Abstract
Objective
The malignant potential of intraepithelial neoplasia of the vulva and vagina after treatment is not well defined. Our objective was to examine risk factors for recurrence and invasive disease.
Methods
Four hundred sixty-four women with biopsy proven high-grade intraepithelial neoplasia of the vulva and vagina were identified in the electronic databases of four colposcopy clinics. Inclusion criteria were a follow-up of more than one year, no history of invasive cancer and no invasive cancer within the first year after initial treatment. We investigated the potential factors associated with recurrence and progression using a logistic regression analysis to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Of the 411 eligible patients, 123 patients (29.9%) recurred later than one year after initial treatment and 24 patients (5.8%) progressed to invasive disease. According to multivariate analyses, the risk factors associated with recurrence were multifocality (OR, 3.33; 95% CI, 2.02 to 5.51), immunosuppression (OR, 2.51; 95% CI, 1.09 to 5.81), excision as initial treatment (vs. laser evaporation; OR, 1.79; 95% CI, 1.11 to 2.91) and smoking (OR, 1.61; 95% CI, 1.02 to 2.55). Risk factors for progression to invasive disease were immunosuppression (OR, 4.00; 95% CI, 1.30 to 12.25), multifocality (OR, 3.05; 95% CI, 1.25 to 7.43) and smoking (OR, 2.97; 95% CI, 1.16 to 7.60), but not treatment modality.
Conclusion
Laser evaporation combined with extensive biopsy is at least as efficacious as initial treatment of intraepithelial neoplasia with excision. Smoking is a risk factor for both recurrence and progression to invasive disease. Hence, smoking cessation should be advised and maintaining a long follow-up period due to late relapses is necessary.
Keywords: Cancer, Intraepithelial neoplasia, Laser evaporation, Vagina, Vulva
INTRODUCTION
The incidence of intraepithelial neoplasia (IN) of the lower genital tract has risen during the last four decades [1]. This increase is most likely due to the increased prevalence of human papillomavirus (HPV) infection which may induce multifocal precancerous epithelial lesions involving the cervix intraepithelial neoplasia (CIN), vagina intraepithelial neoplasia (VAIN), vulva intraepithelial neoplasia (VIN), and anus intraepithelial neoplasia (AIN) [2]. However, not all IN of the lower genital tract are associated with a persistent infection of high risk HPV. VIN can be classified as the usual type VIN which is commonly associated with carcinogenic genotypes of HPV or the differentiated type VIN associated with vulvar dermatologic conditions such as lichen sclerosus [3].
Natural history and treatment options of CIN have been extensively studied; consequently, widely accepted guidelines for diagnosis, treatment and surveillance have been established. Despite the rise in recent years, the incidence rate of VIN is 2.86 per 100,000 women per year, which is ten times lower than that of CIN; and the incidence rates of VAIN and AIN are even lower [1]. As a result of these low rates, management recommendations for IN of the vagina, vulva and anus are based on relatively small prospective studies and retrospective series [4-6]. Although spontaneous regression of VIN may occur, there is consensus that IN should be treated due to its invasive potential as recommended by the Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists [6-9]. Additionally, IN seems to have an adverse impact on the patients' quality of life and sexual functioning [10]. Nevertheless, the risk factors for the development of recurrent or invasive disease after treatment of vulvovaginal IN have not been well established.
The aim of our multicenter retrospective cohort study was to determine the invasive potential, recurrence rates and corresponding risk factors of treated vulovaginal IN.
MATERIALS AND METHODS
In this retrospective cohort study, patients with biopsy-proven, high-grade VIN, or VAIN were identified in the electronic databases of four colposcopy clinics (University Hospitals of Berne and Zurich, Cantonal Hospitals of Bruderholz Basel and Frauenfeld). Patients who simultaneously had anal IN were also included. The following variables were extracted from the patients' medical records: age at first diagnosis; unifocal or multifocal disease; immune status at first diagnosis (history of organ transplantation, human immunodeficiency virus [HIV] positivity, immunosuppressive medication); diagnosis of an invasive cancer of the vulva, vagina, or anus; type of initial and subsequent therapy (vulvectomy, partial vulvectomy, biopsy plus CO2 laser evaporation, topical medical treatment); and smoking habits (more than 10 cigarettes per day). Follow-up visits were usually scheduled every six months for the first five years, and then on an annual basis in subsequent years.
