Abstract
Background
Distant metastases at presentation are rare in well-differentiated thyroid cancer (WDTC). The objective of this study was to report outcomes for patients presenting with distant metastases managed by thyroidectomy and radioactive iodine (RAI) therapy.
Methods
Fifty-two patients with distant metastases from thyroid cancer diagnosed before thyroid surgery (n=32) or on a postoperative RAI scan after thyroid surgery (n=20) were identified from a database of patients with WDTC treated between 1985 and 2005. The median age was 58 years (range 12–83 years), with a male-to-female ratio of 3:2. Forty-seven patients (90%) had total thyroidectomy and two (4%) had thyroid lobectomy, and three patients (6%) were found to be unresectable. Distant metastases were classified into pulmonary and extrapulmonary. Overall survival (OS), disease-specific survival (DSS), and locoregional recurrence-free survival were calculated by the Kaplan–Meier method. Factors predictive of the outcome were determined by univariate and multivariate analyses.
Results
Thirty-nine patients (75%) were diagnosed with pulmonary metastases alone and 13 (25%) with extrapulmonary metastases. The sites of extrapulmonary metastases were bone in nine, mediastinum in one, pyriform sinus in one and skin in one, and one patient had synchronous lung, bone, and intracerebral metastases. After thyroid surgery, 47 patients (90%) were treated with RAI alone, and 2 patients had external beam radiation in addition to RAI. With a median follow-up after surgery of 78.5 months, the 5-year OS and DSS were 65% and 68%, respectively. Twenty-nine patients (56%) died during follow-up, of whom 24 (46%) died of thyroid cancer. Six patients (12%) developed recurrent disease in the lateral neck, and three patients (6%) developed recurrence in the thyroid bed. Over 45 years, follicular pathology and extrapulmonary metastases were predictive of lower 5-year DSS (56% vs. 100%, p<0.001; 50% vs. 70%, p=0.004; and 46% vs. 75%, p=0.013, respectively).
Conclusion
Approximately half of patients with WDTC presenting with distant metastases die of disease within 5 years of initial diagnosis despite thyroid surgery and RAI. Age over 45 years, extrapulmonary metastases, and follicular pathology were significant predictors of the poor outcome.
Introduction
Well differentiated thyroid cancer (WDTC) most commonly presents with a disease confined to the thyroid gland with or without involvement of regional lymph nodes. Distant metastases occur during follow-up in 6%–20% of patients (1–5). The presence of distant metastatic disease at presentation is less common with rates between 3% and 15% (6–11). Within this group, surgical intervention for the primary thyroid tumor is still relevant in the majority of patients, largely to facilitate radioactive iodine (RAI) therapy, but also to control disease in the central compartment. The objective of this study was to present our experience in managing patients with WDTC with distant metastases at initial diagnosis and to report outcomes and identify factors predictive of outcome.
Methods
After approval by the Institutional Review Board, 52 patients (3%) who had thyroid surgery for WDTC and had distant metastases at presentation were identified from our institutional database of 1810 patients who underwent initial surgery for WDTC at the Memorial Sloan-Kettering Cancer Center between 1986 and 2005. Patients who underwent thyroid surgery elsewhere before referral, or those who were considered unresectable at the time of referral, were excluded. Patients were listed as having distant metastases (M1) at presentation if the metastases were identified in the preoperative period or within 6 months of thyroid surgery.
