent, or branch artery atheroma or intrinsic small vessel disease) be better differentiated with the use of neuroimaging?
-
•
What proportion of infarcts cavitate, become a white matter hyperintensity, or disappear on conventional MRI, and what are the factors that affect infarct conversion between the different states?
Lacunes of presumed vascular origin
-
•
How can methods for distinguishing between lacunes and perivascular spaces be improved?
-
•
How do secondary degenerative changes near lacunes affect their size and shape in the long term?
-
•
How important is the presence of a hyperintense rim when differentiating between lacunes and other small cystic structures—eg, perivascular spaces?
White matter hyperintensity of presumed vascular origin
-
•
How different are the mechanisms and clinical consequences of periventricular white matter hyperintensities and deep white matter hyperintensities? To address this question, clear definitions and standardised protocols are needed to distinguish periventricular white matter hyperintensities from deep white matter hyperintensities.
-
•
How can newer imaging techniques, such as measurement of T1 water content, help to improve characterisation of white matter hyperintensities and determine the effect of white matter hyperintensities on connected brain regions (eg, cortex), and on clinical symptoms?
-
•
Is there pathological or epidemiological justification for distinguishing between hyperintensities in grey matter and those in white matter?
Perivascular space
-
•
What are the mechanisms that cause multiple occurrences of perivascular spaces?
-
•
What is the link between perivascular space diameter and risk factors and potential clinical consequences? Can a perivascular space diameter be identified so that perivascular spaces of greater diameter can be thought of as pathologically enlarged?
-
•
How are perivascular spaces linked to small vessel disease-related vascular and parenchymal lesions?
-
•
How are perivascular spaces linked to brain atrophy and neurodegenerative pathological changes?
-
•
How can the protocols for detecting and quantifying perivascular spaces be improved?
-
•
What are the clinical consequences of perivascular spaces?
-
•
What is the clinical value of assessing perivascular spaces?
Cerebral microbleed
-
•
How do different neurodegenerative or vascular disorders affect the pathology and spatial distribution of microbleeds and their appearance on imaging?
-
•
How reliable, sensitive, or specific is the distribution of cerebral microbleeds as a marker for cerebral amyloid angiopathy or hypertension?
-
•
What is the predictive value of imaging cerebral microbleeds when investigating cognitive and functional decline?
-
•
What is the clinical value of imaging of cerebral microbleeds to guide treatment decisions (eg, thrombolysis and antithrombotic therapy)?
-
•
How do results from different protocols for imaging and quantifying microbleeds compare, and how can findings made using different protocols be integrated into a common result.
Brain atrophy
-
•
To what extent do vascular lesions related to small vessel disease affect measures of brain atrophy (eg, cortical vs subcortical regions, grey vs white matter?)
-
•
To what extent does brain atrophy (cortical and subcortical, and grey and white matter) affect volumentric measures of vascular lesions?
-
•
How do vascular lesions mediate secondary brain atrophy?
-
•
What are the clinical consequences of secondary brain atrophy that was mediated by vascular lesions?
Other vascular lesions
-
•
How frequently do microinfarcts occur in neurodegenerative diseases? What are the clinical consequences of microinfarcts? What are the imaging signatures of acute microinfarcts during clinical scanning at 1·5 T and 3·0 T? How frequent are microinfarcts in Alzheimer's disease?
-
•
How frequently does acute sulcal subarachnoid haemorrhage result in siderosis? To what extent does superficial siderosis affect cortical neuronal function? What are the clinical consequences of superficial siderosis (including the frequency of subsequent symptomatic subarachnoid haemorrhage or intracerebral haemorrhage)?
General
-
•
How can direct imaging of small intracerebral arteries be improved?
-
•
How can subtle changes of vascular origin be differentiated from changes mediated by other pathologies (eg, neurodegeneration), and what are the exact histopathological substrates?
-
•
To what extent do lesions related to small vessel disease cause secondary neurodegeneration, and what are the mechanisms for this process?