Only patients with a follow-up of twelve months or longer after initial diagnosis were included in the analysis. If a patient had both excision and biopsy combined with laser evaporation during the first year, laser evaporation was considered the initial treatment since it is the more comprehensive type of therapy. It was the policy of all colposcopy clinics involved in our study to re-excise involved margins. Exclusion criteria were a history of either invasive vulvar, vaginal, anal or cervical cancer, but not a history of CIN. Patients with the diagnosis of invasive cancer within one year from initial diagnosis of IN were also excluded in order to minimize falsely detecting preexisting invasive disease due to incorrect initial diagnosis. Low grade IN (formerly VIN I) was not considered an inclusion criterion since it had been omitted from the classification system for VIN developed by the International Society for the Study of Vulvovaginal Diseases (ISSVD) in 2005 [3].
Recurrence was defined as biopsy-proven IN one year and later after initial treatment. We applied this definition in order to exclude patients with inappropriate and/or insufficient initial treatment requiring immediate retreatment at the centers. The study's primary endpoints were progression to invasive disease and biopsy-proven recurrence.
Both univariate and multivariate data analyses were conducted in IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). The Kaplan-Meier estimator was used to estimate the survival function from data on recurrence of IN and progression to cancer. Multinomial logistic regression models (stepwise backward) were used to control for potential confounding factors (age at initial diagnosis, immune status, focality, grade [grade 2 vs. 3], type of treatment and smoking behavior [>10 cigarettes per day]) on discrete outcomes. Pearson's chi squared tests were used to detect differences in non-parametric variables. All tests were performed at a significance level of α=0.05 and confidence intervals were computed at a level of 95%. The study protocol was reviewed and approved by the local Institutional Review Boards (StV Nr. 08/2006) and written informed consent for review of patient charts was obtained.
RESULTS