Patient-, tumor-, and treatment-related characteristics were collected by a retrospective review of patient charts. In the 52 patients with distant metastases, the median age was 58 years (range 12–83 years), with a male to female ratio of 3:2. Forty-seven patients (90%) had total thyroidectomy and two (4%) had thyroid lobectomy, and three patients (6%) were found to have unresectable disease. Of the two patients who underwent lobectomy, one had lobectomy as an accessory procedure to stabilize the cervical spine in the presence of bone metastasis, but was considered unfit for further surgery. There was no information available for the remaining lobectomy patient. Seven patients had a central neck dissection alone (13%), 25 patients underwent central and lateral neck dissection (48%), and 20 patients (38%) had no neck dissection. Pathological T status was T1 in 10 (19%), T2 in 4 (8%), T3 in 18 (34.5%), T4a in 18 (34.5%), and T4b in 2 (4%) patients. Thirty-two patients were pN1 (62%) of whom 8 patients had disease confined to the central compartment (pN1a=25%), and 24 had lateral neck metastases (pN1b=75%). Forty-six patients had papillary carcinoma (88%), and six patients had follicular carcinoma (12%). Of the 46 patients with papillary carcinoma, nine had tall-cell carcinoma. This histological subtype was not routinely described until the mid-1990s. It is likely that some patients who had surgery before this date were coded as papillary carcinoma, and therefore this stratification in to a tall-cell variant was not included in our analysis.
Sites of distant metastases were classified into pulmonary and extrapulmonary sites. Details of treatment of distant metastases were recorded. Overall survival (OS) and disease-specific survival (DSS) stratified by the site of distant metastases were calculated by the Kaplan–Meier method. Factors predictive of outcome were determined by univariate analyses using the log-rank test and a multivariate analysis by the Cox proportional hazards model. Statistical analysis was carried out using JMP statistical package (SAS Institute, Inc., Cary, NC) and SPSS (IBM Company Headquarters, Chicago, IL).
Results
Distant metastases were identified before thyroid surgery in 31 patients (60%), and on postoperative imaging in the remaining 21 patients (40%). All 21 patients who had metastatic disease identified postoperatively had asymptomatic pulmonary metastases. Twenty of these 21 patients had disease identified on radioiodine whole-body scan, and the other remaining patient had a metastatic lung lesion identified on postoperative chest computed tomography (CT) imaging. This patient was a 75-year-old with follicular carcinoma. At surgery, the disease was found to be widely infiltrative with involvement of the prevertebral fascia and great vessels. The integrity of the recurrent nerve was in question, and so a remnant of the contralateral thyroid gland was left. Postoperative radioiodine scanning did not reveal any metastases, and all iodine was concentrated in the contralateral thyroid bed. However, CT scan demonstrated lung metastases. After surgery, the patient was readmitted with severe hemoptysis and received external beam radiotherapy to the lung lesions. This patient went on to die of his pulmonary disease.
Of the patients identified preoperatively, 6 (19%) had metastatic disease diagnosed by nuclear medicine scanning (3 RAI scans and 3 bone scans), 16 (52%) after CT or magnetic resonance imaging scanning, 6 (19%) on chest X-ray, and 1 patient with skin metastasis was identified clinically (3%). In two patients (7%), we were unable to determine the precise method of diagnosis from records provided by external referring hospitals, where the preoperative investigation had been arranged.
Thirty-nine patients (75%) were classified as having pulmonary metastases alone, and 13 (25%) had metastases to extrapulmonary sites. The sites of extrapulmonary metastases were bone in nine patients (17%), mediastinum in one (2%), pyriform sinus in one (2%), and skin in one (2%). One patient (2%) had synchronous lung, bone, and intracerebral metastases. Patient, tumor, and treatment characteristics stratified by the site of metastases are shown in Table 1. Patients with extrapulmonary metastases were more likely to be over 45 years old and have follicular carcinoma, but less likely to have an extrathyroid extension or cervical nodal disease. Sixty-seven percent of patients with papillary carcinoma were over the age of 45 years, whereas 100% of those with follicular carcinoma were over the age of 45 years (p=0.1). Although not significant, this observation may suggest an association between age and the histological subtype if the sample size was greater.
Table 1.