-
•
How do vascular lesions interact with neurodegenerative pathological changes to cause cognitive decline and other clinical manifestations of neurodegeneration, particularly physical disability, gait disturbance, and depression?
-
•
Is there any suitable imaging scale that integrates many imaging findings for small vessel disease and how would this scale be used in research and clinical practice (eg, for risk prediction and treatment stratification)?
-
•
How can the imaging of vascular manifestations be used to improve the selection of individuals in future clinical trials and when measuring outcomes?
-
•
How can the protocols for measuring blood–brain barrier integrity be improved, and how do changes related to small vessel disease and neurodegenerative pathological changes interact to affect blood–brain barrier integrity?
-
•
What is the link between changes in cerebral blood flow and volume and ischaemic brain pathological changes?
Conclusions
Our neuroimaging standards for SVD are intended to harmonise current disparate terminology and analysis methods. We encourage other researchers to use these standards. They could provide a useful introduction to the principles of neuroimaging in SVD, which could help investigators to incorporate measurement of SVD into studies of neurodegenerative diseases or the pathological basis of ageing. These standards should allow cross-study comparisons of findings, accelerating the translation of new findings into practice. Additionally, standardised approaches could enable formal between-study meta-analyses, which are increasingly needed to obtain the sample sizes necessary for some types of research such as genetic association studies. We suggest that our reporting standards could be a useful guide to authors, peer reviewers, and editors, and might raise the methodological quality of neuroimaging studies of SVD as have analogous reporting standards (eg, the CONSORT guidelines) increased the quality of other types of research. Finally, although the primary purpose of our standards was for research, we hope that these standards will have a positive effect on clinical diagnosis and the reporting of SVD lesions in routine clinical practice.
These standards were developed on the basis of consensus in a large group of experts using rigorous methods. However, we acknowledge that in some parts of this rapidly evolving specialty, our recommendations are based on small studies, unvalidated findings, or expert opinion without a strong body of supporting evidence. More work is essential to validate these standards. In particular, the lack of radiological–pathological association studies is a key limitation to our understanding of the neuroimaging findings in SVD. Additionally, best standards for neuroimaging of SVD will change over time and, therefore, periodic revision of these standards will be needed.
Acknowledgments
Acknowledgments
The following institutions provided funding: the Centres of Excellence in Neurodegeneration (COEN) concordat (UK Medical Research Council, the German Centre for Neurodegenerative Disease [DZNE], and the Canadian Institutes of Health Research [CIHR]) reference COEN017; Royal Society of Edinburgh; Scottish Imaging Network, a Platform for Scientific Excellence (SINAPSE) Collaboration; UK Cross-Council Centre for Cognitive Ageing and Cognitive Epidemiology, Edinburgh, UK; Carl Friedrich von Siemens Foundation; Vascular Dementia Research Foundation; NIHR Biomedical Research Unit in Dementia awarded to Cambridge University Hospitals NHS Trust and the University of Cambridge, Cambridge, UK; NIHR Biomedical Research Unit in Dementia awarded to UCL Hospitals NHS Trust and UCL, London UK; FP6 ERA-NET NEURON grant (01 EW1207); and the Canadian Stroke Network. We thank M Henderson, K Shuler, L Thomas, I Wittko, and Anna Charlton for administrative support.