Of 464 patients identified from 1977 to 2011, 411 patients (88.6%) had a follow-up longer than one year and were included in the analysis. Mean age at diagnosis was 46 years (±14; range, 17 to 90 years). With regard to the type of IN, 381 patients (92.7%) presented with high-grade vulvar and 30 patients (7.3%) with vaginal anal IN. Patient and treatment characteristics are presented in Table 1. Among the immunosuppressed patients, 19 patients (4.6%) were HIV positive, 9 patients (2.2%) were recipients of organ transplantation and were taking immune-suppressing medication, and 5 patients (1.2%) were under long-term steroid medication.
Table 1.
Associations of patient characteristics with recurrence and progression of the disease more than 12 months after initial diagnosis of intraepithelial neoplasia
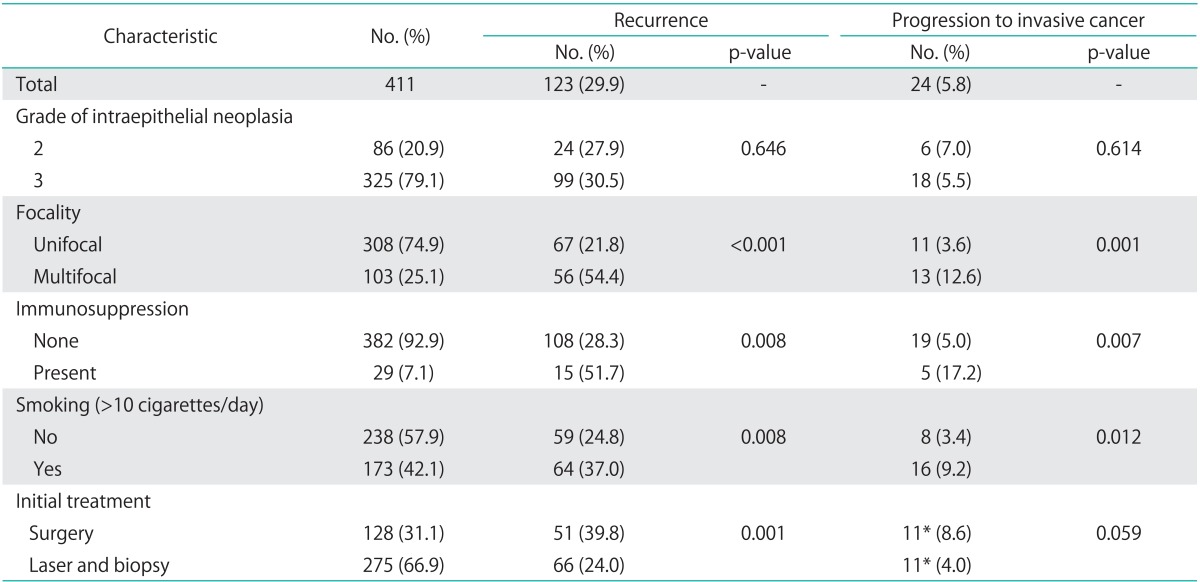
*Two patients who progressed to invasive cancer underwent medical treatment as initial treatment. They both had a recurrence prior to progression which was treated by laser surgery in one case and surgical excision in the other.
Initial treatment during the first year after diagnosis was surgical biopsy combined with CO2 laser evaporation in 270 patients (65.7%), surgical excision alone in 114 patients (27.7%), and vulvectomy in 19 patients (4.6%). Laser evaporation was used consistently throughout the study period, although a peak was noted in the early part of the decade 2000. The use of excisions peaked in the middle of the 1990s, yet continued to be used on a relatively frequent basis in more recent years. For eight patients (1.9%), the initial treatment within the first 12 months was photodynamic therapy or local drug application such as imiquimod. Mean follow-up time was 85 months (±56; range, 13 to 389 months).
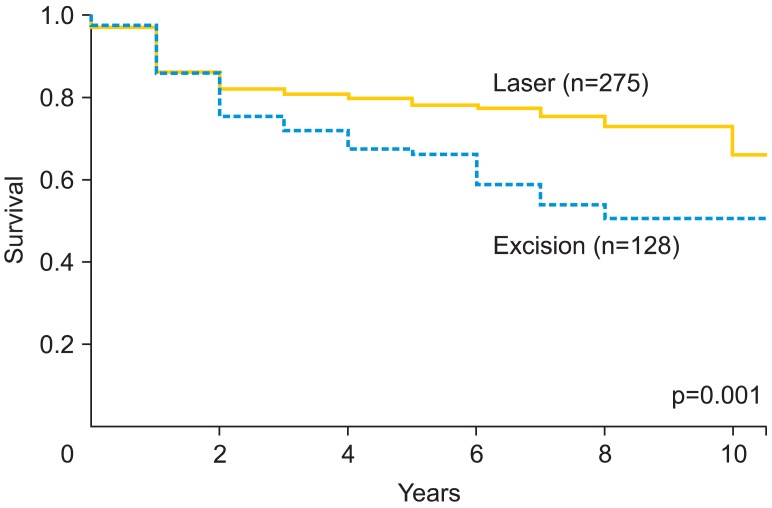
Recurrence occurred later than one year after initial diagnosis in 123 patients (29.9%), and 24 patients (5.8%) progressed to invasive disease. One of 30 women (3.3%) with an initial diagnosis of VAIN progressed to invasive cancer, and 23 of 377 patients (6.1%) with VIN progressed (p=0.999). The types of cancer detected during follow-up were vulvar (16), vaginal (4), and perianal (4). In the 241 patients with a follow-up longer than five years, 34.9% (84) were still experiencing recurrences. Based on multivariate analyses, risk factors associated with recurrence included immunosuppression, multifocal disease, excision as initial treatment and smoking (Table 2). Furthermore, risk factors for progression to invasive disease were immunosuppression, multifocal disease and smoking. The survival analysis for recurrence of IN by initial treatment modality (excision versus biopsy with laser evaporation) indicated a more favorable outcome for those patients treated with laser evaporation (Fig. 1).
