Abstract
In this study, ethanolic extracts from Hericium erinaceum cultivated with Artemisia capillaris (HEAC) were assessed for their ability to lower the cholesterol levels of male Sprague-Dawley rats fed a high-fat diet. Rats were randomly subdivided into seven test groups. Each group contained eight rats fed a high-fat diet during a growth period lasting 4 wk. Supplementation with the extracts was performed once a day for 2 wk after the high-fat diet. The control group (rats fed a high-fat diet) showed a high efficiency ratio (feed efficiency ratio) value compared to the normal group. Biochemical parameters, including total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-c), and triglyceride (TG) levels dramatically increased in the control group compared to the normal group. High-density lipoprotein-cholesterol (HDL-c) content in the control group was also significantly lower relative to the normal group. Two positive control groups, treated with simvastatin and atorvastatin, had lowered TC, LDL-c, and TG levels, and increased HDL-c content compared to the control group. Treatment with the tested extracts, including HEAC, ethanolic extracts from Hericium erinaceum, and ethanolic extracts from Artemisia capillaris reduced TC, LDL-c, and TG levels and elevated HDL-c content in the hyperlipidemia rats. The atherogenic index and cardiac risk factor values for the HEAC-treated group were 0.95 and 1.95, respectively. Simvastatin- and atorvastatin-treated groups showed atherogenic index values of 1.56 and 1.69, respectively, and cardiac risk factor values of 2.56 and 2.69, respectively. These results show HEAC possesses an ability to cure hyperlipidemia in rats and may serve as an effective natural medicine for treating hyperlipidemia in humans.
Keywords: Atorvastatin, Hericium erinaceum, Hyperlipidemia, Simvastatin
The incidence of lifestyle-related diseases, such as atherosclerosis, hypertension, and hyperlipidemia, has been increasing in the last few decades [1]. Hyperlipidemia is a major worldwide health problem related to the development of various diseases, including diabetes [2]. Reducing the level of cholesterol in blood plasma is an effective method to treat hyperlipidemia-related diseases. The currently used medicines, such as fibrates and statins, are characterized by their high lipid-lowering speed and efficacy [3], but can potentially evoke adverse effects [4]. In contrast to those drugs, plant-derived natural products are have minimal adverse effects and affect multiple targets for preventing and curing hyperlipidemia [5].
Ethanolic extracts of Hericium erinaceum cultivated with Artemisia capillaris (HEAC) have biological activites, including the inhibition of aflatoxin B1 biotransformation to aflatoxin B1-8,9-epoxide and the inhibition of vascular smooth muscle cell proliferation [6, 7]. HEAC has shown its potent protection effects on CCl4-induced acute hepatotoxicity in rats [7].
Herein, we investigated the cholesterol-lowering activity of HEAC and other ethanolic extracts, including H. erinaceum (HE) and Artemisia capillaris (AC), using male Sprague-Dawley rats fed a high-fat diet (HFD) for 4 wk for hyperlipidemia induction. The physiological parameters determined were body weight gain and the feed efficiency ratio (FER). The biochemical parameters determined were total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), triglyceride levels (TG), the atherogenic index (AI), and the cardiac risk factor (CRF).
MATERIALS AND METHODS
Chemicals
Simvastatin and atorvastatin calcium anhydrous were obtained from Chongkundang Pharm Company (Seoul, Korea). HEAC, HE, and AC were supplied by Cosis Bio Company (Chungju, Korea).
Cultivation and fermentation
The strain of HE maintained on a potato dextrose agar (PDA) slant was produced (as previously described) by fermentation of HE mycelia with the following modification [7]. A YMPG medium (consisting of KH2PO4 2.0 g/L, MgSO4·7H2O 1.0 g/L, glucose 10.0 g/L, malt extract 10.0 g/L, yeast extract 2.0 g/L, and agar 20.0 g/L) was used as media for seed cultivations carried out for 7 days at 25℃ and 120 rpm. The extracts from the dried leaves of A. capillaria (in a two-fold volume of water) were prepared as a media for the solid fermentation and 200 mL of the seed culture broth were then added to initiate the fermentation. After 40 days of cultivation, mycelia and culture media were harvested by filtration of the culture broth. For further solvent extraction, the harvested mycelia was left to dry at room temperature for 24 hr, and the dried mycelia (145 g) extracted with 30% ethanol (3 L) at room temperature for 2 wk. The extracted mycelia, designated as HEAC, was filtered and lyophilized prior to its use in experiments. The mycelia of HE, cultivated with PDA media and the dried leaves of A. capillaria, were extracted using the same procedure and were designated as HE and AC, respectively.