Contributors
All authors contributed to the Position Paper through attendance at the two workshops, participation in specific workgroups, writing and editing the report, and providing approval for final submission. JMW, MDi, and EES coordinated the overall process, obtained funding, organised the two workshops, coordinated the report preparation, and finalised the paper for submission. PBG, CCh, BN, MvB, and CS were external advisers who attended the second workshop, read the draft standards in detail, provided in-depth critique, and approved the final paper for submission. EES, FD, MDi, JMW, AV, JTOB, SEB, and RIL did the literature searches. Workgroup participants were as follows: recent small subcortical infarcts (JMW [chair], RvO, VH, BCMS, VM, LP, and FD), lacunes of presumed vascular origin (RIL [chair], HC, MB, CB, VM, and ORB), white matter hyperintensities of presumed vascular origin (CCo [chair], SEB, CDC, F-EdL, FF, JTOB, and LP), perivascular space (FF [chair], VH, AV, GJB, RvO, and FB), cerebral microbleeds (JTOB [chair], DW, MB, CCo, RIL, and RF), brain atrophy (GJB [chair], NF, IK, SG, ST, SEB, and FB), image acquisition and analysis (RF, OS, SEB, MDu, and JMW), clinical relevance (ORB and LP), other lesions (EES [chair], DW, and AV), and future directions (MDi and MDu).
Conflicts of interest
GJB consults for and receives research support from Boehringer Ingelheim. FF serves on scientific advisory boards for Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Pfizer, Novartis, and Teva Pharmaceutical Industries, and as scientific adviser for Perceptive Informatics, he serves on the editorial boards of Cerebrovascular Diseases, Multiple Sclerosis, the Polish Journal of Neurology and Neurosurgery, Stroke, and the Swiss Archives of Neurology and Psychiatry, and has received speaker honoraria and support from Biogen Idec, Bayer Schering, Merck Serono, Novartis, Sanofi-Aventis, and Teva Pharmaceutical Industries. RF receives operating research funding from the Canadian Institutes of Health Research, the Alberta and Pfizer Translational Research Program, the Hotchkiss Brain Institute, and the University of Calgary to support work on imaging SVD. JTOB has been a consultant for GE Healthcare, Lilly, Bayer Healthcare, and TauRx, and has received speakers' honoraria from Pfizer, GE Healthcare, Eisai, Shire, Lundbeck, Lilly, and Novartis. SEB has had research funds to the Cognitive Neurology Research and Stroke Research Units from Pfizer (Wyeth Research), GlaxoSmithKline, Lundbeck, Roche, and Novartis. She has received speakers' honoraria for continuing medical education from Pfizer, Eisai, and Novartis, and honoraria for ad-hoc consulting from Pfizer, Novartis, GlaxoSmithKline, Roche, Elan, and Bristol-Myers Squibb. HC has received honoraria for consultancy from Servier, Lundbeck, and Janssen Research and Development. F-EdL is supported by a Vidi grant (016·126·351) from the Netherlands Organisation for Health Research and Development. LP is a member of the editorial boards of Acta Neurologica Scandinavica, International Journal of Alzheimer Disease, and Cerebrovascular Diseases and editor of the vascular cognitive impairment section of Stroke. ST served as a member of the advisory board for Lilly Deutschland. MDi is a consultant for Lilly Deutschland and Boehringer Ingelheim, Biologische Heilmittel Heel, Bristol-Myers Squibb, and Ever Neuro Pharma. He has received honoraria from Bayer Vital, Boehringer Ingelheim Pharma Biologische Heilmittel Heel, Bristol-Myers Squibb, Lundbeck, Sanofi-Aventis Deutschland, Shire Deutschland, German Centre for Neurodegenerative Diseases (DZNE), Georg Thieme Verlag, UpToDate, and W Kohlhammer. He has received Principal Investigator and Research Funding (industry) from Bayer Vital, Eisai Medical Research, Eisai, Essex Pharma, Ferrer Internacional, and ICON Clinical Research. All other authors declare that they have no conflicts of interest.
Contributor Information
Joanna M Wardlaw, Email: joanna.wardlaw@ed.ac.uk.
Martin Dichgans, Email: martin.dichgans@med.uni-muenchen.de.