Table 2.
Multivariate regression analysis for the development of recurrence or invasive disease more than 12 months after initial diagnosis of intraepithelial neoplasia
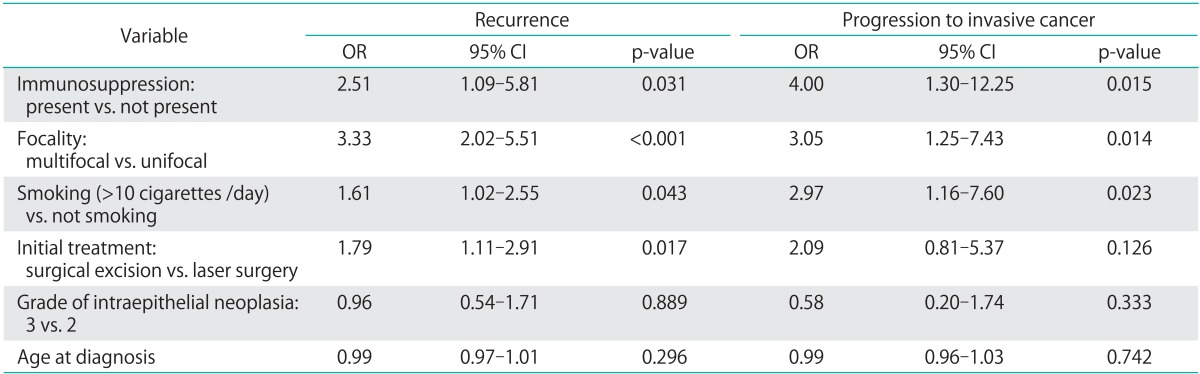
CI, confidence interval; OR, odds ratio.
Fig. 1.
Recurrence-free survival analysis of vulvovaginal intraepithelial neoplasia by initial treatment modality of either laser evaporation or surgical excision.
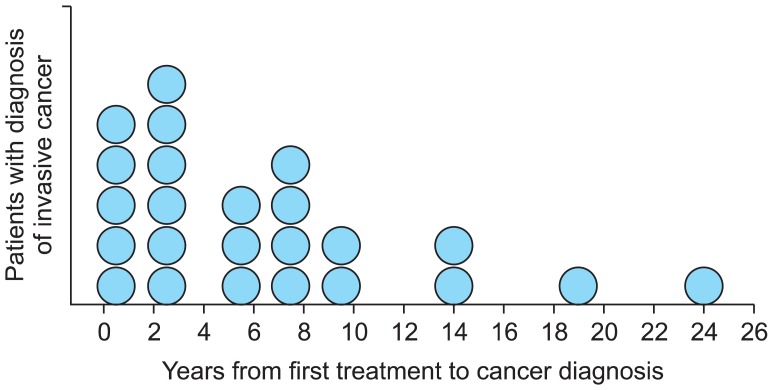
The mean time from first diagnosis to invasive disease was 82 months (±74; range, 13 to 290 months). However, as shown in Fig. 2, two peaks were observed: the first peak was within the second and third years after initial treatment and the second peak was within the seventh and eighth years. In 46% (11/24) of patients with progression to invasive disease, cancer was diagnosed within the first three years, whereas disease progression occurred five years or later after initial diagnosis in 54% (13/24). Twenty-two of the 24 patients developing invasive cancer had a second treatment for recurring IN before cancer diagnosis. Cancer stages were as follows: 14 patients had microinvasive vulvar cancer (pT1a), three patients had pT1b pN0 vulvar cancer, three patients had pT2 pN0 vulvar cancer, three patients had T1 pN0 vaginal cancer and one patient had pT1, pN1 vaginal cancer. The patient with node positive vaginal cancer died of disease at age forty.
Fig. 2.
Years to invasive disease after first treatment for intraepithelial neoplasia (n=24).
