Abstract
Objective
To analyze the prognostic factors related to the recurrence rate of vulvar cancer.
Methods
Retrospective study of 87 patients diagnosed of vulvar squamous cell carcinoma diagnosed at a tertiary hospital in Madrid between January 2000 and December 2010.
Results
The pathological mean tumor size was 35.1±22.8 mm, with stromal invasion of 7.7±6.6 mm. The mean free margin after surgery was 16.8±10.5 mm. Among all patients, 31 (35.6%) presented local recurrence (mean time 10 months; range, 1 to 114 months) and 7 (8%) had distant metastases (mean time, 5 months; range, 1 to 114 months). We found significant differences in the mean tumor size between patients who presented a relapse and those who did not (37.6±21.3 mm vs. 28.9±12.1 mm; p=0.05). Patients with free margins equal or less than 8 mm presented a relapse rate of 52.6% vs. 43.5% of those with free margin greater than 8 mm (p=0.50). However, with a cut-off of 15 mm, we observed a local recurrence rate of 55.6% vs. 34.5%, respectively (p=0.09). When the stromal invasion cut-off was >4 mm, local recurrence rate increased up to 52.9% compared to 37.5% when the stromal invasion was ≤4 mm (p=0.20).
Conclusion
Tumor size, pathologic margin distance and stromal invasion seem to be the most important predictors of local vulvar recurrence. We consider the cut-off of 35 mm of tumor size, 15 mm tumor-free surgical margin and stromal invasion >4 mm, high risk predictors of local recurrence rate.
Keywords: Margin distance, Prognostic factors, Recurrence rate, Vulvar carcinoma
INTRODUCTION
Vulvar cancer represents 5% of gynecologic cancers and 1% of all cancers in women, with an incidence of 1-2/100,000 women [1]. It classically affects to older women, with an average age of 65-70 years [2], although lastly has been detected an increase in the incidence among women younger than 50 years old [3].
Ninety percent of vulvar cancers are squamous cell carcinomas and the remaining 10% includes a wide variety of tumors such as melanoma, adenocarcinoma of Bartholin gland and Paget disease [4]. Survival rates are related to inguinal nodal status at the time of diagnosis. In patients with operable disease without nodal involvement, the overall survival (OS) is 90%; however, in patients with nodal involvement, the 5-year OS rate is approximately 50%-60% [5].
The most important risk factors for node metastasis are the clinical node status, age, differentiation grade, tumor stage, tumor size, depth stromal invasion and presence of lymphovascular space invasion [6]. The survival rate of vulvar cancer has increased in the last decades due to the introduction of radical vulvectomy en bloc with inguinofemoral and pelvic lymphadenectomy, replacing the simple excision of the lesion. However, due to the high morbidity of this surgery, in the last 20 years the treatment of vulvar cancer has moved to a more conservative and tailored treatment [7].
The first step in reducing morbidity was performed by Byron, who introduced the triple incision technique, which showed same oncological outcomes, with a recurrence of the skin bridge at only 2.4%, but a greater morbidity reduction [8]. Posteriorly, the implementation of tumoral wide excision and the sentinel node (SN) biopsy, a great reduction in morbidity has been achieved. The current standard of pathological margin of at least 8 mm in patients with early stage disease has added benefits to this aspect [7,9-11]. The objective of our study was to evaluate the prognostic factors associated with local recurrence in squamous cell carcinoma of the vulva.
MATERIALS AND METHODS
After Institutional Review Board approval, a retrospective study of patients diagnosed of vulvar cancer was performed. We found 96 patients treated at La Paz University Hospital in Madrid from January 2000 until December 2010, although we selected just 87 vulva epithelial tumors (90.6%) [12]. Nine were excluded, because we found 4 melanomas, 3 sarcomas, 1 granular tumor, and 1 gastrointestinal mesenchymal tumor. Patients with microinvasive carcinoma or intraepithelial neoplasia were excluded as well. For staging purposes the 2009 International Federation of Gynecology and Obstetrics (FIGO) classification was used [13].
The preoperative evaluation included a general examination, complete blood count and coagulation, biochemistry, chest x-ray and electrocardiogram. All patients underwent gynecological examination, pelvic ultrasound, cervical cytology and urinary cytology if the tumor had urethral involvement. A colposcopy was carried out if cytology was pathologic and vulvoscopy in selected cases. MRI and/or abdominal-pelvic CT scan, as well as PET and bone scan was considered in advanced stages (II-IV). Colonoscopy and cystoscopy in cases of suspected rectal and bladder invasion, respectively.