References
- 1.Norrving B. Lacunar infarcts: no black holes in the brain are benign. Pract Neurol. 2008;8:222–228. doi: 10.1136/jnnp.2008.153601. [DOI] [PubMed] [Google Scholar]
- 2.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Laakso M. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43:3137–3146. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- 5.Brayne C, Richardson K, Matthews FE. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge City over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 6.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuropathology Group of the Medical Research Council Cognitive function and ageing study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 8.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 9.Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke. 2010;42:359–366. doi: 10.1161/STROKEAHA.110.594754. [DOI] [PubMed] [Google Scholar]
- 10.Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42:1140–1145. doi: 10.1161/STROKEAHA.110.600114. [DOI] [PubMed] [Google Scholar]
- 11.Hachinski V, Iadecola C, Petersen RC. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 12.Gorelick PB, Scuteri A, Black SE. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Editorial A united approach to vascular disease and neurodegeneration. Lancet Neurol. 2012;11:293. doi: 10.1016/S1474-4422(12)70050-9. [DOI] [PubMed] [Google Scholar]
- 14.Kang DW, Han MK, Kim HJ. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79:848–855. doi: 10.1212/WNL.0b013e3182648a79. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury D, Wardlaw JM, Dennis MS. Are multiple acute small subcortical infarctions caused by embolic mechanisms? J Neurol Neurosurg Psychiatry. 2004;75:1416–1420. doi: 10.1136/jnnp.2004.038653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross sectional study. J Neurol Neurosurg Psychiatry. 2011;82:540–542. doi: 10.1136/jnnp.2009.190298. [DOI] [PubMed] [Google Scholar]
- 17.Potter GM, Doubal FN, Jackson CA. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke. 2010;41:267–272. doi: 10.1161/STROKEAHA.109.566307. [DOI] [PubMed] [Google Scholar]
- 18.Moreau F, Patel S, Lauzon ML. Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence. Stroke. 2012;43:1837–1842. doi: 10.1161/STROKEAHA.111.647859. [DOI] [PubMed] [Google Scholar]
- 19.Koch S, McClendon MS, Bhatia R. Imaging evolution of lacunar stroke–leukoariosis or lacune? Neurology. 2011;77:1091–1095. doi: 10.1212/WNL.0b013e31822e1470. [DOI] [PubMed] [Google Scholar]
- 20.Donnan GA, Bladin PF, Berkovic SF, Longley WA, Saling MM. The stroke syndrome of striatocapsular infarction. Brain. 1991;114:51–70. [PubMed] [Google Scholar]
- 21.Fisher CM. Lacunar infarcts—a review. Cerebrovasc Dis. 1991;1:311–320. [Google Scholar]
- 22.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 23.Santos M, Gold G, Kovari E. Differential impact of lacunes and microvascular lesions on poststroke depression. Stroke. 2009;40:3557–3562. doi: 10.1161/STROKEAHA.109.548545. [DOI] [PubMed] [Google Scholar]
- 24.Choi P, Ren M, Phan TG. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke. 2012;43:1505–1510. doi: 10.1161/STROKEAHA.111.647271. [DOI] [PubMed] [Google Scholar]
- 25.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 26.Franke CL, van Swieten JC, van Gijn J. Residual lesions on computed tomography after intracerebral hemorrhage. Stroke. 1991;22:1530–1533. doi: 10.1161/01.str.22.12.1530. [DOI] [PubMed] [Google Scholar]
- 27.Vermeer SE, den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people. The Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 29.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouw AA, Seewann A, van der Flier WM. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 31.de Laat KF, Tuladhar AM, van Norden AGW, Norris DG, Zwiers MP, de Leeuw F-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 32.Windham BG, Griswold ME, Shibata D, Penman A, Catellier DJ, Mosley TH., Jr Covert neurological symptoms associated with silent infarcts from midlife to older age: the Atherosclerosis Risk in Communities study. Stroke. 2012;43:1218–1223. doi: 10.1161/STROKEAHA.111.643379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saini M, Ikram K, Hilal S, Qiu A, Venketasubramanian N, Chen C. Silent stroke: not listened to rather than silent. Stroke. 2012;3:3102–3104. doi: 10.1161/STROKEAHA.112.666461. [DOI] [PubMed] [Google Scholar]
