Abstract
Background
The tall-cell variant (TCV) of papillary thyroid carcinoma (PTC) is considered a more aggressive variant of PTC, with a poor prognosis. This is largely due to the tendency for TCV to present at an older age and with extrathyroidal extension (ETE). When these two variables are controlled for, it is unclear whether tall-cell histology alone portends a poor prognosis. Because previous studies have been underpowered to adequately answer this question, we hypothesized that TCV may have poorer prognosis than PTC. Our objective was to utilize a large cancer registry to obtain sufficient power to differentiate between outcomes in cases of TCV and PTC.
Methods
Using the National Cancer Institute's Surveillance, Epidemiology, and End Results database, we identified 278 TCV patients and 2522 classical PTC patients with sufficient information for a detailed matched-pair analysis. Each TCV patient was matched with a PTC patient for age, sex, extent of ETE, regional and distant metastases, surgical and adjuvant therapy, and year of diagnosis. The TCV cohort was then compared against all PTC cases and matched PTC cases.
Results
Compared with classical PTC, TCV patients presented at an older age (54.3 years vs. 46.3 years, p < 0.0001) had a higher rate of ETE (53.6% vs. 30.2%, p < 0.0001) and poorer 5-year disease-specific survival (81.9% vs. 97.8%, p < 0.0001). In the matched-pair analysis comparing TCV patients to the matched PTC cohort, 5-year disease-specific survival was poorer in the TCV cohort (81.9% vs. 91.3%, p = 0.049). The number of deaths in the TCV cohort was higher than in the matched PTC cohort (p = 0.043).
Conclusions
TCV exhibits poorer survival than classical PTC. When the major prognostic factors for thyroid cancer are controlled for, including age and ETE, tall-cell histology alone remains a significant prognostic factor for disease-specific death.
Introduction
Tall-cell variant (TCV) of papillary thyroid carcinoma (PTC) is defined by the World Health Organization as being composed predominantly of tall cells with height at least twice width, eosinophilic cytoplasm, and basally oriented nuclei. As a variant of PTC, the characteristic nuclear features of PTC are also present (1).
TCV is reported to behave aggressively compared with classical PTC, presenting with an increased incidence of regional metastases and poorer radioactive iodine avidity, ultimately demonstrating poorer prognosis (2–7). Other groups, however, have argued that TCV's poorer prognosis is mainly attributable to its tendency to present at an older age, with a higher frequency of extrathyroidal extension (ETE), and that when age and ETE are controlled for, tall-cell histology per se does not portend an inferior prognosis (8–10). Therefore, the thyroid cancer literature is not in agreement about the true impact of TCV histology on prognosis.
TCV is a relatively rare variant of PTC, representing between 3% and 19% of cases (1,3,5,6). Because thyroid cancer mortality is relatively low, identifying survival differences between rare variants such as TCV and classical PTC requires large cohorts of patients and long periods of follow-up. However, fewer than 300 TCV cases have been reviewed in the literature (2,3,7,10–15), with the majority of published series comprising cohorts of fewer than 20 patients. As such, these studies have been underpowered to identify divergent outcomes between TCV and PTC. Larger patient cohorts and sufficient follow-up are required to identify any survival differences.
Cancer registries, such as the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) registry, allow investigators to access large patient cohorts as well as follow-up data. To identify any difference in outcome between TCV and classical PTC, we carried out a matched-pair analysis of TCV and PTC patients using the NCI SEER database. We report that, when major prognostic factors including age and ETE are controlled for, tall-cell histology itself is associated with poorer survival.
Materials and Methods
SEER program
The National Cancer Institute's SEER program is considered the gold standard cancer registry, having collected clinical data since 1973 and now capturing 26% of the U.S. population. The SEER registry collects detailed information on patient demographics, tumor characteristics, and initial therapy. All patients are followed for vital status to permit analysis of survival. Quality control is an integral part of the SEER program (16). Comparison studies have confirmed that pathologic, surgical, and radiation data are accurately recorded (17). However, there are some limitations of SEER data when compared with large institutional prospective studies. Some pathologic and clinical details are not recorded, including cancer recurrence. There is no centralized pathology review and pathologic data rely on medical records from the treating institutions. In thyroid cancer cases, recurrence data are not available, and only limited data are available regarding adjuvant therapy. However, the database does provide a detailed breakdown of thyroid cancer by histologic type, including variants of PTC. The SEER database has recorded TCV histology since 1999. Because SEER is a de-identified dataset, the NCI does not require institutional review board oversight.
