Abstract
Objective
In cervical intraepithelial neoplasia (CIN), p16INK4a immunohistochemistry has been reported to be a useful diagnostic biomarker. However, limited information is available about the association between the p16INK4a immunohistochemistry and the outcomes of CIN. Here, we report p16INK4a immunohistochemistry as an effective biomarker to predict the outcomes of CIN.
Methods
p16INK4a immunohistochemistry was performed in patients with CIN from January 2000 to August 2009. Among these patients, we have performed a retrospective analysis of the medical records to evaluate the outcome of CIN 1-2 and performed statistical analysis to determine the correlation between p16INK4a expression and the outcomes. We also performed HPV genotyping and analyzed the relation between the infecting human papillomavirus (HPV) genotype and the outcomes.
Results
A total of 244 patients, including 82 with CIN 1, 60 with CIN 2, and 102 with CIN 3, were examined. The rate of p16INK4a overexpression increased with increasing CIN grade, 20.7% for CIN 1, 80.0% for CIN 2, and 89.2% for CIN 3, with significant differences between CIN 1 and CIN 2-3 group. In the 131 CIN 1-2 patients, the progression rate was significantly higher for the patients showing p16INK4a overexpression than for those not showing p16INK4a overexpression (p=0.005); the regression rate was also found to be significantly lower for the patients showing p16INK4a overexpression (p=0.003). High-risk HPV genotypes were detected in 73 patients (73.7%). Both progression and regression rates were not significantly different between the high-risk HPV-positive and HPV-negative groups (p=0.401 and p=0.381, respectively).
Conclusion
p16INK4a overexpression was correlated with the outcome of CIN 1-2, and p16INK4a is considered to be a superior biomarker for predicting the outcome of CIN 1-2 compared with HPV genotyping.
Keywords: Biomarker, Cervical intraepithelial neoplasia, Human papillomavirus, Immunohistochemistry, p16INK4a
INTRODUCTION
Cervical cancer is the second most common malignancy in women worldwide [1]. The incidence of cervical cancer in young women has been increasing recently. Cervical intraepithelial neoplasia (CIN) is a precancerous lesion that can be treated effectively to prevent progression to cervical cancer. CIN 1 lesions are usually followed up without treatment; however, 10% of CIN 1 lesions progress to CIN 3 or cervical cancer. In patients of CIN 2, 20% of CIN 2 lesions progress to CIN 3 or cervical cancer, and 40% of CIN 2 lesions regress spontaneously [2].
For many gynecologists, the management of patients with CIN 1-2 is controversial [2]: should they observe patients until spontaneous regression or treat patients with ablative or excisional procedures? Although it may be appropriate to treat only patients that are at high risk of progression, and to observe low risk patients that may regress spontaneously, it is not easy to predict the outcome of each patient. There is a growing need to establish an effective biomarker that would serve as a reliable predictor of the outcomes.
One of the important biomarkers for CIN and cervical cancer is the human papillomavirus (HPV) genotyping. HPV DNA is found positive in more than 90% of cervical cancer patients [3], and especially, high-risk genotypes of HPV are considered to be associated with development of CIN and cervical cancer [3-5]. It has been shown that HPV genotyping can detect women with cytological abnormalities and it has a potential role in identifying women at risk of residual or recurrent disease after treatment of CIN [6]. However, HPV genotyping is less specific than cytology, because many infections regress without progressing to high-grade lesions and a positive HPV test does not necessarily discriminate between transient and chronic infection [7,8].