- 34.Haley AP, Hoth KF, Gunstad J. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry. 2009;17:976–985. doi: 10.1097/JGP.0b013e3181b208ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inzitari D, Pracucci G, Poggesi A. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:279–282. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hachinski VC, Potter P, Merskey H. Leukoaraiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 37.Pollock H, Hutchings M, Weller RO, Zhang E-T. Perivascular spaces in the basal gangli of the human brain: their relationship to lacunes. J Anat. 1997;191:337–346. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48:745–754. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 39.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–454. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 40.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071–1086. doi: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 41.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–2490. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 42.MacLullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poirier J, Derouesne C. Cerebral lacunae. A proposed new classification. Clin Neuropathol. 1984;3:266. [PubMed] [Google Scholar]
- 44.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MDF. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol. 1989;10:929–936. [PMC free article] [PubMed] [Google Scholar]
- 45.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 46.Rouhl RPW, van Oostenbrugge RJ, Knottnerus ILH, Staals JEA, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255:692–696. doi: 10.1007/s00415-008-0777-y. [DOI] [PubMed] [Google Scholar]
- 47.Zhu YC, Dufouil C, Mazoyer B. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011;32:709–713. doi: 10.3174/ajnr.A2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Song X, Zhang Y. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. AJNR Am J Neuroradiol. 2011;32:1490–1495. doi: 10.3174/ajnr.A2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 50.Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998;245:116–122. doi: 10.1007/s004150050189. [DOI] [PubMed] [Google Scholar]
- 51.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 52.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32:528–534. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 53.De Reuck J, Auger F, Cordonnier C. Comparison of 7·0-T T*-magnetic resonance imaging of cerebral bleeds in post-mortem brain sections of Alzheimer patients with their neuropathological correlates. Cerebrovasc Dis. 2011;31:511–517. doi: 10.1159/000324391. [DOI] [PubMed] [Google Scholar]
- 54.Fazekas F, Kleinert R, Roob G. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 55.Dichgans M, Holtmannspotter M, Herzog J, Peters N, Bergmann M, Yousry TA. Cerebral microbleeds in CADASIL: a gradient-echo magnetic resonance imaging and autopsy study. Stroke. 2002;33:67–71. doi: 10.1161/hs0102.100885. [DOI] [PubMed] [Google Scholar]
- 56.Tatsumi S, Shinohara M, Yamamoto T. Direct comparison of histology of microbleeds with postmortem MR images: a case report. Cerebrovasc Dis. 2008;26:142–146. doi: 10.1159/000139661. [DOI] [PubMed] [Google Scholar]
- 57.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 58.Vernooij MW, van der Lugt A, Ikram MA. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 59.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 60.Greenberg SM, Vernooij MW, Cordonnier C. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sperling RA, Jack CR, Jr, Black SE. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lammie GA, Lindley R, Keir S, Wiggam MI. Stress-related primary intracerebral hemorrhage: autopsy clues to underlying mechanism. Stroke. 2000;31:1426–1448. doi: 10.1161/01.str.31.6.1426. [DOI] [PubMed] [Google Scholar]
- 63.Broderick J, Connolly S, Feldmann E. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2202. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 64.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 65.Jackson CA, Sudlow CL. Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry. 2006;77:1244–1252. doi: 10.1136/jnnp.2006.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhattathiri PS, Gregson B, Prasad KSM. Reliability assessment of computerized tomography scanning measurements in intracerebral hematoma. Neurosurg Focus. 2003;15:1–5. doi: 10.3171/foc.2003.15.4.6. [DOI] [PubMed] [Google Scholar]