Case selection
Using SEER*Stat release 6.4.4 (April 2008; NCI Cancer Statistics Branch, Bethesda, MD), cases were identified using the appropriate International Classification of Diseases for Oncology (18) codes for “papillary carcinoma of thyroid” (8260) and “papillary carcinoma, tall cell” (8344), arising within the thyroid gland. Other variants of PTC were not included. This identified 336 cases of TCV between 1999 and 2006, and 10,609 contemporaneous PTC cases.
Cases were limited to patients who had been recorded as receiving a total thyroidectomy under surgery of primary site. Data for analysis included age, sex, size of primary tumor in greatest dimension, presence of gross ETE, presence of cervical metastases, presence of distant metastases, year of diagnosis, and administration of adjuvant radioactive iodine. Only gross ETE into the strap muscles or surrounding structures was considered ETE. For many patients, data regarding the performance and extent of lymph node dissection were not recorded, and this was not included in the study. This left 278 TCV patients and 2522 classical PTC patients.
Matching procedure
Each TCV patient was matched to a PTC patient for age (within 10 years), sex, presence of gross extrathyroidal disease, regional and distant metastatic stage (cervical N0 vs. N+; distant M0 vs. M1), surgical therapy (all received total thyroidectomy), adjuvant radioactive iodine or external beam radiotherapy, and year of diagnosis (within 5 years). TCV and PTC cases were matched 1:1. Multiple matched PTC cases were not possible because of limited numbers of candidate PTC cases in older age groups.
Statistical analysis
Three cohorts were constructed: TCV (n = 278), matched PTC (n = 278), and all PTC (n = 2522). The TCV cohort was compared with the entire PTC and matched PTC cohorts using the chi-squared test for categorical variables, t-test for continuous variables, the McNemar method for matched pairs, and the Kaplan–Meier method of survival analysis. The survival statistic of interest was disease-specific survival at up to 7 years of follow-up, which was compared using a one-tailed log-rank test. The a priori level of significance was p = 0.05. All analyses were carried out in SPSS version 16.0 (SPSS, Chicago, IL).
Results
TCV versus all PTC cases
Patient and tumor characteristics for TCV and all PTC patients are summarized in Table 1. Because TCV histology has only been recorded since 1999, mean duration of follow-up for these three cohorts ranged from 26.1 to 28.0 months.
Table 1.
Tall-Cell Variant Versus All Papillary Thyroid Carcinoma Cases
| Parameter | TCV | All PTC | TCV vs. all PTC |
|---|---|---|---|
| Patients | 278 | 2522 | |
| Mean follow-up (months) | 28.0 (26.0–30.0) | 26.1 (25.3–26.8) | p = 0.28 |
| Mean age | 54.3 (52.4–56.2) | 46.3 (45.5–47.1) | p < 0.0001 |
| Age ≥ 45 years | 69.8% (64.5–75.1) | 50.1% (49.9–50.3) | p < 0.0001 |
| Male | 25.5% (20.4–30.6) | 26.1% (24.6–27.7) | p = 0.84 |
| Gross extrathyroidal extension | 53.6% (50.6–56.6) | 30.2% (29.3–31.1) | p < 0.0001 |
| Mean tumor size (mm) | 25.9 (23.9–27.9) | 27.1 (26.1–28.1) | p = 0.28 |
| Cervical metastases | 39.6% (33.9–45.3) | 36.5% (34.6–38.4) | p = 0.35 |
| Distant metastases | 8.3% (5.0–11.5) | 13.1% (11.8–14.4) | p = 0.021 |
| Adjuvant RAI treatment | 55.0% (49.2–60.8) | 48.8% (46.9–50.7) | p = 0.05 |
Patient and tumor characteristics: TCV and all PTC cases. Data expressed as mean with 95% confidence interval.
PTC, papillary thyroid carcinoma; RAI, radioactive iodine; TCV, tall-cell variant.