The surgery included lesion excision with radical vulvectomy (including excision of the vulvar skin and subcutaneous tissue to the fascia lata), hemivulvectomy, simple vulvectomy or wide excision (at least with 1-cm of macroscopic free margin) if possible depending on the extent and location of the disease. SN biopsy was performed by means of instillation of blue dye in cases which satisfied the next criteria: single squamous cell carcinoma of the vulva with infiltration >1 mm, tumoral size <4 cm, negative inguinal exploration or suspicious inguinal nodes <1.5 cm diameter in imaging techniques (CT-scan or MRI). Systematic inguinal uni- or bilateral lymphadenectomy was performed if contraindication to SN biopsy or positive result. Inguinal lymphadenectomy was performed according to the "classic" technique (including superficial and deep inguinal node dissection) until 2004 (17 cases). Posteriorly just superficial inguinal lymphadenectomy (without opening the cribriform fascia) was performed. Inguinal #12 Blake drains placement and compressive dressing in all patients. The patients were administered anti-thrombotic prophylaxis the first 3-5 days with subcutaneous enoxaparin 40 mg/24 hours.
Adjuvant radiotherapy was performed in the surgical patients with pathologic margins affected or when the tumor spread to urethra or anus. Pelvic radiotherapy was carried out in patients with more than one metastatic lymph node with cumulative dose of 50.4 Gy. The surgical specimen was examined after fixation in formalin and all specimens were embedded in paraffin. The distance of the margins was determined from tissue sections stained with hematoxylin and eosin. To ensure accurate measurements, multiple sections at the closest margin to the lesion were performed. The free margin of the nearest tumor was established taking into account the pathological margins in both the deep (basal) and the peripheral tissue. The term "surgical margin" refers to the distance between the lesion and the surgical incision, which corresponds to disease-free tissue. The follow-up of patients consisted in general and gynecological examination, and vulvar and cervical cytology biannually the first five years, and then, once a year. An annual CT-scan was also considered. Recurrence was defined as the appearance of tumor in a new location after treatment, or in the same location after a minimum disease-free period of 6 months.