Biological materials and experimental design
Male Sprague-Dawley rats (200~220 g) were used in this study. The rats were housed under normal laboratory conditions (23 ± 2℃, 55 ± 15%, 12/12 hr light-dark cycle) with free access to a standard pellet (AIN-93G) (Feedlab, Guri, Korea) and water ad libitum during the experiments. After the rats had adapted to their environment, eight rats were randomly selected from the normal control group and fed a standard pellet (AIN-93G), while other rats were fed with a HFD with 45 kcal % fat primarily lard equivalent to a fat content of 236 g per kg diet (Feedlab) during an experimental period of 4 wk. Rats fed a HFD were subdivided into six groups: the control, simvastatin, atorvastatin, HEAC, HE, and AC groups, with each group consisting of eight rats. At the dose level of 100mg/kg per day, oral administration was performed by infusion with the same volume of water (2 mL). The room temperature was maintained at 25℃. All experimental procedures were performed in accordance with the guide for the care and use of experimental animals.
Determination of weight gain, food intake, and FER
The starting date of the experiment was set as day "0" and the animal body weight was measured every 12 hr. Body weight was measured until the final date of the experiment. The FER was obtained from the amount of feed intake and the body weight increase during the entire experimental period; FER = increase of body weight (g)/amount of feed intake (g) during the experiment.
Determination of biochemical parameters of hyperlipidemia
TC was measured by the method previously reported [8] and TG was measured by the colorimetric method reported [9]. LDL-c was measured by the method of Friedewald et al. [10] and HDL-c was determined by the method of Zlatkis and Zak [11].
Determination of the AI and the CRF
The AI was calculated as (TC-HDL-c)/HDL-c and the CRF was calculated as (TC/HDL-c) [12].
Hepatic morphology
Livers were removed from the rats and put in a buffer solution containing 4% formalin. Fixed tissues were routinely processed for paraffin embedding and 4 µm sections were prepared and dyed with hematoxylineosin; stained areas were viewed using an optical microscope at 400×.
Statistical analysis
Statistical analysis was performed using the SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as means ± SD. The effects of drug treatments were statistically evaluated using the one-way analysis of variance (one-way ANOVA) followed by the Dunnett post-hoc test to rectify for multiple comparison treatments. Statistical significance was set at the p < 0.05 level.
RESULTS
Effects of HEAC extract on body weight gain and FER
Male Sprague-Dawley rats, fed a HFD, were randomly divided into six test groups: a control group for rats fed the HFD, two positive control groups supplemented with two currently used medicines (simvastatin and atorvastatin), and three test groups supplemented with HEAC, HE, or AC. Each group consisted of eight rats, with the normal group receiving a control diet, and the other six groups fed a HFD during a growth period of 4 wk. Simvastatin, atorvastatin, HEAC, HE, and AC were supplemented once a day for 2 wk, after a continuous control diet of 4 wk for a HFD treatment. After supplementation of the tested materials for 2 wk, rats were starved overnight, weighed, and sacrificed.
Body weight gain and the FER value are shown in Table 1. The high-fat control group dramatically increased in weight gain compared to the normal group. However, the food intake during the experimental period in the control group significantly decreased relative to the normal group. Therefore, the FER value of the control group was higher than the normal group. Two medicines currently used showed very similar results, as the FER values did not significantly change. HEAC and AC did not change the FER values compared to the control groups. Only HE showed a different FER value close to the value of the normal group.
Table 1.
Effects of HEAC on the body weight gain, food intake, and FER values of rats with hyperlipidaemia (from a high-fat diet after 4 wk)
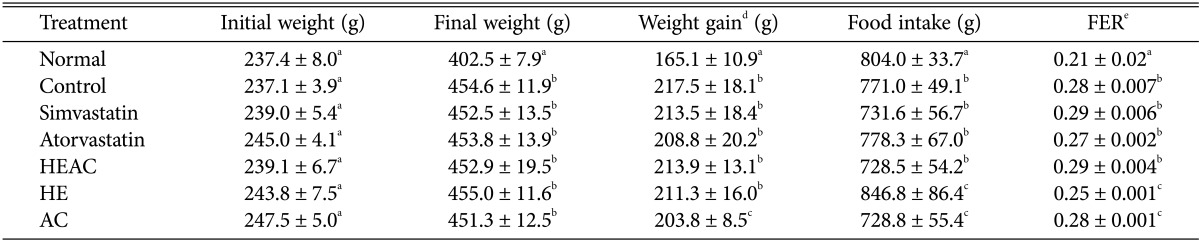
HEAC, ethanolic extracts from Hericium erinaceus cultivated with Artemisia capillaris; HE, ethanolic extracts from Hericium erinaceus; AC, ethanolic extracts from Artemisia capillaris.