DISCUSSION
Treatment of vulvovaginal IN is associated with high recurrence rates. A systematic review of 3,322 published patients with VIN III showed a recurrence rate of 19% after vulvectomy, 18% after partial vulvectomy, 22% after local excision, and 23% after laser evaporation [6]. The higher recurrence rate observed in our study (29.9%) may be due to the substantially longer mean follow-up time of 85 months compared to 39 months in the above-mentioned review. In addition, our results are consistent with the results of a recently published prospective study on VIN recurrence rates [4]. We do not think that our higher recurrence rate is overestimated since our definition of recurrence was histologically proven disease more than 12 months after initial diagnosis. Hence, an incomplete excision followed by re-excision within a year was counted as one treatment. Additionally, 34.9% of those patients with long follow-up periods had recurrences at least five years after initial treatment, which highlights the importance of maintaining a long follow-up period for this disease.
Other researchers have found significantly lower recurrence rates if the surgical margins were free of IN (17%) than with involved margins (47%) [6,11]. Due to the long retrospective time frame of our study, we were not able to re-examine the excised tissue specimen. Initial treatment subsumed all treatments within the first year after initial diagnosis. Therefore, we had patients who had local excision as first treatment followed by laser evaporation due to involved margins. In this case, initial treatment was considered to be laser evaporation since both treatments were within the first year. We chose this definition since many patients had some sort of incomplete treatment prior to referral to our centers and they were immediately retreated until free margins were achieved.
Herod et al. [12] reported a higher recurrence rate in 133 women with high-grade VIN following laser evaporation than after local excision (75% vs. 40%). Moreover, the systematic review by van Seters et al. [6] did not find a significant difference between these two treatment modalities. In contrast, our study showed superior treatment results for laser evaporation with a 24% recurrence rate compared to 39.8% for surgical excision (p=0.001). In multivariate analyses, this result was confirmed. Again, we believe that the longer follow-up time in our study might explain these differences. We consider this result important since normal morphology are often better preserved using laser treatment than using surgical excision. Since this is a retrospective cohort study, treatment results may have been influenced by selection bias. Selection bias would imply that more severe, larger or multifocal IN lesions would have been treated by excision resulting in a higher recurrence rate of this treatment modality. However, in practice, larger or multifocal lesions are more likely to be treated by biopsy and laser evaporation in order to maintain normal vulvar morphology.
Besides initial treatment, this analysis identified other independent risk factors for recurrence including cigarette smoking, immunosuppression and multifocality. Multifocal disease has been described as a risk factor for recurrence in other studies [8,13-15]. Multifocal IN is known to be HPV-related, whereas surgical treatment of the visible lesion may not clear the HPV-infection [16,17]. Accordingly, persistence of HPV infection has been described as a risk factor for recurrence of VIN [13]. Immunosuppression is a known risk factor for both occurrence and recurrence of IN of the lower anogenital tract [18-21].
Cigarette smoking is among the most well established HPV cofactors in the etiology of cervical and vulvar cancer [22]. The biological mechanisms whereby cigarette smoking increases cervical and vulvar cancer risk remain largely undetermined. One possibility is that smoking enhances immunosuppression since constituents of cigarette smoke and their metabolites are present in the cervical mucus and depress populations of cervical Langerhans cells and T lymphocytes [23-25]. The risk associated with smoking is modified by genetic variation in Interleukin 2 which propagates a T lymphocyte-mediated immune response to HPV and tumor antigens [26]. On the other hand, smoking cessation has been shown to have an effect on colposcopic lesion size of CIN grade 1 or less [27]. However, to the best of our knowledge, the present study is the first to demonstrate the risk of smoking on recurrence and progression of VIN and VAIN using a multivariate analysis. Smokers had a 1.6 and 3 times higher risk of recurrence and progression to invasive cancer, respectively.
Progression to cancer at least one year after initial treatment occurred in 5.8% of our treated patients. This is consistent with findings from Jones et al. [8] who observed a progression rate of 3.8% in 342 treated patients, with half of the women followed for more than five years. In a population-based analysis of the Cancer Registry of Norway, 3.4% of 468 women with VIN developed invasive squamous cell carcinoma of the vulva later than seven months after initial diagnosis of VIN [28]. In this study, it is not clear if invasive recurrences in the vaginal or anal skin did not occur or were not considered.