Concerning statistical analysis, normally distributed quantitative data were presented with mean±SD while asymmetric data are presented with median and range. Qualitative variables were presented with absolute values and percentages. Quantitative data between groups were compared using the Student t-test and ANOVA. Categorical variables were compared using chi-square test. Survival analysis was performed using Kaplan-Meier curves.
RESULTS
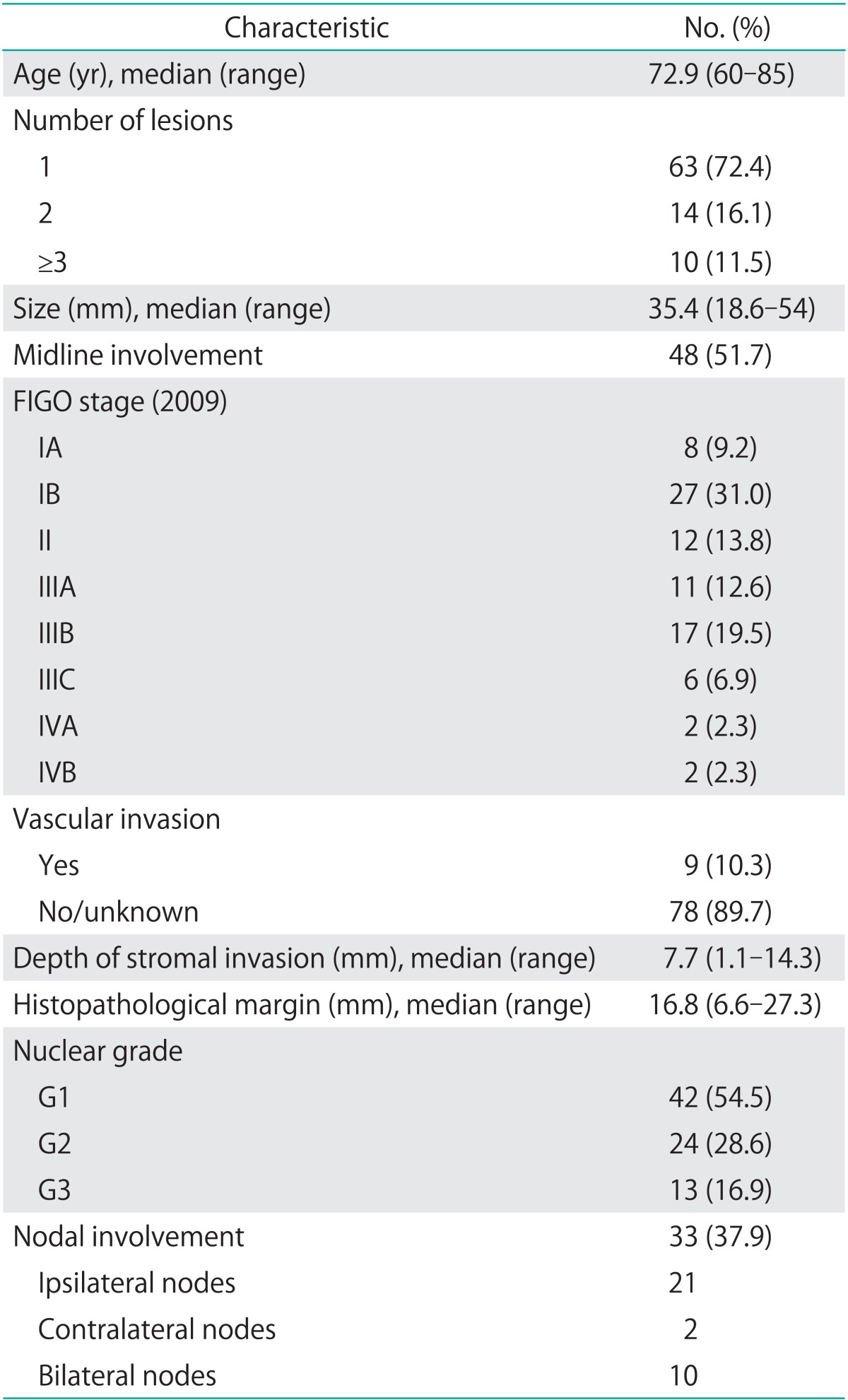
The charts of 87 patients were analyzed. Clinicopathological and surgical characteristics are summarized in Table 1. The mean age at diagnosis was 72.9±12.1 years. Regarding the histological subtypes (according to WHO 2003 classification) we observed: 84 squamous tumors (96.6%), 1 glandular tumor (1.1%), and 2 tumors of skin appendage origin (2.3%). Among all squamous we found 67 keratinizing, 2 non-keratinizing, 5 basaloids, 4 verrucous, 1 bowenoid, 1 sarcomatoid, and 4 basal cell carcinomas. The glandular one corresponded to a Paget disease, and the last two were 1 sebaceous carcinoma and 1 malignant sweat gland tumor.
Table 1.
Clinicopathologic and surgical characteristics
FIGO, International Federation of Gynecology and Obstetrics.
Thirty one patients (35.6%) had previous vulvar pathology and 2 patients (2.3%) presented previous cervical disease (one cervical carcinoma treated 40 years before without relapse, and one HG-SIL). The lesions had an average size of 35.4±18.6 mm in diameter, among them 51.7% affected the midline. Inguinal exploration was positive in 41.4% of patients. When we compared the rate of inguinal positivity to involvement of midline, we observed that inguinal positivity was significantly higher in patients with midline involvement (62.2% vs. 19%, respectively; p<0.001). However, we did not find significant differences between the relapse rate when midline was affected compared to when it was free of tumor (42.9% vs. 48.5%, respectively; p=0.64). Regarding surgical procedures, radical vulvectomy was carried out in 58.6% of patients, simple vulvectomy in 9.2%, hemivulvectomy in 2.3% and local wide excision in 29.9%.