Patient, Tumor, and Treatment Characteristics Stratified by the Site of Metastases
| Variable | Overall (n=52) | Pulmonary metastases (n=39) | Extrapulmonary metastases (n=13) | p-Value |
|---|---|---|---|---|
| Age | ||||
| <45 years | 15 (29%) | 14 (93%) | 1 (7%) | 0.05 |
| >45 years | 37 (71%) | 25 (68%) | 12 (32%) | |
| Gender | ||||
| Female | 22 (42%) | 17 (77%) | 5 (23%) | 0.752 |
| Male | 30 (58%) | 22 (73%) | 8 (27%) | |
| Extrathyroid extension | ||||
| No | 27 (52%) | 17 (63%) | 10 (37%) | 0.038 |
| Yes | 25 (48%) | 22 (88%) | 3 (12%) | |
| Histology | ||||
| Papillary Ca | 46 (88%) | 38 (83%) | 8 (17%) | <0.001 |
| Follicular Ca | 6 (12%) | 1 (17%) | 5 (83%) | |
| pT Stage | ||||
| T1 | 15 (29%) | 7 (47%) | 8 (53%) | 0.184 |
| T2 | 5 (10%) | 2 (40%) | 3 (60%) | |
| T3 | 12 (23%) | 12 (100%) | 0 (0%) | |
| T4 | 20 (38%) | 18 (90%) | 2 (10%) | |
| pN Stage | ||||
| N0 | 20 (38%) | 9 (45%) | 11 (55%) | <0.001 |
| N1 | 32 (62%) | 30 (94%) | 2 (6%) | |
Boldface indicates statistical significance (p≤0.05) according to χ2 test.
Ca, carcinoma; pT/N, pathological status of tumors/nodes.
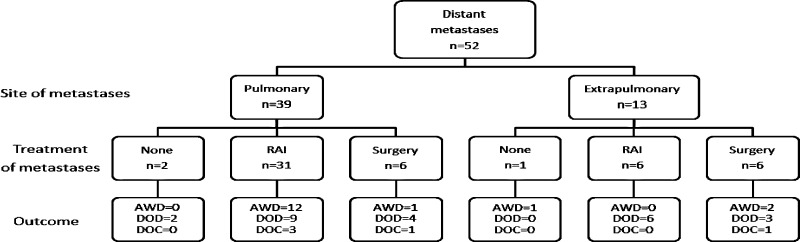
A flowchart showing treatment of metastases after thyroid surgery is shown in Figure 1. Forty-six patients (88%) were treated with RAI, which was given during follow-up, in addition to the RAI given for remnant ablation. The median cumulative dose of RAI was 205 mCi (range 30–496 mCi), given as a single dose in 15 patients, 2 doses in 14 patients, and 3 or more doses in the remaining 28 patients. The median time between doses for patients receiving more than 1 dose of RAI was 21 months (range 4–127 months). Nine of these patients also had planned surgical metastasectomy. These included five pulmonary resections, one mediastinal node resection via mediastinoscopy, one total laryngopharyngectomy for metastasis to the upper esophagus, one resection of a metastasis of the clavicle, and one hemipelvectomy for metastasis to the hip. Of the remaining six patients (12%), who did not receive RAI therapy, two were lost to follow-up, one was felt to be too ill for additional therapy, and three had surgical treatment of a metastasis, but were not fit for further treatment. Of the three patients treated surgically, one had pulmonary resection, one had a hemipelvectomy, and the remaining patient had resection of a cervical vertebral metastasis, which was identified as thyroid origin on frozen section. Five patients had external beam radiotherapy, two to the pelvis, two to long bones, and one to the thyroid bed.
FIG. 1.
Flowchart of treatment of metastases after thyroidectomy. RAI, radioactive iodine; AWD, alive with disease; DOD, died of disease; DOC, died of other causes.
Entire cohort
The 5-year OS and DSS for the whole group were 65% and 68%, respectively. Twenty-nine patients (56%) died during follow-up, of whom 24 (46%) died of thyroid cancer. Six patients (12%) developed recurrent disease in the lateral neck and three patients (6%) developed recurrence in the thyroid bed. Analysis of our data showed that age over 45 years, follicular pathology and extrapulmonary metastases were predictive of lower DSS (Table 2). Only one patient presenting under the age of 45 years had extrapulmonary metastasis, and none have died of disease with a median follow-up of 80 months in that subgroup (range 16–188 months).
Table 2.