Previous experimental and epidemiologic studies have shown that expression of E6 and E7 genes of the high-risk genotypes of HPV in the squamous epithelial cells of the uterine cervix may result in neoplastic growth, and that infection with high-risk HPV results in the expression of p16INK4a [9,10]. p16INK4a is one of the cyclin-dependent kinase inhibitors that prevents phosphorylation of retinoblastoma protein (Rb) and therefore plays an important role in the regulation of the mammalian cell cycle [11]. Although p16INK4a protein is considered to be a tumor suppressor, paradoxical p16INK4a overexpression has frequently been observed in CIN lesions associated with high-risk HPV infection [12]. p16INK4a overexpression is associated with dysfunction of the pRb protein through naturally arising mutations, or its binding to the HPV 16 E7 protein and E7 protein might induce both abnormal cell cycle progression and p16INK4a overexpression [9]. Previous studies have mentioned that p16INK4a immunohistochemistry could be a biomarker to predict the outcomes of CIN lesions, although the predominance as a biomarker compared with HPV genotyping was not fully elucidated [13-16]. Therefore, we examined p16INK4a immunohistochemistry of the uterine cervix as an effective biomarker compared with HPV genotyping in patients with CIN 1-2 by retrospective nested cohort study.
MATERIALS AND METHODS
1. Patient selection
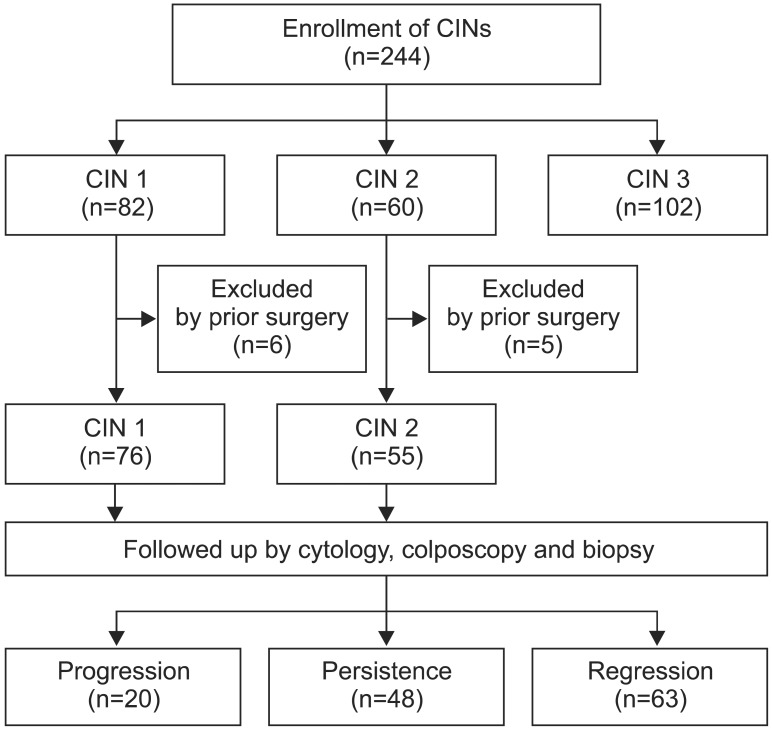
This retrospective study was approved by the Ethics Committee of Keio University School of Medicine (No. 20080180). Between January 2000 and August 2009, 244 consecutive patients with CIN were examined and treated at the Department of Obstetrics and Gynecology, Keio University Hospital, Tokyo, Japan. The number of patients with CIN was as follows: CIN 1, 82 patients; CIN 2, 60 patients; CIN 3, 102 patients. Conventional cytology, colposcopically directed biopsy, histological examination and p16INK4a immunohistochemistry were conducted in all the patients for their initial visits. The initial cervical exfoliated cells for Pap smear also collected in PreservCyt (Hologic Inc., Bedford, MA, USA) were utilized for HPV genotyping. Patients diagnosed with CIN 1-2 were followed up at three months intervals and received conventional Pap smear and colposcopic examinations. At each visit, if any abnormal lesions were suspected, colposcopically directed biopsy was performed. Patients with CIN 3 were immediately treated by laser vaporization or cone resection without follow-up. While reviewing the medical records, we collected data including the results of subsequent cytological or histological examination results and subsequent treatments if the patient underwent any procedures. Among CIN 1 and CIN 2 patients, eleven patients with past history of treatments such as laser vaporization or laser conization were excluded and we evaluated the outcomes of the CIN 1 and CIN 2 lesions after the initial diagnosis. Consequently, a total of 131 patients were enrolled, including 76 patients with CIN 1 and 55 patients with CIN 2 lesions (Fig. 1).