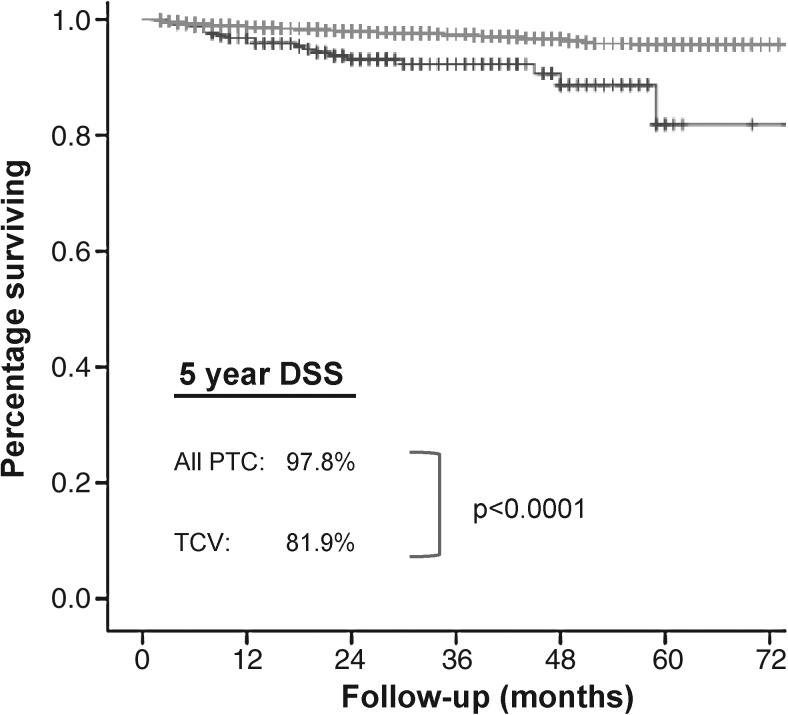
As expected, TCV presented with a higher rate of ETE (53.6% vs. 30.2%, p < 0.0001) and at an older age (54.3 years vs. 46.3 years, p < 0.0001). The TCV cohort had a higher proportion of patients presenting at age 45 or older (69.8% vs. 50.1%, p < 0.0001). TCV exhibited a similar rate of regional metastases (39.6% vs. 36.5%, p = 0.35) but a lower rate of distant metastases (8.3% vs. 13.1%, p = 0.02), compared with all PTC. Tumor size and sex were similar. TCV patients were more likely to be treated with adjuvant radioiodine (55.0% vs. 48.8%, p = 0.05). The 5 year disease-specific survival of TCV patients was poorer than all PTC patients (81.9% vs. 97.8%, p < 0.0001) (Fig. 1).
FIG. 1.
Kaplan–Meier plot of DSS. Five-year DSS for TCV (81.9%) versus all PTC (97.8%) differs significantly on log-rank test (p < 0.0001). DSS, disease-specific survival; PTC, papillary thyroid carcinoma; TCV, tall-cell variant.
TCV versus matched PTC cases
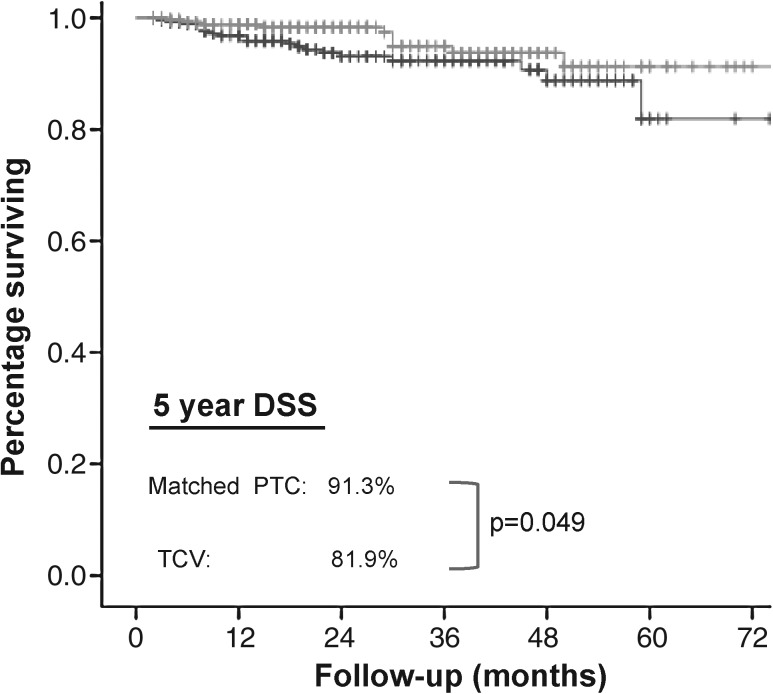
The patient and tumor characteristics for TCV and matched PTC patients are summarized in Table 2. Comparing the TCV cohort with the matched PTC cohort, the 5 year disease-specific survival remained poorer (81.9% vs. 91.3%, p = 0.049) (Fig. 2). There was a higher proportion of disease-specific deaths in the TCV group, compared with the matched PTC group (19 vs. 10 out of 278, p = 0.043). Of the 19 patients in the TCV group who died of thyroid cancer, 15 had tumors with ETE and 4 had tumors without gross ETE. In comparison, all 10 patients in the matched PTC group who died had tumors with gross ETE.