a-cThe data (mean ± SE values) were determined from three replicates. Means in the column followed by the same letter are not significantly different (p < 0.05).
dBody weight gain: final weight (g) - initial weight (g).
eFeed efficiency ratio (FER) = body weight gain (g)/food intake (g).
Effects of HEAC on TC, HDL-c, LDL-c, and TG levels
Serum TC, HDL-c, LDL-c, and TG levels of the normal group were 87.0, 49.8, 29.6, and 38.2 mg/dL, respectively. TC, LDL-c, and TG levels of the control group increased to 166.9, 117.7, and 100.3 mg/dL, respectively. HDL-c in the control group was 29.1 mg/dL. Therefore, the level of TC, LDL-c and TG in the control group was significantly elevated relative to the normal group (Table 2). The level of HDL-c in the control group significantly decreased compared to the normal group. These results showed that the serum lipid content in rats changed after a HFD. Supplementation with simvastatin, atorvastatin, HEAC, HE, and AC significantly reduced TC, LDL-c, and TG levels (p < 0.05) and all except AC increased HDL-c levels (p < 0.05) (Table 2). TC, LDL-c, and TG levels in the simvastatin-treated group were 98.3, 50.1, and 48.3 mg/dL, respectively, and the HDL-c level was 38.5 mg/dL. TC, LDL-c, and TG levels in the atorvastatin-treated group were 89.9, 48.0, and 41.3 mg/dL, respectively, and the HDL-c level was 33.7 mg/dL. TC, LDL-c, and TG levels in the HEAC-supplemented group were 97.5, 37.0, and 51.5 mg/dL, respectively, and the HDL-c level was 50.2 mg/dL. Therefore, HEAC showed a potent cholesterol-lowering activity compared to simvastatin and atorvastatin.
Table 2.
Effects of HEAC on biochemical parameters of hyperlipidaemic rats (induced by a high-fat diet after 4 wk)
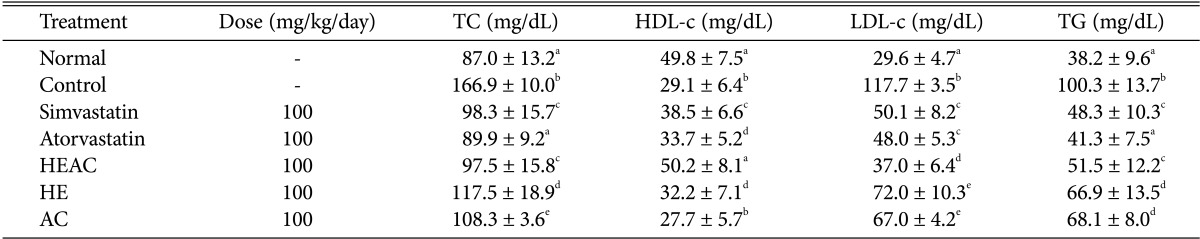
HEAC, ethanolic extracts from Hericium erinaceus cultivated with Artemisia capillaris; TC, total cholesterol; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; TG, triglyceride levels;; HE, ethanolic extracts from Hericium erinaceus; AC, ethanolic extracts from Artemisia capillaris.
a-cThe data (mean ± SE values) were determined from three replicates. Means in the column followed by the same letter are not significantly different (p < 0.05).
Effects of HEAC on the AI and CRF
The AI (defined as the base 10 logarithm of the ratio of TG to HDL-c) was employed for predicting cardiovascular risk. After a HFD, each group had a lower AI compared to the hyperlipidemia group (Table 3). The AI and CRF for the normal group were 0.75 and 1.75, respectively, while the control group had an AI of 5.31 and a CRF of 6.31. The positive control groups treated with simvastatin and atorvastatin exhibited an ability to lower the AI and the CRF compared to the control groups. The HEAC-treated group had an AI of 0.95 and a CRF of 1.95. The HE- and AC-treated groups had a lower AI and CRF.
Table 3.