Fig. 1.
Patients. CIN, cervical intraepithelial neoplasia.
2. Definition of CIN progression and regression
Progression was defined as the appearance of histologically confirmed CIN 3 or more during the follow-up period. We defined regression as at least two consecutive conventional cytology with normal colposcopical findings. Women were regarded as having persistent lesions when they did not have either regression or progression over the follow-up period [17].
3. p16INK4a immunohistochemistry and interpretation of results
All samples were prepared from colposcopically directed punch biopsy specimens. The immunohistochemistry was performed as follows. Formalin-fixed and paraffin-embedded specimens were stained on a Dako Autostainer (Dako Norden A/S, Glostrup, Denmark) using a CINtec p16INK4a Histology Kit for the DakoCytomation Autostainer according to the manufacturer's instructions. Briefly, antigen retrieval was performed by heating in a water bath at 95℃ for 20 minutes. After blocking endogenous peroxidase activity, the slides were incubated with primary antibody (E6H4 clone). Then, a secondary antibody reagent for visualization was employed from the above-mentioned kit. The slides were incubated with DAB (3,3'-diaminobenzidine). Substrate-chromogen solution and counterstaining was performed with Mayer's hematoxylin before coverslipping.
The evaluation of immunoreactivity was reported previously. Briefly, immunoreactivity was evaluated on the basis of nuclear and/or cytoplasmic staining, 1) evaluation of the staining intensity: -, no staining; +, weak staining; ++, moderate staining; +++, strong staining, and 2) evaluation of the percentage of positively stained cells in each section. Immunoreactivity for p16INK4a was then classified as negative, weakly positive, moderately positive, and strongly positive (Table 1, Fig. 2). The p16INK4a overexpression rate was calculated by adding the number of moderately and strongly positive specimen.
Table 1.
Evaluation of p16INK4a immunohistochemistry
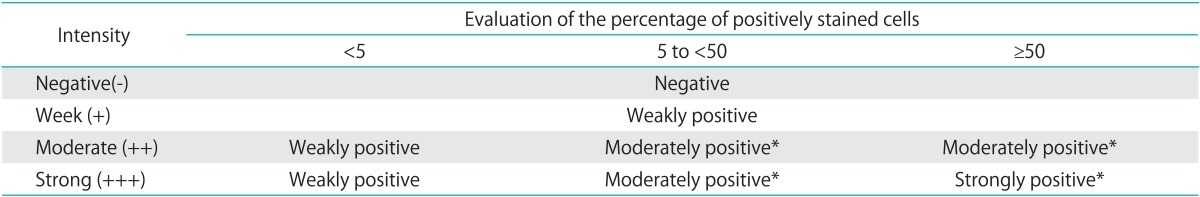
*Indicates p16INK4a overexpression.
Fig. 2.
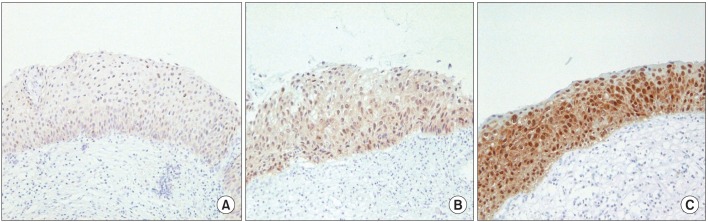
p16INK4a immunohistochemistry of cervical biopsy specimens. Immunoreactivity for p16INK4a was classified as negative, weakly positive (A), moderately positive (B), or strongly positive (C) (microscope objective: ×10).