- 67.Linn J, Halpin A, Demaerel P. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar N, Cohen-Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology. 2006;66:1144–1152. doi: 10.1212/01.wnl.0000208510.76323.5b. [DOI] [PubMed] [Google Scholar]
- 69.Jack CR., Jr Alliance for aging research AD biomarkers work group: structural MRI. Neurobiol Aging. 2011;32:S48–S57. doi: 10.1016/j.neurobiolaging.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters RJ, Fox NC, Crum WR, Taube D, Thomas DJ. Haemodialysis and cerebral oedema. Nephron. 2001;87:143–147. doi: 10.1159/000045903. [DOI] [PubMed] [Google Scholar]
- 71.Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–550. doi: 10.1212/01.WNL.0000150542.16969.CC. [DOI] [PubMed] [Google Scholar]
- 72.Bobinski M, de Leon MJ, Wegiel J. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 73.Jagust WJ, Zheng L, Harvey DJ. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black S, Gao F, Bilbao J. Understanding white matter disease. Imaging–pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 75.Appelman AP, Exalto LG, van der Graaf Y, Biessels GJ, Mali WP, Geerlings MI. White matter lesions and brain atrophy: more than shared risk factors? A systematic review. Cerebrovasc Dis. 2009;28:227–242. doi: 10.1159/000226774. [DOI] [PubMed] [Google Scholar]
- 76.Aribisala BS, Valdes Hernandez MC, Royle NA. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2013;23:1084–1092. doi: 10.1007/s00330-012-2677-x. [DOI] [PubMed] [Google Scholar]
- 77.Mungas D, Jagust WJ, Reed BR. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy-Cooperman N, Ramirez J, Lobaugh NJ, Black SE. Misclassified tissue volumes in Alzheimer disease patients with white matter hyperintensities: importance of lesion segmentation procedures for volumetric analysis. Stroke. 2008;39:1134–1141. doi: 10.1161/STROKEAHA.107.498196. [DOI] [PubMed] [Google Scholar]
- 79.Carmichael O, Schwarz C, Drucker D. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wardlaw JM, Brindle W, Casado AM. A systematic review of the utility of 1·5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research. Eur Radiol. 2012;22:2295–2303. doi: 10.1007/s00330-012-2500-8. [DOI] [PubMed] [Google Scholar]
- 81.Potter G, Doubal F, Jackson C, Sudlow C, Dennis M, Wardlaw J. Associations of clinical stroke misclassification (‘clinical–imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis. 2010;29:395–402. doi: 10.1159/000286342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valdes Hernandez MC, Morris Z, Dickie DA. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology. 2012;40:13–22. doi: 10.1159/000341859. [DOI] [PubMed] [Google Scholar]
- 83.Gao FQ, Swartz RH, Scheltens P. Complexity of MRI white matter hyperintensity assessments in relation to cognition in aging and dementia from the Sunnybrook Dementia Study. J Alzheimers Dis. 2011;26(suppl 3):379–388. doi: 10.3233/JAD-2011-0058. [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Valdes Hernandez MC, Doubal F, Chappell FM, Wardlaw JM. How much do focal infarcts distort white matter lesions and global cerebral atrophy measures? Cerebrovasc Dis. 2012;34:336–342. doi: 10.1159/000343226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong KS, Gao S, Chan YL. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 86.Seifert T, Enzinger C, Storch MK, Pichler G, Niederkorn K, Fazekas F. Acute small subcortical infarctions on diffusion weighted MRI: clinical presentation and aetiology. J Neurol Neurosurg Psychiatry. 2005;76:1520–1524. doi: 10.1136/jnnp.2005.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Longstreth WT, Jr, Manolio TA, Arnold A. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 88.Wahlund LO, Barkhof F, Fazekas F. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 89.Bocti C, Swartz RH, Gao FQ, Sahlas DJ, Behl P, Black SE. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke. 2005;36:2126–2131. doi: 10.1161/01.STR.0000183615.07936.b6. [DOI] [PubMed] [Google Scholar]