Fifty-four percent of invasive cancers in our study were diagnosed more than five years after initial diagnosis of IN. Jones et al. [8] proposed the term "treatment failure" for invasive cancer which develops within seven years following initial diagnosis of IN and "new field cancers" for cancers occurring later. As in our series, about half of invasive cancers were considered to be "new field cancers" in the latter study. Independent risk factors for the development of an invasive cancer of the vulva or vagina were multifocal IN, immunosuppression and smoking. Twenty-two of the 24 patients who experienced invasive cancer had at least one treatment for recurring IN prior to cancer diagnosis. Hence, it seems plausible that risk factors for recurrence and progression to invasive disease overlap. Additionally, immunosuppression and smoking may facilitate the development of "new field cancers" as discussed earlier.
The results of this study are limited by the retrospective design. However, it is the largest dataset of patients with IN of the vulva and vagina on which a multivariate analysis was performed and the follow-up period is long. Since 35% of relapses occurred after five years, maintaining a long follow-up period is necessary. Unfortunately, information about whether patients stopped or continued to smoke after initial diagnosis of IN was not available. However, since our results showed that cigarette smoking increased the risk of recurrence as well as progression to invasive disease, counseling women with genital IN to stop smoking seems advisable. Smoking cessation counseling is feasible in this patient population, and results on the effect of smoking cessation on recurrence and progression rate are eagerly awaited [29].
ACKNOWLEDGMENTS
Supported by a research grant of the University of Zurich, Switzerland. We thank JoEllen Welter, Clinical Research Coordinator, Cantonal Hospital Frauenfeld, for help in statistical analysis of the data.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 2.Jacyntho CM, Giraldo PC, Horta AA, Grandelle R, Goncalves AK, Fonseca T, et al. Association between genital intraepithelial lesions and anal squamous intraepithelial lesions in HIV-negative women. Am J Obstet Gynecol. 2011;205:115.e1–115.e5. doi: 10.1016/j.ajog.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005;50:807–810. [PubMed] [Google Scholar]
- 4.Frega A, Sopracordevole F, Scirpa P, Biamonti A, Lorenzon L, Scarani S, et al. The re-infection rate of high-risk HPV and the recurrence rate of vulvar intraepithelial neoplasia (VIN) usual type after surgical treatment. Med Sci Monit. 2011;17:CR532–CR535. doi: 10.12659/MSM.881941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 6.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97:645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Gynecologic Practice of American College Obstetricians and Gynecologists. ACOG Committee opinion no. 509: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118:1192–1194. doi: 10.1097/AOG.0b013e31823b17c2. [DOI] [PubMed] [Google Scholar]
- 8.Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106:1319–1326. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson RD, Denehy TR. Rapid spontaneous regression of acute-onset vulvar intraepithelial neoplasia 3 in young women: a case series. J Low Genit Tract Dis. 2012;16:56–58. doi: 10.1097/LGT.0b013e31822d93ee. [DOI] [PubMed] [Google Scholar]
- 10.McFadden KM, Sharp L, Cruickshank ME. The prospective management of women with newly diagnosed vulval intraepithelial neoplasia: clinical outcome and quality of life. J Obstet Gynaecol. 2009;29:749–753. doi: 10.3109/01443610903191285. [DOI] [PubMed] [Google Scholar]
- 11.Modesitt SC, Waters AB, Walton L, Fowler WC, Jr, Van Le L. Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstet Gynecol. 1998;92:962–966. doi: 10.1016/s0029-7844(98)00350-0. [DOI] [PubMed] [Google Scholar]