Table 2.
Tall-Cell Variant Versus Matched Papillary Thyroid Carcinoma Cases
| Parameter | TCV | Matched PTC | TCV vs. matched PTC |
|---|---|---|---|
| Patients | 278 | 278 | |
| Mean follow-up (months) | 28.0 (26.0–30.0) | 27.3 (25.3–29.3) | p = 0.71 |
| Mean age | 54.3 (52.4–56.2) | 54.4 (52.5–56.3) | p = 0.96 |
| Age ≥45 years | 69.8% (64.5–75.1) | 69.8% (64.5–75.1) | p = 0.99 |
| Male | 25.5% (20.4–30.6) | 25.2 (20.1–30.3) | p = 0.99 |
| Gross extrathyroidal extension | 53.6% (50.6–56.6) | 53.2% (50.2–56.2) | p = 0.99 |
| Mean tumor size (mm) | 25.9 (23.9–27.9) | 27.3 (25.2–29.4) | p = 0.93 |
| Cervical metastases | 39.6% (33.9–45.3) | 39.9% (34.1–45.6) | p = 0.99 |
| Distant metastases | 8.3% (5.0–11.5) | 8.3% (5.0–11.5) | p = 0.99 |
| Adjuvant RAI treatment | 55.0% (49.2–60.8) | 55.0% (49.2–60.8) | p = 0.99 |
Patient and tumor characteristics: TCV and matched PTC cases. Data expressed as mean with 95% confidence interval.
FIG. 2.
Kaplan–Meier plot of DSS. Five-year DSS for TCV (81.9%) vs. matched PTC (91.3%) differs significantly on log-rank test (p = 0.049).
Discussion
TCV is defined by the World Health Organization as being composed predominantly of tall cells with height at least twice width, eosinophilic cytoplasm, and basally oriented nuclei. As a variant of PTC, the characteristic nuclear features of PTC are also present (1,2). Thresholds for the requisite percentage of tall cells range from 30% to 70%, according to different investigators (2). It is known to be underdiagnosed, with multiple authors reporting that the majority of TCV cases are missed by pathologists (2,3,10,15).
Traditionally, TCV has been considered a more aggressive variant of PTC. However, it has been argued that only clinical staging, not histology, determines outcome in variants of PTC (8–10,18). The clinical observations that TCV presents at an older age and with a much higher rate of ETE have been cited as the sole reasons for its poorer prognosis. Akslen and LiVolsi (9) reported that TCV patients (n = 7) presented at an older age and with more frequent ETE, and experienced worse survival; however, on multivariate analysis, histology did not independently affect outcome. Michels et al. (10) similarly reported that TCV patients (n = 56) were older, with larger tumors and more frequent metastases and ETE. However, when clinical stage was controlled for, histology did not affect survival or recurrence. Prendiville et al. (11) compared TCV patients (n = 20) to a historical control group, finding more common ETE with TCV, and no differences in survival or recurrence rates. Similar results were also reported by Flint et al. (19) in 11 TCV patients.
In contrast, a number of investigators have reported the contrary (2,3,5,6). Terry et al. (3) studied 19 TCV patients, finding larger tumors and more frequent ETE. On multivariate analysis, tall-cell histology independently predicted higher rates of recurrence. Pilotti et al. (6) reported that a group of poorly differentiated thyroid carcinomas (n = 44; 39 TCV patients) experienced inferior outcomes on Cox multiple regression analysis, with a hazard ratio of 4.80 for relapse and 5.05 for death. Similar results were published by Ito et al. (5), demonstrating higher rates of recurrence (odds ratio 2.55) and cause-specific death (odds ratio 6.67) for TCV on multivariate analysis. Using an alternative study design to eliminate the confounding effect of TCV's high rate of ETE, Ghossein et al. (2) at Memorial Sloan-Kettering Cancer Center limited their analysis only to patients with no ETE. This study included 47 TCV patients, who presented at older age, with higher rates of nodal metastases and multifocality in comparison to PTC patients. On multivariate analysis, the only independent factor associated with nodal metastases was tall-cell histology.