- 90.Prins ND, van Straaten ECW, van Dijk EJ. Measuring progression of cerebral white matter lesions on MRI. Visual rating and volumetrics. Neurology. 2004;62:1533–1539. doi: 10.1212/01.wnl.0000123264.40498.b6. [DOI] [PubMed] [Google Scholar]
- 91.Kapeller P, Barber R, Vermeulen RJ. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke. 2003;34:441–445. doi: 10.1161/01.str.0000049766.26453.e9. [DOI] [PubMed] [Google Scholar]
- 92.Fazekas F, Barkhof F, Wahlund LO. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2003;13:31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez J, Gibson E, Quddus A. Lesion Explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963–973. doi: 10.1016/j.neuroimage.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 94.Jeerakathil T, Wolf PA, Beiser A. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 95.van den Heuvel DMJ, ten Dam VH, de Craen AJ. Measuring longitudinal white matter changes: comparison of a visual rating scale with a volumetric measurement. AJNR Am J Neuroradiol. 2006;27:875–878. [PMC free article] [PubMed] [Google Scholar]
- 96.Valdes Hernandez MC, Ferguson KJ, Chappell FM, Wardlaw JM. New multispectral MRI data fusion technique for white matter lesion segmentation: method and comparison with thresholding in FLAIR images. Eur Radiol. 2010;20:1684–1691. doi: 10.1007/s00330-010-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Firbank MJ, Minett T, O'Brien JT. Changes in DWI and MRS associated with white matter hyperintensities in elderly subjects. Neurology. 2003;61:950–954. doi: 10.1212/01.wnl.0000086375.33512.53. [DOI] [PubMed] [Google Scholar]
- 98.Barnes SR, Haacke EM, Ayaz M, Boikov AS, Kirsch W, Kido D. Semiautomated detection of cerebral microbleeds in magnetic resonance images. Magn Reson Imaging. 2011;29:844–852. doi: 10.1016/j.mri.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seghier ML, Kolanko MA, Leff AP, Jager HR, Gregoire SM, Werring DJ. Microbleed detection using automated segmentation (MIDAS): a new method applicable to standard clinical MR images. PLoS One. 2011;6:e17547. doi: 10.1371/journal.pone.0017547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cordonnier C, Potter GM, Jackson CA. Improving interrater agreement about brain microbleeds. Development of the brain observer microbleed scales (BOMBS) Stroke. 2009;49:94–99. doi: 10.1161/STROKEAHA.108.526996. [DOI] [PubMed] [Google Scholar]
- 101.Gregoire SM, Chaudhary UJ, Brown MM. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 102.Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D. Qualitative assessment of cerebral atrophy on MRI: inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol. 1997;37:95–99. doi: 10.1159/000117417. [DOI] [PubMed] [Google Scholar]
- 103.Farrell C, Chappell F, Armitage PA. Development and initial testing of normal reference MR images for the brain at ages 65–70 and 75–80 years. Eur Radiol. 2008;19:177–183. doi: 10.1007/s00330-008-1119-2. [DOI] [PubMed] [Google Scholar]
- 104.Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2:79–88. doi: 10.1016/s1474-4422(03)00304-1. [DOI] [PubMed] [Google Scholar]
- 105.O'Sullivan M, Jouvent E, Saemann PG. Measurement of brain atrophy in subcortical vascular disease: a comparison of different approaches and the impact of ischaemic lesions. Neuroimage. 2008;43:312–320. doi: 10.1016/j.neuroimage.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 106.de Bresser J, Portegies MP, Leemans A, Biessels GJ, Kappelle LJ, Viergever MA. A comparison of MR based segmentation methods for measuring brain atrophy progression. Neuroimage. 2011;54:760–768. doi: 10.1016/j.neuroimage.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 107.Frisoni GB, Jack CR. Harmonization of magnetic resonance-based manual hippocampal segmentation: a mandatory step for wide clinical use. Alzheimers Dement. 2011;7:171–174. doi: 10.1016/j.jalz.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 108.Duering M, Righart R, Csandi E. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025–2028. doi: 10.1212/WNL.0b013e3182749f39. [DOI] [PubMed] [Google Scholar]
- 109.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Veluw SJ, Zwanenburg JJ, Engelen-Lee J. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2012;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]