- 12.Herod JJ, Shafi MI, Rollason TP, Jordan JA, Luesley DM. Vulvar intraepithelial neoplasia: long term follow up of treated and untreated women. Br J Obstet Gynaecol. 1996;103:446–452. doi: 10.1111/j.1471-0528.1996.tb09771.x. [DOI] [PubMed] [Google Scholar]
- 13.Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100:271–275. doi: 10.1016/j.ygyno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Kuppers V, Stiller M, Somville T, Bender HG. Risk factors for recurrent VIN: role of multifocality and grade of disease. J Reprod Med. 1997;42:140–144. [PubMed] [Google Scholar]
- 15.Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93–99. doi: 10.1016/s0002-9378(97)80018-x. [DOI] [PubMed] [Google Scholar]
- 16.van Beurden M, ten Kate FJ, Smits HL, Berkhout RJ, de Craen AJ, van der Vange N, et al. Multifocal vulvar intraepithelial neoplasia grade III and multicentric lower genital tract neoplasia is associated with transcriptionally active human papillomavirus. Cancer. 1995;75:2879–2884. doi: 10.1002/1097-0142(19950615)75:12<2879::aid-cncr2820751214>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 18.Petry KU, Köchel H, Bode U, Schedel I, Niesert S, Glaubitz M, et al. Human papillomavirus is associated with the frequent detection of warty and basaloid high-grade neoplasia of the vulva and cervical neoplasia among immunocompromised women. Gynecol Oncol. 1996;60:30–34. doi: 10.1006/gyno.1996.0007. [DOI] [PubMed] [Google Scholar]
- 19.Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 20.Dedes KJ, Beneder C, Samartzis N, Muller MD, Fink D, Fehr MK. Outcome of treated anogenital intraepithelial neoplasia among human immunodeficiency virus-infected women. J Reprod Med. 2008;53:947–951. [PubMed] [Google Scholar]
- 21.Massad LS, Xie X, Darragh T, Minkoff H, Levine AM, Watts DH, et al. Genital warts and vulvar intraepithelial neoplasia: natural history and effects of treatment and human immunodeficiency virus infection. Obstet Gynecol. 2011;118:831–839. doi: 10.1097/AOG.0b013e31821a0f4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis: role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–28. [PubMed] [Google Scholar]
- 23.Sasson IM, Haley NJ, Hoffmann D, Wynder EL, Hellberg D, Nilsson S. Cigarette smoking and neoplasia of the uterine cervix: smoke constituents in cervical mucus. N Engl J Med. 1985;312:315–316. doi: 10.1056/nejm198501313120516. [DOI] [PubMed] [Google Scholar]
- 24.Poppe WA, Ide PS, Drijkoningen MP, Lauweryns JM, Van Assche FA. Tobacco smoking impairs the local immunosurveillance in the uterine cervix. An immunohistochemical study. Gynecol Obstet Invest. 1995;39:34–38. doi: 10.1159/000292372. [DOI] [PubMed] [Google Scholar]
- 25.Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1988;2:652–654. doi: 10.1016/s0140-6736(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SK, Madeleine MM, Johnson LG, Du Q, Malkki M, Wilkerson HW, et al. Cervical and vulvar cancer risk in relation to the joint effects of cigarette smoking and genetic variation in interleukin 2. Cancer Epidemiol Biomarkers Prev. 2008;17:1790–1799. doi: 10.1158/1055-9965.EPI-07-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szarewski A, Jarvis MJ, Sasieni P, Anderson M, Edwards R, Steele SJ, et al. Effect of smoking cessation on cervical lesion size. Lancet. 1996;347:941–943. doi: 10.1016/s0140-6736(96)91417-8. [DOI] [PubMed] [Google Scholar]
- 28.Iversen T, Tretli S. Intraepithelial and invasive squamous cell neoplasia of the vulva: trends in incidence, recurrence, and survival rate in Norway. Obstet Gynecol. 1998;91:969–972. doi: 10.1016/s0029-7844(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 29.Santoso JT, Crigger M, English E, Wan J, Likes W. Smoking cessation counseling in women with genital intraepithelial neoplasia. Gynecol Oncol. 2012;125:716–719. doi: 10.1016/j.ygyno.2012.02.018. [DOI] [PubMed] [Google Scholar]