Effects of HEAC on atherogenic index and cardiac risk factor of hyperlipidaemic rats induced by high-fat diet feeding for 4 wk
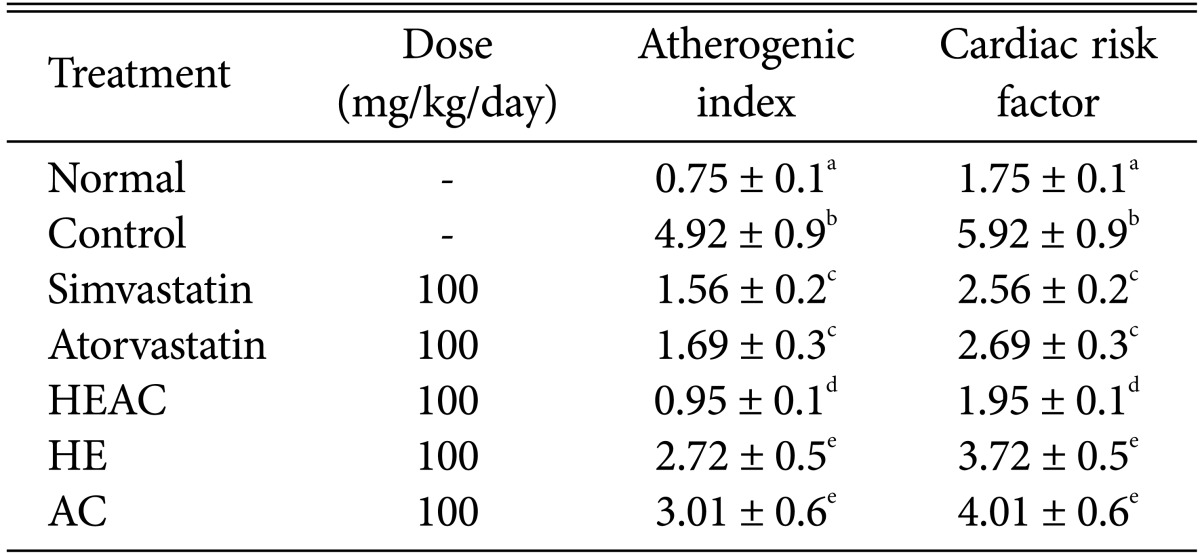
HEAC, ethanolic extracts from Hericium erinaceus cultivated with Artemisia capillaris; HE, ethanolic extracts from Hericium erinaceus; AC, ethanolic extracts from Artemisia capillaris.
a-cThe data (mean ± SE values) were determined from three replicates. Means in the column followed by the same letter are not significantly different (p < 0.05).
Effects of HEAC on hepatic morphology after hyperlipidemia induced by a HFD
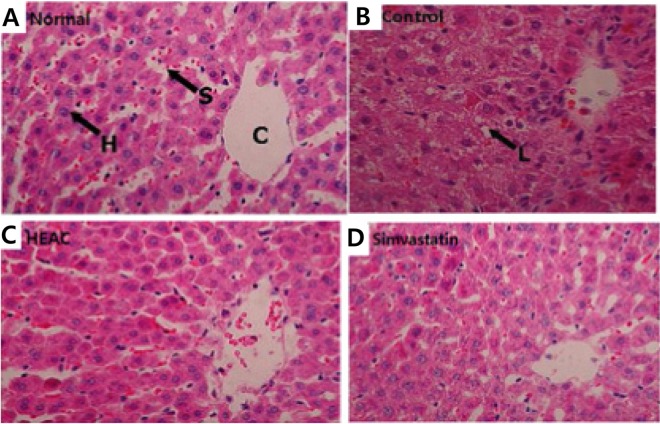
Histological analysis was taken of liver sections from four groups of rats (Fig. 1). Livers from the hyperlipidemia rats contained severe centrilobular hepatic steatosis with numerous lipid droplets appearing in the cytoplasm of the centrilobular hepatocytes (Fig. 1A and B). Treatment with simvastatin and HEAC decreased hepatic steatosis in the obese rats and lipid droplets disappeared with the statin treatment. The histological analysis showed a clear relationship between drug treatment and cholesterol-lowering activity. Histological analysis of the periepididymal fat pad further confirmed this result and indicated that increases in the area and diameter of the periepididymal fat pad in the HFD mainly result from the accumulation of lipids (Fig. 1).
Fig. 1.