SN biopsy was carried out in 19 patients (21.8%), 21.1% of them were positive and all ipsilateral to the lesion. Inguinal lymphadenectomy was performed in 55 patients (63.2%), 7 of them (12.7%) unilaterally and 47 (87.3%) bilaterally. The great majority of lymphadenectomies were performed with separate incisions 49 (89.1%). On the one hand, 17 cases included complete removal of deep inguinal nodes (6 by single incision and 11 by separate ones). Among these patients, there were 6 dehiscence of the suture (35%) and 2 surgical wound infections (11%). Among patients with deep lymphadenectomy 9 (52%) relapsed, 8 in the vulva and one at inguinal level. On the other hand, 34 superficial lymphadenectomy were performed, all with separate incisions. Fifteen patients (44.1%) experimented a suture dehiscence and 5 patients (14.7%) wound infections. There were 23 recurrences in this group (57.5%), 16 in the vulva and 4 of them were inguinal relapses. There were no significant differences between the two kind of inguinal lymphadenectomies in terms of relapses (p=0.31), location of the recurrence (p=0.26), dehiscences (p=0.55) or wound infections (p=0.77). Among the 5 inguinal relapses. 2 of them were contralateral recurrences after unilateral inguinal lymphadenectomies (one of them included inguinal radiation therapy). Among the other three patients, 2 underwent bilateral lymphadenectomy and adjuvant radiotherapy, but the last one did not undergo lymph node dissection or radiation therapy.
Adjuvant radiotherapy was administered in 35 patients (40.2%; all with positive inguinal nodes) with a dose of 50.4 Gy. Radiation therapy was focused on pelvic, inguinal and vulvar areas in 34 patients (97.1%) and only one exclusively in the surgical site for comorbidity. Just adjuvant cisplatinum-based chemotherapy was administered in 3 patients (3.4%).
Thirty six patients (41.4%) experienced postoperative complications. The mean time from surgery to the adverse event was 11.2±7.2 days. Among all complications, 31 (86.1%) were during the first 30 postoperative days. The most common complication was surgical wound dehiscence that was observed in 22 patients (61.1%) and its association to inguinal wound infection than occurred in 9 additional cases (25%). The pathological mean tumor size was 35.1±22.8 mm, with a mean stromal invasion of 7.7±6.6 mm. The mean distance from the free margin in the surgical specimen was 16.8±10.5 mm. Only 10.3% of cases presented positive lymphovascular space invasion. Nuclear grade differentiation was G1 in 54.5%, G2 in 28.6% and G3 in 16.9%. Mean follow-up of patients was 32.3±30.7 months after surgery, with 25% of patients followed longer than 5 years. Fifty-six patients (64.4%) at the last contact remained free of disease, 16 (18.4%) were alive with disease and 15 patients (17.2%) died. Two patients died of other disease and 13 due to their vulvar cancer.
Among all patients, we observed 31 local recurrences (35.6%) and 7 distant metastases (8%). The median time from surgery until the appearance of the first local recurrence was 10 months (range, 1 to 114 months) and until the appearance of distant metastases 5 months (range, 1 to 114 months). Among all local recurrences, 8 (25.8%) were inguinal relapses and the rest in the surgical site. Distant metastases distributed as follows: 2 in the lung, 2 in femoral vessels, 2 pelvic, and 1 in para-aortic nodes. Among the 31 local recurrences, 19 (61.3%) were treated with surgical excision, 8 (25.8%) with radiotherapy, and 4 (12.9%) with chemotherapy. Regarding the management of the 7 distant metastases, only one was amenable to surgery with posterior radiation therapy and chemotherapy, and the rest were treated with palliative radio- or chemotherapy.
When exploring the characteristics of the lesions related to recurrence status, we found significant in the average size. The group of patients who did not relapse had a mean size of 28.9±12.1 mm vs. 37.6±21.3 mm of those who did (p=0.05). The number of lesions did not influence the rate of recurrence, we found a mean number of lesions similar in both groups: 1.2±0.5 lesions in non-recurrence group compared to 1.5±0.9 lesions in the recurrence group (p=0.15). No significant differences were found in the rate of local recurrence in patients with single lesion versus multiple lesions (42% vs. 55.6%, respectively; p=0.32), but it seemed to be higher in the group of multiple lesions.