Small sample size is the most likely cause for the contradictory results of these studies. Because of the low incidence of TCV, ranging from 3% to 19% of PTC cases (6,10), these single-institution studies have accrued small numbers of patients. This raises the possibility of type II error (failure to reject the null hypothesis and detect a true difference) resulting from insufficient sample size and statistical power. In addition to small sample size, a second obstacle to survival analysis in thyroid cancer is the excellent prognosis and low number of deaths with differentiated thyroid cancers. This makes it difficult to identify divergence between survival curves. Large cancer registries offer the advantage of providing data from large numbers of patients. However, any investigator using the SEER registry must ensure that relevant covariates, which need to be controlled for, are available for analysis.
In our study, we carried out a matched-pair analysis to control for age, ETE, and all other major prognostic factors for thyroid carcinoma. Matched-pair analyses and multivariate regression models are two alternatives for studying prognostic factors that might influence survival. In cases where there are insufficient patient numbers to permit a match for every case, regression analysis is generally used to control for relevant covariates, but with less statistical power. An advantage to the SEER database is access to enough patients to permit precise matching, which permits a matched-pair analysis to be carried out. Using the SEER database, we were able to strictly match TCV-PTC pairs according to the major prognostic factors for thyroid carcinoma: age, sex, tumor size, gross ETE, cervical metastases, distant metastases, extent of surgery, and radioiodine treatment. Summary data from the TCV and matched PTC cohorts confirmed that the groups were matched for all of these parameters (Table 2).
In agreement with previous reports, the TCV cohort presented at an older age, with a higher rate of ETE and with poorer disease-specific survival, compared with all (unmatched) PTC patients. In our matched-pair analysis, we found that TCV patients experienced poorer disease-specific survival, even when age and ETE were controlled for. The resultant 5-year disease-specific survival rates was poorer in the TCV cohort compared with the matched PTC cohort (81.9% versus 91.3%, p = 0.049).
Because TCV was not separately recorded prior to 1999, our data were only able to provide 7 years of survival data. The absolute difference in disease-specific survival was modest at 9.4%, but this does arguably represent a clinically significant distinction for well-differentiated thyroid cancer. In absolute numbers, the mortality rate nearly doubled, from 10 to 19 cases out of 278. These data therefore support the contention that tall-cell histology inherently represents a more aggressive variant of PTC, even when clinical stage and risk factors are controlled for. Perhaps, because of the rarity of this tumor and difficulty in achieving statistical power with small sample sizes, several hitherto performed studies have failed to identify this difference.
There is a reasonable biologic basis for our conclusions. The scientific literature reports several molecular characteristics of TCV, which would confer more aggressive behavior. For example, Muc1 protein, which mediates cell–cell adhesion, is overexpressed in many cancers, including nearly all TCV tumors, but few PTC tumors; this is believed to promote cellular dissociation and cancer progression (20). Type IV collagenase, which promotes tumor invasion and metastasis, is also overexpressed in TCV compared with PTC (21). Recent data have also correlated BRAF point mutations (22) and RET/PTC3 rearrangements (23) with TCV carcinoma. Together, these findings support the notion that neoplastic tall cells are not merely morphologically different, but more biologically aggressive at a molecular level. TCV is also believed to be less responsive to radioactive iodine, as most recurrent cases do not take up iodine (4,24).
It is important to mention a number of limitations to this study. There may have been underdiagnosis of TCV in the SEER registry, as it has occurred at major academic institutions. In Michel's series (10), 80% of TCV cases were missed by the pathologist; in Terry's series (3), 90% were not recognized initially. At Memorial Sloan-Kettering Cancer Center, 53% of TCV cases had been initially read as classical PTC (2). In our cohort of SEER patients, the incidence of TCV was 2.6%. This incidence is at the lower end of the 3–19% range reported in the literature, suggesting that there was possibly underdiagnosis occurring in the cases we reviewed. This is a limitation of any cohort study of TCV, although we hasten to clarify that underdiagnosis of TCV would tend to dilute the differences between groups and thus understate any differences. The presence of TCV cases within the PTC cohort would attenuate the differences between the two groups and bias PTC survival statistics downward.