4. HPV genotyping by polymerase chain reaction
The procedure of HPV genotyping has been described previously [18,19] . Briefly, genomic DNA was extracted from exfoliated cells, using proteinase K and phenol-chloroform treatment. The specimens were tested for the presence of HPV DNA by polymerase chain reaction (PCR) analysis with consensus primer pairs (L1C1 and L1C2) designed to amplify an approximately 250-bp segment of the viral DNA. The consensus primer pairs target the HPV L1 open reading frame and detect a broad range of genital HPVs. The HPV genotyping was determined by the direct sequencing method [19], which was determined based on an approximately 200 bases of L1 sequence and a search of the NCBI database (GenBank sequences; http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) using Sequencing Analysis ver. 3.3 (The Perkin-Elmer Co., Norwalk, CT, USA) [18].
5. Statistical analysis
Statistical analysis was performed using SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). The χ2 test was used to assess the association among the p16INK4a expression status, HPV genotyping and the histological diagnosis. Kaplan-Meier analysis was used to estimate the rates of progression and regression of the disease. A log-rank test was used to determine the statistical significance. A p-value<0.05 was considered to denote statistical significance.
RESULTS
A total of 244 patients ranged in age from 22 to 83 years, with a median age of 37.0 years. Among the patients, we evaluated the outcomes of those with CIN 1-2, including 76 patients with CIN 1 and 55 patients with CIN 2. The median follow-up period of the patients with CIN 1-2 was 40 months (range, two to 104 months). The rate of p16INK4a overexpression increased with increasing CIN grade: 17 patients with CIN 1 (20.7%), 48 patients with CIN 2 (80.0%), and 91 patients with CIN 3 (89.2%). The rates of p16INK4a overexpression were significantly higher in the CIN 2-3 patients than in the CIN 1 patients (p<0.001).
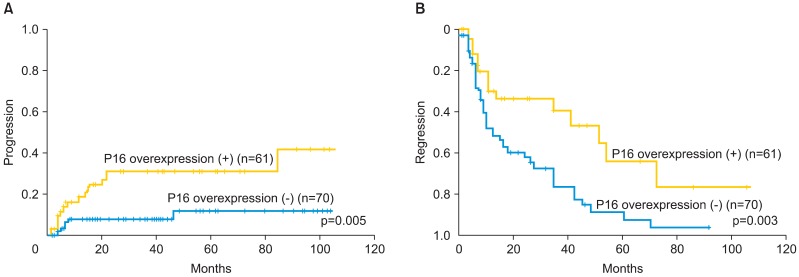
Of all the patients examined, there were 61 patients (46.6%) showing p16INK4a overexpression. During the follow-up period, 14 patients (23.0%) with p16INK4a overexpression showed progression to higher-grade lesions, whereas only 6 (8.6%) among the patients without p16INK4a overexpression showed progression. Two CIN 1 cases and 12 CIN 2 cases with p16 overexpression showed progression to higher-grade lesions. According to the results of the Kaplan-Meier analysis, there was a significant difference in the progression rate between the patients with p16INK4a overexpression and those not showing p16INK4a overexpression (p=0.005) (Fig. 3). A total of 17 (27.9%) patients with p16INK4a overexpression showed regression, whereas 46 patients (65.7%) not showing p16INK4a overexpression showed regression. The difference in the regression rate between these two groups was also significant (p=0.003) (Fig. 4).
Fig. 3.
(A) Kaplan-Meier analysis of the cumulative progression rate and follow-up periods in the patients with cervical intraepithelial neoplasia (CIN) 1-2. The progression rate for the patients showing p16INK4a overexpression was significantly higher than that for patients showing no p16INK4a overexpression (p<0.05). (B) The cumulative regression rate and follow-up periods in patients with CIN 1-2. The regression rate for the patients showing p16INK4a overexpression was significantly lower than that for the patients showing no p16INK4a overexpression (p<0.05).