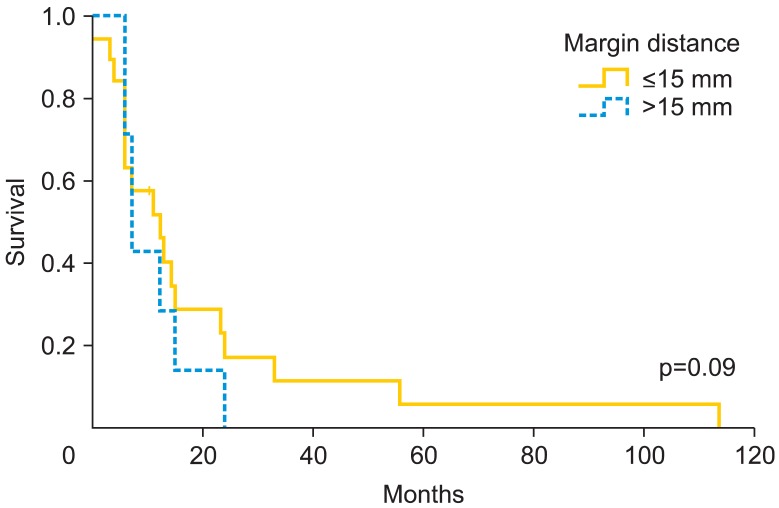
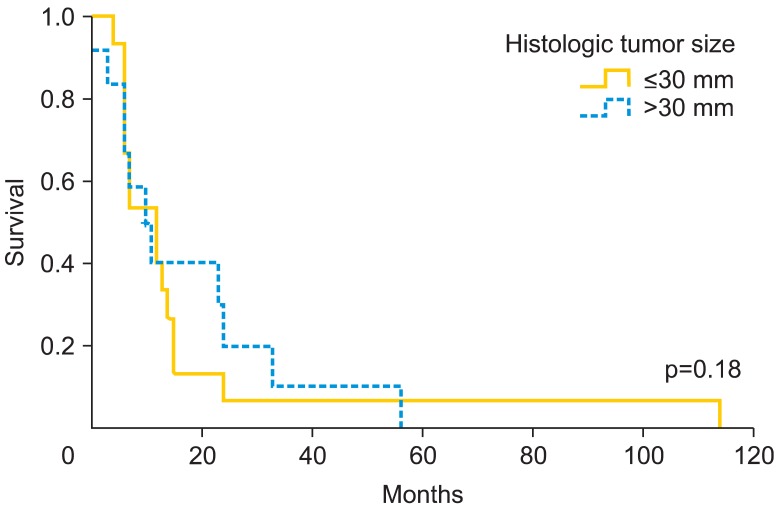
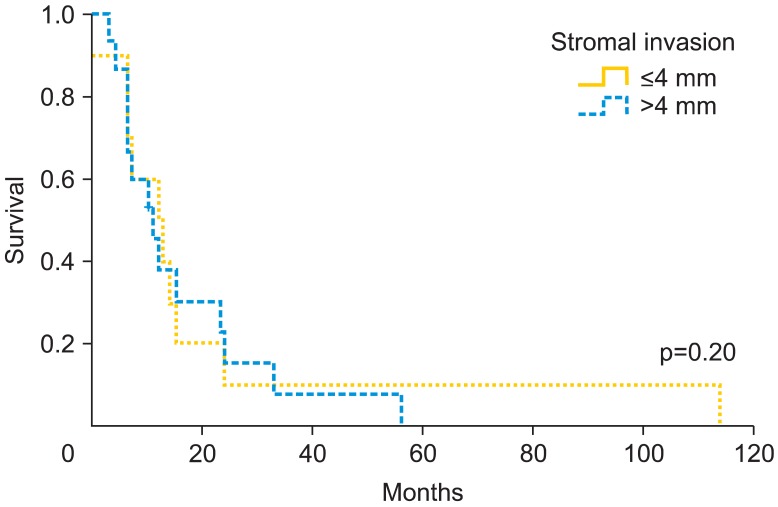
Patients who underwent radical vulvectomy presented higher relapse rate than those who underwent simple vulvectomy (55% vs. 33.3%, respectively; p=0.27). In fact, there were significant differences regarding the type of treatment according to FIGO staging [13]. Among patients who underwent simple vulvectomy, 75% were early stage (FIGO I-II), while those who underwent radical vulvectomy only the 47.1% were early stage (p<0.001). Patients with unilateral lymphadenectomy recurred locally in the 85.7% vs. those with bilateral 47.2% (p=0.062). We did not find significant differences in the rate of nodal positivity according to the laterality, unilateral inguinal lymphadenectomy presented 50% of positive lymph nodes compared to 62.5% in bilateral lymphadenectomy (p=0.55). If we analyze the recurrence rate according to nodal positivity, we did not find significant differences (60% recurrence if positive vs. 37.5% if negative; p=0.12). Despite finding no significant differences (p=0.18), patients with tumors larger than 30 mm have a 16.6% of increase in local recurrence. Moreover, local recurrence related to stromal invasion increases at the cut-off >4 mm. We observed a relapse rate of 52.9% compared to 37.5% when stromal invasion was ≤4 mm (p=0.2). Patients with free margins of 8 mm or less relapsed 52.6% vs. 43.5% with more than 8 mm (p=0.5). However, at cutoff of 15 mm, the local recurrence rate was 55.6% vs. 34.5% (p=0.09). The Kaplan-Meier curves on local relapse didn't showed statistical significance with the Mantel-Cox test (Figs. 1-3). Moreover, we observed significant higher recurrence rate among patients who did receive radiation therapy (61.5% vs. 35.7%, respectively; p=0.038).