Further, the threshold of tall cells required for the diagnosis of TCV has not been strictly defined, and there is variability in the definitions of TCV used by different pathologists. This may potentially cause heterogeneity within our TCV cohort and conceivably could lead to the inclusion of PTCs with 30% or fewer tall cells in the TCV cohort. However, heterogeneity or misdiagnosis within the TCV cohort would tend to dilute any differences between the TCV group and the classical PTC group and thus lead to results, which understate any true differences. In general, variability in cancer registry data makes clinical associations more difficult to identify than in single-institution studies (17).
Other limitations of this study are attributable to the database itself. Although most pathologic data are recorded, patient slides were obviously not reviewed. The lack of centralized review by an experienced thyroid pathologist is a weakness of cancer registry studies. If poorly differentiated thyroid cancers were misdiagnosed, and significantly more likely to be classified as either TCV or classical PTC, the group would falsely appear to have poorer outcomes. Although SEER data have been shown to be accurate, the variables recorded are limited (17). At a minimum, we were able to limit our analysis to patients receiving total thyroidectomy and control for the administration of adjuvant treatment, but we were unable to analyze further details, such as surgical margin status, extent of nodal dissection performed, or dose, timing, and completion of radioiodine treatment. If one group was significantly less likely to receive complete surgery, or received lower doses of radioactive iodine, poorer prognosis might instead be simply explained by inadequate treatment. The SEER database also does not record data regarding recurrence of disease, preventing analysis of recurrence rates and disease-free survival. Finally, the SEER database did not record tall-cell histology prior to 1999, limiting our follow-up, and therefore, the magnitude of the survival difference observed. We suspect that, as survival data mature, the TCV and PTC curves will diverge further. Any research using the SEER database should consider possible sources of systematic bias, which might influence results. In this study, we do not believe that any of these registry limitations would lead to directional bias favoring one group over the other.
We report that patients with TCV of PTC experience poorer survival compared with patients with classical papillary cancer. This is primarily due to the fact that TCV presents with ETE nearly twice as often as PTC, and TCV patients present nearly a decade older than PTC patients. Nevertheless, when ETE, age, and all other major clinical prognostic factors in thyroid cancer are controlled for, tall-cell histology still represents an independent risk factor for death. The TCV is thus an aggressive subtype of PTC, and its accurate diagnosis is important in determining prognosis and treatment of these patients.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.World Health Organization. International Histologic Classification of Tumours: Histological Typing of Thyroid Tumors. 2nd. Springer; Berlin: 1988. [Google Scholar]
- 2.Ghossein RA. Leboeuf R. Patel KN. Rivera M. Katabi N. Carlson DL. Tallini G. Shaha A. Singh B. Tuttle RM. Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: biologic behavior and clinical implications. Thyroid. 2007;17:655–661. doi: 10.1089/thy.2007.0061. [DOI] [PubMed] [Google Scholar]
- 3.Terry JH. St. John SA. Karkowski FJ. Suarez JR. Yassa NH. Platica CD. Marti JR. Tall cell papillary thyroid cancer: incidence and prognosis. Am J Surg. 1994;168:459–461. doi: 10.1016/s0002-9610(05)80099-6. [DOI] [PubMed] [Google Scholar]
- 4.Leung AK. Chow S. Law SC. Clinical features and outcome of the tall cell variant of papillary thyroid carcinoma. Laryngoscope. 2008;118:32–38. doi: 10.1097/MLG.0b013e318156f6c3. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y. Hirokawa M. Fukushima M. Inoue H. Yabuta T. Uruno T. Kihara M. Higashiyama T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Miyauchi A. Prevalence and prognostic significance of poor differentiation and tall cell variant in papillary carcinoma in Japan. World J Surg. 2008;32:1535–1543. doi: 10.1007/s00268-007-9406-7. [DOI] [PubMed] [Google Scholar]