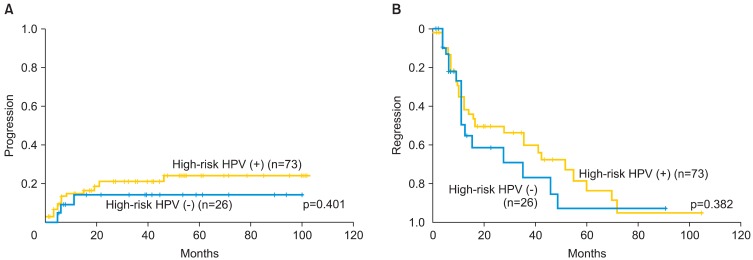
Fig. 4.
(A) Kaplan-Meier analysis of the cumulative progression rate and follow-up periods (mo) for patients with CIN 1-2 lesions with prevalent high-risk human papillomavirus (HPV) infection. The progression rate in patients with prevalent high-risk HPV was not significantly different from that in patients who were negative for high-risk HPV. (B) The cumulative regression rate and follow-up periods (mo) for patients with CIN 1-2 with high-risk HPV infection. The regression rate in the patients with prevalent high-risk HPV was not significantly different from that in patients who were negative for high-risk HPV infection.
The HPV genotyping of 131 patients with CIN 1-2 revealed infection by the following sixteen HPV genotypes; 6, 16, 18, 31, 33, 35, 51, 52, 56, 58, 59, 68, 70, 71, 84, and 90. In this study, we defined HPV16, 18, 31, 33, 35, 51, 52, 56, and 58 as high-risk HPVs prevalent in Japanese women [18,20,21]. Among 201 patients who were tested with HPV genotyping, 162 patients (80.0%) were identified to have infection with high-risk HPV genotypes. The rate of infection with high-risk HPV also increased with increasing CIN grade, with significant differences between CIN 1 (65.1%) and CIN 2-3 lesions (87.7%) (p<0.001).
Among the patients, 73 had infection with prevalent high-risk HPV, 10 had infection by other genotypes of HPV (6, 59, 68, 70, 71, 84), 26 were negative for HPV infection, and 32 were not tested for the HPV infection status. Although patients infected with prevalent high-risk HPV showed a higher tendency towards progression of the CIN lesions, there was no significant difference in the rate of progression of the lesions between high-risk HPV-positive and negative groups (Fig. 4). There were also no significant differences in the rate of progression or regression of the lesions between patients infected with HPV 16 or 18 and those infected with neither of these HPV genotypes (p=0.60) (Table 2).
Table 2.
Outcomes of patients with HPV 16 and 18 infection
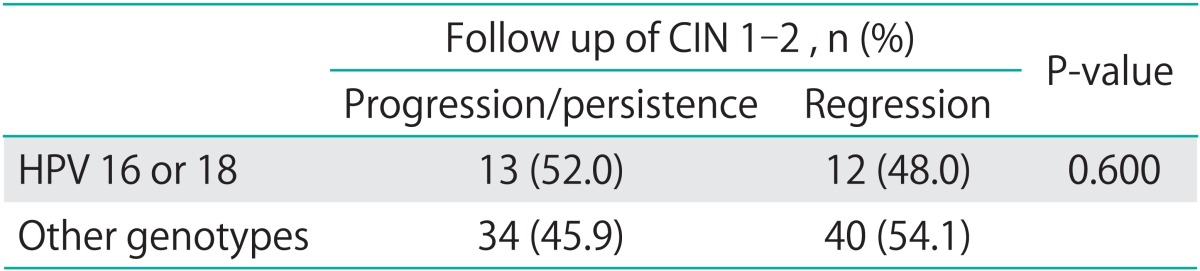
The definition of progression/persistence and regression was described in the manuscript.
CIN, cervical intraepithelial neoplasia.
Among 76 patients with CIN 1, 58 patients were evaluated for HPV genotyping. The associated HPV genotypes with CIN 1 patients were 16, 18, 31, 33, 35, 51, 52, 56, 58, 59, 68, 70, 84, and 90. There was also no significant difference in the rate of progression and regression between high-risk HPV-positive and negative groups among the CIN 1 patients according to the Kaplan-Meier analysis (p=0.951, p=0.652, respectively).