Factors Predictive of Disease-Specific Survival on Univariate and Multivariate Analysis
| Variable | n (%) | 5-year DSS | Univariate p-Value | Multivariate HR [95% CI]; p-value |
|---|---|---|---|---|
| Age | ||||
| <45 years | 15 | 100% | ||
| >45 years | 37 | 56% | <0.001 | NA |
| Gender | ||||
| Female | 22 | 64% | ||
| Male | 30 | 71% | 0.954 | NA |
| Extrathyroid extension | ||||
| No | 27 | 69% | NA | |
| Yes | 25 | 67% | 0.854 | |
| Histology | ||||
| Papillary Ca | 46 | 70% | Referent | |
| Follicular Ca | 6 | 50% | 0.004 | 2.6 [0.9–7.4]; p=0.078 |
| pT Stage | ||||
| T1 | 15 | 72% | NA | |
| T2 | 5 | 80% | ||
| T3 | 12 | 70% | ||
| T4 | 20 | 61% | 0.929 | |
| pN Stage | ||||
| N0 | 20 | 68% | ||
| N1 | 32 | 83% | 0.010 | |
| Site of metastases | ||||
| Pulmonary | 39 | 75% | Referent | |
| Extrapulmonary | 13 | 46% | 0.013 | 2.1 [0.9–5.4]; p=0.13 |
Boldface indicates statistical significance (p≤0.05) according to χ2 test.
DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval.
Pulmonary metastasis-only group
Of the 39 patients with pulmonary metastases alone, 30 had total thyroidectomy and postoperative RAI, 1 had unresectable disease in the central compartment and had debulking surgery followed by RAI, 6 underwent total thyroidectomy and metastectomy, and postsurgical treatment details were unavailable for 2 cases. Of the 30 patients with pulmonary metastases who had total thyroidectomy and postoperative RAI, only 5 died of disease (17%) with a median follow-up of 86 months (range 4–281 months).
Of the six patients who had surgical resection of the pulmonary metastasis and total thyroidectomy, four also received postoperative RAI (two of whom received both ablative RAI and additional treatments). One patient had postoperative external beam radiotherapy to the neck and was then lost to follow-up; the remaining patient had an advanced urological malignancy diagnosed postoperatively and received no further treatment for thyroid cancer. Of the four patients who received metastasectomy and RAI after total thyroidectomy, two have since died of disease (50%).
Extrapulmonary metastases
Thirteen patients had extrapulmonary metastases, and post-thyroidectomy treatment details were available for 12 of these patients. Six were treated with total thyroidectomy and metastectomy. Four of these patients also received postoperative RAI, of whom only one had died of disease. Two patients were not fit for further treatment. Six patients were treated with thyroidectomy and postoperative RAI alone, all of who have since died of disease with a median follow-up of 59 months (range 1–162 months).
Radioiodine avidity
In 42 patients, the results of postoperative RAI scanning were available. DSS stratified by the site of metastases and RAI avidity are shown in Table 3. As expected, DSS was superior in patients with RAI-avid metastases for both pulmonary and extrapulmonary sites, although this was not statistically significant due to the small number of patients. For pulmonary metastases, the 5-year DSS for avid versus nonavid patients was 85% vs. 62%, p=0.146. For extrapulmonary metastases, the 5-year DSS of avid versus nonavid patients was 100% vs. 40% p=0.139.
Table 3.
Disease-Specific Survival Stratified by Site of Metastases and Radioactive Iodine Avidity
| RAI avidity | No. of patients | 5-year DSS | p-Value |
|---|---|---|---|
| All metastases (n=42) | |||
| Avid | 27 | 77% | 0.700 |
| Nonavid | 15 | 66% | |
| Pulmonary metastases (n=35) | |||
| Avid | 22 | 85% | 0.146 |
| Nonavid | 13 | 62% | |
| Extrapulmonary metastases (n=7) | |||
| Avid | 5 | 100% | 0.131 |
| Nonavid | 2 | 40% | |
RAI, radioactive iodine.