Fig. 1.
Recurrence-free survival rate according to pathologic tumor-free margin.
Fig. 3.
Recurrence-free survival rate according to tumor size.
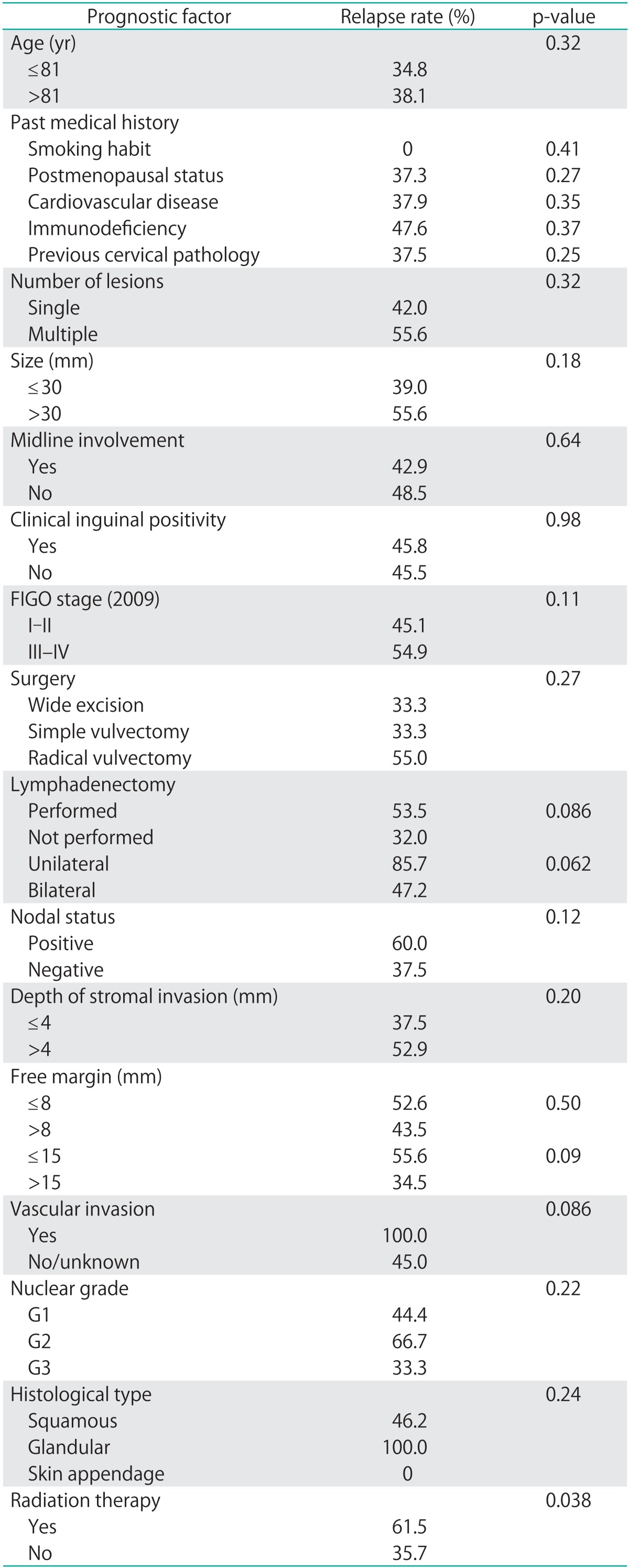
All studied prognostic factors have been summarized in Table 2.
Table 2.
Relapse rates according to the different prognostic factors
DISCUSSION
To our knowledge, there are few articles in the literature that have examined the patterns of recurrence derived from longer series of vulvar carcinoma than ours [14,15]. Current literature [9-17] sets that tumor size, the greater stromal invasion and the lower free margin of resection are considered prognostic factors of recurrence. However, it is not clearly defined the limits to consider each patient on high risk for recurrence.