DISCUSSION
In this study, the rate of p16INK4a overexpression increased with increasing CIN grade, indicating that p16INK4a overexpression could reflect the potential of malignant transformation of cervical epithelial cells. In our previous report, increase in the rate of p16INK4a overexpression may suggest more severe inactivation of pRb by high-risk HPVs which frequently cause progression from CIN 1 to higher grade, and p16INK4a overexpression may be a reliable diagnostic marker for high-risk CIN lesions [22]. We also have shown here in this study there was a significant difference in the progression rate between patients showing p16INK4a overexpression and those not showing p16INK4a overexpression, and a significant difference in the regression rate between these two groups. These results suggest that p16INK4a overexpression was correlated with the outcome of the CIN 1-2 lesions, and that p16INK4a immunohistochemistry may be a promising biomarker for predicting the outcome of CIN 1-2.
Recent publications have demonstrated a correlation between p16INK4a overexpression and the risk of progression or regression of CIN and cervical cancer [13-15,23]. Among these reports, Omori et al. [13] indicated that the p16INK4a expression level was associated with CIN 2 progression, while other studies demonstrated that the p16INK4a expression level was only correlated with the CIN 1 outcomes. Negri et al. [23] mentioned that CIN 1 patients with p16INK4a overexpression showed a significantly higher tendency towards progression to CIN 3 than those that did not show p16INK4a expression. However, clinical information was limited because they only selected CIN 1 patients that showed regression or progression to CIN 3, not including persistent patients. Here in this study, we evaluated both CIN 1 and CIN 2 patients for their outcomes which could be predicted by p16INK4a immunohistochemistry.
From the results of our study, p16INK4a overexpression could be superior biomarker to the HPV genotyping for predicting the outcomes of CIN 1-2; however, we need to consider several aspects of this result. One point is that this study is a retrospective cohort study which has relatively small number of patients and 32 patients were not tested for HPV genotyping. Also, there is a possibility that the direct PCR sequencing for HPV genotyping could not fully cover the entire HPV infections. Therefore, we may have failed to identify all the cases with high-risk HPV infection. Considering more definitive and reliable method of HPV genotyping is available, we could reevaluate the correlation between infecting HPV genotypes and the outcomes CIN 1-2. The last point is that, during the course of the follow-up, the progression and regression rates reached plateaus which were shown in Figs. 3, 4. This partly reflects the surgical interventions, such as laser conization or ablation, and that both progression and regression rates could be different if these procedures were not undertaken. These aspects may lead to the lack of significant differences between the patients infected with high-risk HPV and those with other HPV genotypes.
The management of CIN still poses a dilemma for gynecologists; therefore, there could be unnecessarily prolonged management, overtreatment, and patients' anxiety. In addition, young patients with CIN tend to be lost to be followed up. If we perform p16INK4a immunohistochemistry as a routine laboratory test for identifying the high-risk patients, especially among young patients, we may reduce the potential rate of progression of CIN to cervical cancer in the future. This is the first single-institution study, until date, to demonstrate that p16INK4a immunohistochemistry may be an more appropriate biomarker than HPV genotyping for predicting the outcomes of CIN 1-2 patients.
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (10218969 and 18591845).
We thank Dr. Murakami, Dr. Ohno, Dr. Nakamura, Dr. Hirao, Dr. Ishikawa, Dr. Tsukazaki, and Ms. Abe for their invaluable collaboration for preparation of this work. We also thank Dr. Kouyama at Center of Clinical Research, Keio University for invaluable advice in statistical analysis.