Patients receiving full planned treatment
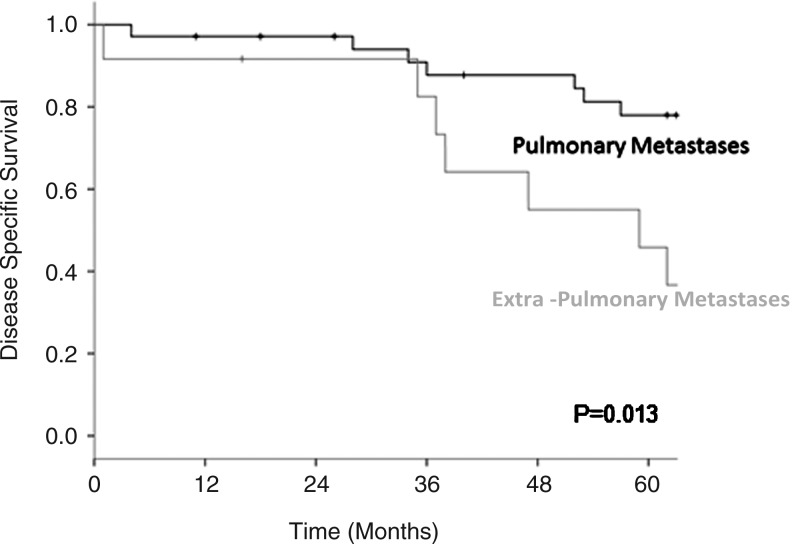
Of the 48 patients considered to have completed the initial planned therapy, 21 have died of disease (45%); 5 have died of other causes (10%); and the remaining 21 are alive (45%), with a median follow-up of 83 months. When this group is stratified by the site of metastasis, listed as pulmonary alone or extrapulmonary, the DSS of the pulmonary group at 5 years was 78% vs. 46% for the extrapulmonary group (p=0.013) (Fig. 2).
FIG. 2.
Disease-specific survival in the 48 patients who received full treatment stratified by the site of metastases at presentation.
Discussion
Although WDTC is a disease with generally a good outcome, patients presenting with distant metastatic disease have less favorable outcomes. For this reason, many risk stratification algorithms consider such cases to be high risk (12–15). Despite the higher chance of poor outcome, current treatment guidelines advocate an aggressive approach to management with surgery and postoperative RAI therapy (14,16,17). Treatment consists of total thyroidectomy, neck dissection as indicated by the detection of disease in the central and/or lateral neck, followed by RAI therapy in most patients.
In keeping with other surgical series of WDTC (1,2,7–11), we report a low rate of distant metastatic disease at presentation. Of 1810 patients studied over a 20-year period, only 1.7% presented with metastases identified before surgery, and a further 1.2% had metastases identified within 6 months of surgery, giving an overall rate of metastases at presentation of 2.9%.
The approach to treatment of such patients at our institution is to resect all detectable neck diseases, with total thyroidectomy and neck dissection as indicated, and then treat with postoperative RAI. This is in keeping with both American (14) and International (16) guidelines. Surgical metastectomy is considered if the lesion is amenable to resection and if the patient is fit for further treatment. Thyroid cancer is quite unlike other cancers, in that patients with distant metastases can still enjoy prolonged survival, with the use of RAI, provided the metastatic lesions are RAI avid. This accounts for why our reported survival outcomes in patients with distant metastases are very good with a 5-year OS and DSS of 65% and 68%, respectively. Avidity to RAI is directly related to the degree of differentiation of thyroid cancer, and this, in turn, is directly related to the age of the patient. For example, in children with thyroid cancer, the tumor is well differentiated and therefore strongly RAI avid. As a consequence, complete responses in children with widespread pulmonary metastases are not unusual, and excellent outcomes have been reported. Loss of RAI avidity occurs when dedifferentiation occurs in thyroid cancer and that has been associated with a poor outcome (18). In our series, we report better outcome in patients with radioiodine-avid disease with 5-year DSS of 77% compared to 66% for nonavid metastases. However, this did not reach statistical significance (p=0.703), most likely due to the small number of cases. DSS was superior in RAI-avid metastases for both pulmonary and extrapulmonary sites of involvement.