- 6.Pilotti S. Collini P. Manzari A. Marubini E. Rilke F. Poorly differentiated forms of papillary thyroid carcinoma: distinctive entities or morphological patterns? Semin Diag Pathol. 1995;12:249–255. [PubMed] [Google Scholar]
- 7.Machens A. Holzhausen H. Lautenschläger C. Dralle H. The tall-cell variant of papillary thyroid carcinoma: a multivariate analysis of clinical risk factors. Langenbecks Arch Surg. 2004;389:278–282. doi: 10.1007/s00423-004-0485-8. [DOI] [PubMed] [Google Scholar]
- 8.Wenig BM. Thompson LD. Adair CF. Shmookler B. Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998;82:740–753. [PubMed] [Google Scholar]
- 9.Akslen LA. LiVolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000;88:1902–1908. [PubMed] [Google Scholar]
- 10.Michels JJ. Jacques M. Henry-Amar M. Bardet S. Prevalence and prognostic significance of tall cell variant of papillary thyroid carcinoma. Hum Pathol. 2007;38:212–219. doi: 10.1016/j.humpath.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Prendiville S. Burman KD. Ringel MD. Shmookler BM. Deeb ZE. Wolfe K. Azumi N. Wartofsky L. Sessions RB. Tall cell variant: an aggressive form of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2000;122:352–357. doi: 10.1016/S0194-5998(00)70047-7. [DOI] [PubMed] [Google Scholar]
- 12.Moreno Egea A. Rodriguez Gonzalez JM. Sola Perez J. Soria Cogollos T. Parrilla Paricio P. Prognostic value of the tall cell variety of papillary cancer of the thyroid. Eur J Surg Oncol. 1993;19:517–521. [PubMed] [Google Scholar]
- 13.Johnson TL. Lloyd RV. Thompson NW. Beierwaltes WH. Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1998;12:22–27. doi: 10.1097/00000478-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski ML. Merino MJ. Tall cell variant of papillary thyroid carcinoma: a reassessment and immunohistochemical study with comparison to the usual type of papillary carcinoma of the thyroid. Am J Surg Pathol. 1996;20:964–974. doi: 10.1097/00000478-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Rüter A. Nishiyama R. Lennquist S. Tall-cell variant of papillary thyroid cancer: disregarded entity? World J Surg. 1997;21:15–20. doi: 10.1007/s002689900187. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A. Clegg LX. Ward E. Ries LA. Wu X. Jamison PM. Wingo PA. Howe HL. Anderson RN. Edwards BK. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 17.Harlan LC. Hankey B. The SEER Program Database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21:2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 18.Fritz A. Percy C. Jack A. International Classification of Diseases for Oncology. 3rd. WHO; Geneva: 2000. [Google Scholar]
- 19.Flint A. Davenport RD. Lloyd RV. The tall cell variant of papillary carcinoma of the thyroid gland. Comparison with the common form of papillary carcinoma by DNA and morphometric analysis. Arch Pathol Lab Med. 1991;115:169–171. [PubMed] [Google Scholar]
- 20.Wreesmann VB. Sieczka EM. Socci ND. Hezel M. Belbin TJ. Childs G. Patel SG. Patel KN. Tallini G. Prystowsky M. Shaha AR. Kraus D. Shah JP. Rao PH. Ghossein R. Singh B. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64:3780–3789. doi: 10.1158/0008-5472.CAN-03-1460. [DOI] [PubMed] [Google Scholar]
- 21.Campo E. Merino MJ. Liotta L. Neumann R. Stetler-Stevenson W. Distribution of the 72-kd type IV collagenase in nonneoplastic and neoplastic thyroid tissue. Hum Pathol. 1992;23:1395–1401. doi: 10.1016/0046-8177(92)90060-g. [DOI] [PubMed] [Google Scholar]
- 22.Adeniran AJ. Zhu Z. Gandhi M. Steward DL. Fidler JP. Giordano TJ. Biddinger PW. Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 23.Basolo F. Giannini R. Monaco C. Melillo RM. Carlomagno F. Pancrazi M. Salvatore G. Chiappetta G. Pacini F. Elisei R. Miccoli P. Pinchera A. Fusco A. Santoro M. Potent mitogenicity of the RET/PTC3 oncogene correlates with its prevalence in tall-cell variant of papillary thyroid carcinoma. Am J Pathol. 2002;160:247–254. doi: 10.1016/S0002-9440(10)64368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosai J. Carcangiu M. DeLellis R. Atlas of Tumor Pathology: Tumors of the Thyroid Gland. 3rd. AFIP; Washington: 1992. Fascicle 5. [Google Scholar]