In our data, we observed a significant 8.9 mm larger mean size in patients who relapsed. Moreover, patients with tumors larger than 30 mm have a 16.6% of increase in recurrence rate. Chan et al. [9] considered high risk patients at a tumor size >50 mm, with a 5-year survival of 88.5% for patients with tumors ≤50 mm and 59.2% for tumor size >50 mm. The Spanish Society of Obstetrics and Gynecologist (SEGO) considers 40 mm the limit between tumors with high and low risk [18], and Maggino et al. [15] set the cut-off at 20 mm, finding a relative risk of 1.2 (p=0.001) for relapse. We consider 30 mm would be a reasonable limit between high and low risk, additionally, with the implementation of wide excision in most of the vulvar cancer, we try to leave a 2 cm macroscopic limit, so in the present situation if we consider the pathological limit greater than 30 mm, they would be al included among high risk artificially. Regarding stromal invasion, we know that over 4 mm local recurrence rate increases from 52.9% until 37.5%. Although in our study, the differences were not significant, we think it could be an additional risk factor for recurrence. The FIGO staging system [13] sets the cutoff point to increase the stage from IA to IB in 1 mm, and Maggino et al. [15] considered better cutoff at 3 mm, with a relative risk of 1.5 (p=0.03). In the same line, Chan et al. [9] observed a 5-year survival of 92.2% when the invasion was less than 4 mm and 73.2% when higher. In our opinion, and according to Chan [9] data, stromal invasion over 4 mm could increase the risk of recurrence. Previous reports that have evaluated the role of surgical free margins in vulvar cancer have shown contradictory results, although the most recommended macroscopic free distance in literature is 2-3 cm [7,9,16,17]. However, data from pathologic reports published by Heaps et al. [10], seemed to indicate that a tumor-free margin >10 mm could be safe in terms of recurrence rate. Chan et al. [9] analyzed 90 vulvar cancers with no local recurrences in the group of patients with a histological free margin >8 mm, whereas a 23% was observed if lower margin. De Hullu et al. [11] focused on relapses at the level of the inguinal skin bridge and reported a mild increase after less radical procedures compared with radical vulvectomy en bloc with lymphadenectomy in women with T1 and T2 lesions. Furthermore, these authors observed that a surgical macroscopic margin of 10 mm corresponded to a histological margin under 8 mm in 50% of cases increasing the rate of local recurrence, same as results reported by Palaia et al. [16] who correlated the macroscopic margin of 10 mm to microscopic of 8 mm. Because of that, it is recommended to obtain a macroscopic surgical margin greater than 2 cm [11] to minimize the chance of obtaining a pathologic margin under 10-15 mm. In contrast, Routzier et al. [19] did not find the margins of resection a significant risk factor for local recurrences as well as Groenen et al. [17] with a cut-off at 8 mm. Tantipalakorn et al. [20] described also same recurrence rates and OS related to free margins groups. In our study, we did not find significant differences at 8 mm. However, when we rise the limit up to 15 mm, closer to macroscopic intended margin, we observed that that the local recurrence rate decreased 21.1% (p=0.09). Our results could be explained by the fact that local recurrences do not depend only on the free margins, but also on multiple associated factors like tumor size, lymph node affectation and stromal invasion. These results could be also explained by an inadequate or different review method of free margins in the specimens, although we consider it an unlikely possibility. Due to the evidence collected, we consider reasonable to try to achieve a macroscopic margin during surgery at least of 20 mm, to approximate as much as possible to a microscopic free margin of 15 mm, which we consider an accurate cut-off to avoid local recurrences.
We observed that patients with unilateral lymphadenectomy recurred locally almost double than those with bilateral lymphadenectomy. This finding could be explained because among those 6 patients with unilateral lymphadenectomy that relapsed (85.7%), we found 3 of them treated with wide excision and positive sentinel lymph node that could affect to the rate of contralateral recurrence. And also we observed 2 cases with tumoral size over 3 cms. which could also affect to the higher rate of contralateral inguinal spread. Our findings agree with previous data reported where no correlation was observed between lymph node metastasis and localization of recurrent disease mostly in vulvar region [21].
Regarding the higher recurrence rate among radiated patient, we could explain it because that group of patients presented 85.8% of advanced FIGO stage, compared to a 15.8% among the patients who did not received radiation therapy; this fact, influenced dramatically on the relapse rate. Moreover, our data showed 54.9% of relapse rate among FIGO III-IV stages compared to 45.1% of early stages.