Micrographs of rat hepatic tissue (hematoxylin and eosin stain, ×400). A, Normal indicates the hepatic tissues from normal rats fed a normal diet; B, Control indicates the hepatic tissues from obese rats fed a high-fat diet. The ethanolic extracts from Hericium erinaceus cultivated with Artemisia capillaris (C) and simvastatin (D) indicate the hepatic tissues from obese rats fed a high-fat diet and treated with HEAC and simvastatin, respectively. C, central vein; S, sinusoidal capillary; H, hepatocyte; L, lipid droplet.
DISCUSSION
In the present study, a HFD successfully induced hyperlipidemia in rats, modulating biochemical parameters (enhancement of TC, LDL-c, and TG levels) and lowering HDL-c levels (Table 2). In the obese rats, the FER value also changed, indicating weight gain and a lower food intake. As two statin compounds were used as positive controls for other treatments, simvastatin and atorvastatin decreased TC, LDL-c, and TG levels, but increased HDL-c levels. The treatment of hyperlipidemia patients with statin compounds works via the inhibition of HMG-CoA reductase activity [13]. Therefore, our results confirmed their cholesterol-lowering activities. However, even if they are widely used in the world, it is worthwhile to expand treatment options for hyperlipidemia using new drugs with new target sites in the lipid-lowering process.
In the present study, AC lowered TC, LDL-c, and TG levels in the obese rats, but increased HDL-c levels. This result is similar to those previously reported using obese rabbits [14]. In 1998, Pan et al. [14] reported that AC extract decreased TC levels and increased HDL levels in mice with hyperlipidemia by improving their metabolism. Chen et al. [15] reported that Artesimia scoparia reduces atherosclerotic lesions in hypercholesterolmic rabbits by decreasing TC and TG levels. Pathology results indicate that Artesimia aucheri extract significantly reduces arterial wall atherosclerotic lesions from high-cholesterol [16].
The ethanolic extract of HE showed very similar results to the AC extract with its cholesterol-lowering activities and ability to increase HDL-c levels. Interestingly, Yang et al. [17] reported the lipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of H. erinaceus in dietary-induced hyperlipidemic rats. They found hypolipidaemic effects of H. erinaceus proportionally related to the increasing concentration of the exo-biopolymer.
Interestingly, HEAC tested in this study showed its hypolipidemia activity against the obese rats. HEAC exhibited a significant decrease in TC, LDL-c, and TG levels, and a significant increase in HDL-c in rats fed a HFD. HEAC showed stronger hypolipidaemic activity in relation to the AC- and HE-treated rats. This is likely due to a certain compound or class of compound over-produced in H. erinaceus during its growth in media containing A. scoparia. One potential compound, scoparone, is reportedly over-produced in HEAC [7, 18]. Scoparone is a primary coumarin present in AC and has hypolipidaemic activity via the retardation of the pathomorphological changes in hypercholesterolaemic diabetic rabbits [19]. Therefore, scoparone in HEAC or exo-polymers in the HEAC medium might explain the hypolipidaemic activity in HEAC.
The AI and CRF from using HEAC showed a recovery close to the normal group (Table 3) and the two values are similar to the currently-used drugs. From liver tissues examined by microscopy (Fig. 1) histological examination of the normal group showed a normal cell architecture (Fig. 1A), while significant morphological changes were observed in the control group (Fig. 1B). Liver sections in the control obese rats showed less cells and lipid vacuolization, (indicated with the black arrow). On the other hand, the accumulation of hepatic lipid droplets appeared to be relatively lower with simvastatin and HEAC treatment (Fig. 1C and D). These results seem to correspond to the serum lipid profiles given in Table 2 and are consistent with previous studies [20, 21].
Taken together, these results demonstrate a potent cholesterol-lowering activity from HEAC. Further studies on the efficacy of HEAC and the identification of active compound(s) from HEAC could therefore lead to the development of therapeutic drugs for controlling hyperlipidemia in humans.
ACKNOWLEDGEMENTS
This work was supported in part by the Soonchunhyang University Research Fund.