In our study, we found that age over 45 years, extra pulmonary metastases, and follicular histology were significant predictors of a poor outcome. Although some investigators have reported age as not predictive of an outcome (2,9), the majority of reports in the literature support an association between young age and good outcome (4–6,8,10,11,19–21) after treatment for metastatic thyroid cancer. In the present series, no patient under the age of 45 years died of thyroid cancer during follow-up. In our series, we found that age and RAI avidity were associated, with higher rates of RAI-avid disease in younger patients, p=0.049 (Table 4). The association with age is directly related to tumor differentiation and hence RAI avidity. As well as age, the site of distant metastases and the type of pathology were also important. The site of metastasis has been reported as significant with a number of studies finding an association between extrapulmonary metastases and poor prognosis (7–9,20). This was in keeping with the findings of our study, which showed improved survival for patients with metastases limited to the lung (p=0.013). Looking in more detail at the group stratified by pulmonary versus extrapulmonary metastases, those with pulmonary metastases were more likely to be younger and to have papillary histology. Interestingly, patients with metastases limited to the lung had higher rates of both extrathyroid extension and cervical nodal disease, which has not been reported previously. We speculate that this may represent the biological predilection of papillary carcinoma for pulmonary metastasis in comparison with follicular carcinoma. There was an association between the presence of nodal metastases and histology. Thirty percent of patients with papillary carcinoma had nodal metastases, versus 0% of follicular carcinomas (p=0.001). An association between extrathyroid extension and histology was also shown, with 52% of papillary and 17% of follicular carcinomas demonstrating extra thyroid extension, although this failed to reach statistical significance, p=0.102. Again, this suggests diversity in the biological behavior of papillary and follicular carcinoma.
Table 4.
Radioactive Iodine Avidity Stratified by Age
| Variable | RAI nonavid | RAI avid | p-Value |
|---|---|---|---|
| Age | |||
| <45 years | 4 | 11 | 0.049 |
| >45 years | 21 | 16 | |
The findings that outcome is dependent on the site of distant metastases have also been reported in other cancers. For example, breast cancer patients with bone metastases have a poorer outcome than those with lung metastases. Indeed, it has recently been reported that the molecular profile for breast cancer patients with bone metastases is very different to that for breast cancer patients with lung metastases (22,23). Since the molecular profile of tumors is different for different sites of distant metastases, it is not surprising that the type of thyroid pathology (follicular versus papillary) would determine the outcome in patients with distant metastases. In our study, we found that follicular histology was associated with a poor outcome (p=0.004), a finding that has been reported by other groups (2,4,5,7,19). We also found that follicular histology was associated more commonly with extrapulmonary metastases (p<0.001) (Table 1). This would therefore suggest that the metastatic gene signature of follicular thyroid cancer must be different from the metastatic gene signature for papillary thyroid cancer. This observation may be important clinically in determining the type of systemic therapy such tumors may be sensitive to.
In summary, we can say that patients who present with metastatic disease are a complex cohort of patients, ranging from those who are young and fit to those who are older and have life-threatening comorbidities. Within our series, there was heterogeneity of treatment, as many patients had significant comorbidities preventing aggressive approaches to management. Of the 48 patients who received full planned treatment, 21 have died of disease (45%); 5 have died of other causes (10%); and the remaining 21 are alive (45%), with a median follow-up of 83 months. Patients who presented with extrapulmonary metastases were found to have significantly poorer DSS at 5 years (46% vs. 75%, p=0.013). We can conclude that although 46% patients with WDTC and distant metastases at presentation die of disease within 5 years of initial diagnosis, over 50% of patients are alive and free of disease after 5 years. The observation that patients with WDTC and distant metastases at presentation can still enjoy prolonged survival is not seen in any other type of solid organ cancer with the possible exception of germ cell tumors. Age over 45 years, extrapulmonary metastases, and follicular pathology are the main predictors of poor outcomes in these patients.
Disclosure Statement
No competing financial interests exist for any author.