Footnotes
The corresponding author Takuma Fujii received lecture fee from MSD Co. (Tokyo, JAPAN).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa H, Sugano K, Fujii T, Kubushiro K, Tsukazaki K, Nozawa S. Frequent detection of human papilloma viruses in cervical dysplasia by PCR single-strand DNA-conformational polymorphism analysis. Anticancer Res. 2002;22:1655–1660. [PubMed] [Google Scholar]
- 5.Fujii T, Tsukazaki K, Kiguchi K, Kubushiro K, Yajima M, Nozawa S. The major E6/E7 transcript of HPV-16 in exfoliated cells from cervical neoplasia patients. Gynecol Oncol. 1995;58:210–215. doi: 10.1006/gyno.1995.1213. [DOI] [PubMed] [Google Scholar]
- 6.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–2545. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996;93:4350–4354. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu L, Guo M, He Z, Thornton J, McDaniel LS, Hughson MD. Human papillomavirus genotyping and p16INK4a expression in cervical intraepithelial neoplasia of adolescents. Mod Pathol. 2005;18:267–273. doi: 10.1038/modpathol.3800290. [DOI] [PubMed] [Google Scholar]
- 11.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 12.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omori M, Hashi A, Nakazawa K, Yuminamochi T, Yamane T, Hirata S, et al. Estimation of prognoses for cervical intraepithelial neoplasia 2 by p16INK4a immunoexpression and high-risk HPV in situ hybridization signal types. Am J Clin Pathol. 2007;128:208–217. doi: 10.1309/0UP5PJK9RYF7BPHM. [DOI] [PubMed] [Google Scholar]
- 14.Hariri J, Oster A. The negative predictive value of p16INK4a to assess the outcome of cervical intraepithelial neoplasia 1 in the uterine cervix. Int J Gynecol Pathol. 2007;26:223–228. doi: 10.1097/01.pgp.0000236942.51840.56. [DOI] [PubMed] [Google Scholar]
- 15.del Pino M, Garcia S, Fuste V, Alonso I, Fuste P, Torne A, et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol. 2009;201:488.e1–488.e7. doi: 10.1016/j.ajog.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki S, Zen Y, Inoue M. Biomarker expression in cervical intraepithelial neoplasia: potential progression predictive factors for low-grade lesions. Hum Pathol. 2011;42:1007–1012. doi: 10.1016/j.humpath.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Oki A, Furuta R, Maeda H, Yasugi T, Takatsuka N, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer. 2011;128:2898–2910. doi: 10.1002/ijc.25630. [DOI] [PubMed] [Google Scholar]
- 18.Masumoto N, Fujii T, Ishikawa M, Mukai M, Saito M, Iwata T, et al. Papanicolaou tests and molecular analyses using new fluid-based specimen collection technology in 3000 Japanese women. Br J Cancer. 2003;88:1883–1888. doi: 10.1038/sj.bjc.6601023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii T, Saito M, Iwata T, Hirao N, Nishio H, Ohno A, et al. Ancillary testing of liquid-based cytology specimens for identification of patients at high risk of cervical cancer. Virchows Arch. 2008;453:545–555. doi: 10.1007/s00428-008-0687-5. [DOI] [PubMed] [Google Scholar]
- 20.Sasagawa T, Basha W, Yamazaki H, Inoue M. High-risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol Biomarkers Prev. 2001;10:45–52. [PubMed] [Google Scholar]
- 21.Onuki M, Matsumoto K, Satoh T, Oki A, Okada S, Minaguchi T, et al. Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer. Cancer Sci. 2009;100:1312–1316. doi: 10.1111/j.1349-7006.2009.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa M, Fujii T, Saito M, Nindl I, Ono A, Kubushiro K, et al. Overexpression of p16 INK4a as an indicator for human papillomavirus oncogenic activity in cervical squamous neoplasia. Int J Gynecol Cancer. 2006;16:347–353. doi: 10.1111/j.1525-1438.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 23.Negri G, Vittadello F, Romano F, Kasal A, Rivasi F, Girlando S, et al. p16INK4a expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch. 2004;445:616–620. doi: 10.1007/s00428-004-1127-9. [DOI] [PubMed] [Google Scholar]