In conclusion tumoral size, pathologic margin distance and stromal invasion seem to be the most important predictors of local vulvar recurrence. We consider the cut-off of 35 mm of tumoral size, 15 mm tumor-free pathological margin and stromal invasion >4 mm, high risk predictors of local recurrence rate.
Fig. 2.
Recurrence-free survival rate according to stromal invasion.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hacker NF. Vulvar cancer. In: Berek JS, Hacker NF, editors. Practical gynecologic oncology. 4th ed. Philadelphia: Williams & Wilkins; 2005. pp. 585–602. [Google Scholar]
- 2.Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, et al. Carcinoma of the vulva: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–S27. doi: 10.1016/S0020-7292(06)60028-3. [DOI] [PubMed] [Google Scholar]
- 3.Joura EA. Epidemiology, diagnosis and treatment of vulvar intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2002;14:39–43. doi: 10.1097/00001703-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Finan MA, Barre G. Bartholin's gland carcinoma, malignant melanoma and other rare tumours of the vulva. Best Pract Res Clin Obstet Gynaecol. 2003;17:609–633. doi: 10.1016/s1521-6934(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 5.Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study) Am J Obstet Gynecol. 1991;164:997–1004. doi: 10.1016/0002-9378(91)90573-a. [DOI] [PubMed] [Google Scholar]
- 6.Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study) Gynecol Oncol. 1993;49:279–283. doi: 10.1006/gyno.1993.1127. [DOI] [PubMed] [Google Scholar]
- 7.de Hullu JA, van der Zee AG. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006;60:38–58. doi: 10.1016/j.critrevonc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Woelber L, Choschzick M, Eulenburg C, Hager M, Jaenicke F, Gieseking F, et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann Surg Oncol. 2011;18:3811–3818. doi: 10.1245/s10434-011-1778-0. [DOI] [PubMed] [Google Scholar]
- 9.Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol. 2007;104:636–641. doi: 10.1016/j.ygyno.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38:309–314. doi: 10.1016/0090-8258(90)90064-r. [DOI] [PubMed] [Google Scholar]
- 11.De Hullu JA, Hollema H, Lolkema S, Boezen M, Boonstra H, Burger MP, et al. Vulvar carcinoma: the price of less radical surgery. Cancer. 2002;95:2331–2338. doi: 10.1002/cncr.10969. [DOI] [PubMed] [Google Scholar]
- 12.Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 13.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Podratz KC, Symmonds RE, Taylor WF. Carcinoma of the vulva: analysis of treatment failures. Am J Obstet Gynecol. 1982;143:340–351. doi: 10.1016/0002-9378(82)90823-7. [DOI] [PubMed] [Google Scholar]
- 15.Maggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva: a multicenter CTF Study. Cancer. 2000;89:116–122. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Palaia I, Bellati F, Calcagno M, Musella A, Perniola G, Panici PB. Invasive vulvar carcinoma and the question of the surgical margin. Int J Gynaecol Obstet. 2011;114:120–123. doi: 10.1016/j.ijgo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Groenen SM, Timmers PJ, Burger CW. Recurrence rate in vulvar carcinoma in relation to pathological margin distance. Int J Gynecol Cancer. 2010;20:869–873. doi: 10.1111/IGC.0b013e3181df7423. [DOI] [PubMed] [Google Scholar]
- 18.Spanish Society of Obstetrics and Gynecologist. Squamous cell invasive vulvar cancer: oncological guidelines. Madrid: SEGO; 2010. [Google Scholar]
- 19.Rouzier R, Morice P, Haie-Meder C, Lhomme C, Avril MF, Duvillard P, et al. Prognostic significance of epithelial disorders adjacent to invasive vulvar carcinomas. Gynecol Oncol. 2001;81:414–419. doi: 10.1006/gyno.2001.6198. [DOI] [PubMed] [Google Scholar]
- 20.Tantipalakorn C, Robertson G, Marsden DE, Gebski V, Hacker NF. Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet Gynecol. 2009;113:895–901. doi: 10.1097/AOG.0b013e31819b413f. [DOI] [PubMed] [Google Scholar]
- 21.Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, et al. Clinicopathological prognostic factors andpatterns of recurrence in vulvar cancer. Anticancer Res. 2009;29:545–552. [PubMed] [Google Scholar]