References
- 1.Dinneen SF. Valimaki MJ. Bergstralh EJ. Goellner JR. Gorman CA. Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80:2041–2045. doi: 10.1210/jcem.80.7.7608252. [DOI] [PubMed] [Google Scholar]
- 2.Lin JD. Huang MJ. Juang JH. Chao TC. Huang BY. Chen KW. Chen JY. Li KL. Chen JF. Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–1235. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 3.Massin JP. Savoie JC. Garnier H. Guiraudon G. Leger FA. Bacourt F. Pulmonary metastases in differentiated thyroid carcinoma. Study of 58 cases with implications for the primary tumor treatment. Cancer. 1984;53:982–992. doi: 10.1002/1097-0142(19840215)53:4<982::aid-cncr2820530427>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Schlumberger M. Challeton C. De Vathaire F. Travagli JP. Gardet P. Lumbroso JD. Francese C. Fontaine F. Ricard M. Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37:598–605. [PubMed] [Google Scholar]
- 5.Mihailovic J. Stefanovic L. Malesevic M. Differentiated thyroid carcinoma with distant metastases: probability of survival and its predicting factors. Cancer Biother Radiopharm. 2007;22:250–255. doi: 10.1089/cbr.2006.313. [DOI] [PubMed] [Google Scholar]
- 6.Casara D. Rubello D. Saladini G. Masarotto G. Favero A. Girelli ME. Busnardo B. Different features of pulmonary metastases in differentiated thyroid cancer: natural history and multivariate statistical analysis of prognostic variables. J Nucl Med. 1993;34:1626–1631. [PubMed] [Google Scholar]
- 7.Haq M. Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol. 2005;63:87–93. doi: 10.1111/j.1365-2265.2005.02304.x. [DOI] [PubMed] [Google Scholar]
- 8.Sugitani I. Fujimoto Y. Yamamoto N. Papillary thyroid carcinoma with distant metastases: survival predictors and the importance of local control. Surgery. 2008;143:35–42. doi: 10.1016/j.surg.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Shaha AR. Shah JP. Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474–476. doi: 10.1016/s0002-9610(97)00158-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee J. Soh E-Y. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis. Ann Surg. 2010;251:114–119. doi: 10.1097/SLA.0b013e3181b7faf6. [DOI] [PubMed] [Google Scholar]
- 11.Showalter TN. Siegel BA. Moley JF. Baranski TJ. Grigsby PW. Prognostic factors in patients with well-differentiated thyroid cancer presenting with pulmonary metastasis. Cancer Biother Radiopharm. 2008;23:655–660. doi: 10.1089/cbr.2008.0501. [DOI] [PubMed] [Google Scholar]
- 12.Shaha AR. Shah JP. Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996;3:534–538. doi: 10.1007/BF02306085. [DOI] [PubMed] [Google Scholar]
- 13.Hay ID. Bergstralh EJ. Goellner JR. Ebersold JR. Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. discussion 1057–1058. [PubMed] [Google Scholar]
- 14.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 15.Cady B. Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 16.Watkinson JC. The British Thyroid Association guidelines for the management of thyroid cancer in adults. Nucl Med Commun. 2004;25:897–900. doi: 10.1097/00006231-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pacini F. Schlumberger M. Dralle H. Ilisea R. Smith Y. Viersinga V. [European consensus on the management of patients with differentiated carcinoma of the thyroid from follicular epithelium] Vestn Khir Im I I Grek. 2008;167:52–56. [PubMed] [Google Scholar]
- 18.Riesco-Eizaguirre G. Gutierrez-Martinez P. Garcia-Cabezas MA. Nistal M. Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 19.Durante C. Haddy N. Baudin E. Leboulleux S. Hartl D. Travagli JP. Caillou B. Ricard M. Lumbroso JD. De Vathaire F. Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 20.Shoup M. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–197. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 21.Mihailovic JM. Stefanovic LJ. Malesevic MD. Erak MD. Tesanovic DD. Metastatic differentiated thyroid carcinoma: clinical management and outcome of disease in patients with initial and late distant metastases. Nucl Med Commun. 2009;30:558–564. doi: 10.1097/MNM.0b013e32832cc2ab. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen DX. Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen DX. Bos PD. Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]