References
- 1.Formiguera X, Cantón A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol. 2004;18:1125–1146. doi: 10.1016/j.bpg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Mizutani T, Inatomi S, Inazu A, Kawahara E. Hypolipidemic effect of Pleurotus eryngii extract in fat-loaded mice. J Nutr Sci Vitaminol (Tokyo) 2010;56:48–53. doi: 10.3177/jnsv.56.48. [DOI] [PubMed] [Google Scholar]
- 3.Eliasson B, Svensson AM, Miftaraj M, Jonasson JM, Eeg-Olofsson K, Sundell KA, Gudbjörnsdóttir S. Clinical use and effectiveness of lipid lowering therapies in diabetes mellitus: an observational study from the Swedish National Diabetes Register. PLoS One. 2011;6:e18744. doi: 10.1371/journal.pone.0018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935–938. doi: 10.1016/j.amjcard.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Elisashvili V. Submerged cultivation of medicinal mushrooms: bioprocesses and products (review) Int J Med Mushrooms. 2012;14:211–239. doi: 10.1615/intjmedmushr.v14.i3.10. [DOI] [PubMed] [Google Scholar]
- 6.Lee SE, Jeong JH, Kim DG, Choi WS. Inhibitory effect on aflatoxin B1-8,9-epoxide formation and anti-complementary activity of methanol extract from Hericium erinaceus cultivated with Artemisia iwayomogi. Food Sci Biotechnol. 2003;12:183–186. [Google Scholar]
- 7.Choi WS, Kim CJ, Park BS, Lee SE, Takeoka GR, Kim DG, Lanpiao X, Kim JH. Inhibitory effect on proliferation of vascular smooth muscle cells and protective effect on CCl4-induced hepatic damage of HEAI extract. J Ethnopharmacol. 2005;100:176–179. doi: 10.1016/j.jep.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Richmond W. Use of cholesterol oxidase for assay of total and free cholesterol in serum by continuous-flow analysis. Clin Chem. 1976;22:1579–1588. [PubMed] [Google Scholar]
- 9.McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–542. [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 11.Zlatkis A, Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969;29:143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]
- 12.Haglund O, Luostarinen R, Willin R, Wilbell I, Salden T. The effects of fish oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in humans supplemented with vitamin E. J Nutr. 1991;121:165–169. doi: 10.1093/jn/121.2.165. [DOI] [PubMed] [Google Scholar]
- 13.Todd PA, Goa KL. Simvastatin: a review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- 14.Pan J, Liu G, Liu H, Qiu Z, Chen L. Effects of Artemisia capillaris on blood glucose and lipid in mice. Zhong Yao Cai. 1998;21:408–411. [PubMed] [Google Scholar]
- 15.Chen YL, Huang HC, Weng YI, Yu YJ, Lee YT. Morphological evidence for the antiatherogenic effect of scoparone in hyperlipidaemic diabetic rabbits. Cardiovasc Res. 1994;28:1679–1685. doi: 10.1093/cvr/28.11.1679. [DOI] [PubMed] [Google Scholar]
- 16.Dinani NJ, Asgary A, Madani H, Naderi G, Mahzoni P. Hypocholesterolemic and antiatherosclerotic effect of Artemisia aucheri in hypercholesterolemic rabbits. Pak J Pharm Sci. 2010;23:321–325. [PubMed] [Google Scholar]
- 17.Yang BK, Park JB, Song CH. 2003. Hypolipidemic effect of an exo-polymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci Biotechnol Biochem. 2003;67:1292–1298. doi: 10.1271/bbb.67.1292. [DOI] [PubMed] [Google Scholar]
- 18.Choi WS, Kim YS, Yang JA, Lee YH, Park BS, Lee SE. Curative effects of extracts of Hericium erinaceum hypha cultivated with Artemisia capillaris (HEAC) and their primary active compounds on rat liver disease. J Korean Soc Appl Biol Chem. 2011;54:531–537. [Google Scholar]
- 19.Huang HC, Weng YI, Lee CR, Jan TR, Chen YL, Lee YT. Protection by scoparone against the alterations of plasma lipoproteins, vascular morphology and vascular reactivity in hyperlipidaemic diabetic rabbit. Br J Pharmacol. 1993;110:1508–1514. doi: 10.1111/j.1476-5381.1993.tb13993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Chen G, Li B, Ji B, Guo Y, Tian F. Evaluation of antioxidative and hypolipidemic properties of a novel functional diet formulation of Auricularia auricula and Hawthorn. Innov Food Sci Emerg Technol. 2009;10:215–221. [Google Scholar]
- 21.Woo MN, Bok SH, Choi MS. Hypolipidemic and body fat-lowering effects of Fatclean in rats fed high-fat diet. Food Chem Toxicol. 2009;47:2076–2082. doi: 10.1016/j.fct.2009.05.041. [DOI] [PubMed] [Google Scholar]