Abstract
Objective
To determine the efficacy, progression-free survival (PFS) and overall survival (OS) for the combination of intravenous bevacizumab and oral cyclophosphamide in heavily pretreated patients with recurrent ovarian carcinoma.
Methods
A retrospective review was performed for all patients with recurrent ovarian carcinoma treated with intravenous bevacizumab 10 mg/kg every 14 days and oral cyclophosphamide 50 mg daily between January 2006 and December 2010. Response to treatment was determined by Response Evaluation Criteria in Solid Tumors criteria and/or CA-125 levels.
Results
Sixty-six eligible patients were identified. Median age was 53 years. Fifty-five patients (83%) had undergone optimal cytoreduction. All patients were primarily or secondarily platinum resistant at the time of administration of bevacizumab and cyclophosphamide. The median number of prior chemotherapy treatments was 6.5 (range, 3 to 16). Eight patients (12.1%) had side effects which required discontinuation of bevacizumab and cyclophosphamide. There was one bowel perforation (1.5%). Overall response rate was 42.4%, including, complete response in 7 patients (10.6%), and partial response in 21 patients (31.8%), while 15 patients (22.7%) had stable disease and 23 patients (34.8%) had disease progression. Median PFS for responders was 5 months (range, 2 to 14 months). Median OS from initiation of bevacizumab and cyclophosphamide was 20 months (range, 2 to 56 months) for responders and 9 months (range, 2 to 51 months) for non-responders (p=0.004).
Conclusion
Bevacizumab and cyclophosphamide is an effective, well-tolerated chemotherapy regimen in heavily pretreated patients with recurrent ovarian carcinoma. This combination significantly improved PFS and OS in responders. Response rates were similar and favorable to the rates reported for similar patients receiving other commonly used second-line chemotherapeutic agents.
Keywords: Anti-angiogenic therapy, Bevacizumab, Cyclophosphamide, Platinum resistant ovarian carcinoma, Recurrent ovarian carcinoma
INTRODUCTION
In 2012, approximately 22,280 women in the United States were diagnosed with ovarian cancer and 15,500 died of their disease, making ovarian cancer the fifth leading cause of cancer death [1]. Although cytoreductive surgery and first-line chemotherapy with a platinum and a taxane have increased disease-free survival and overall survival (OS) for patients with ovarian, fallopian tube and primary peritoneal cancer, disease recurrence is common in this patient population. After recurrence, response rates to second-line chemotherapy for platinum-sensitive patients are 30% or higher; however, patients with platinum-resistant disease have significantly lower response rates of 10%-25% to chemotherapeutic agents, such as liposomal doxorubicin, topotecan, taxanes, etoposide, and gemcitabine [2].
Given the poor response of recurrent disease to traditional cytotoxic agents, approaches with biologic agents that target the mechanisms of tumor growth and spread have been pursued. One of these agents is bevacizumab, a recombinant humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF). VEGF is a proangiogenic molecule that increases tumor growth and vascular permeability in ovarian cancer models. In humans, increased expression of VEGF is associated with a poor prognosis and a decreased disease-free interval [3-5].
In recurrent ovarian cancer, response rates of 16%-21% with an additional 39%-55% of patients exhibiting stable disease (SD) been reported for single-agent bevacizumab [6,7]. Addition of cytotoxic therapy to anti-angiogenic therapy in preclinical models has suggested a synergistic effect [8]. Specifically, the combination of metronomic cytotoxic chemotherapy and anti-VEGF therapy has been hypothesized to increase drug access to tumor sites, lead to chemo-sensitization, and increase rates of tumor cell apoptosis [9,10]. Clinical experience has supported this hypothesis. Several phase II clinical trials of bevacizumab have shown the drug to be well tolerated with response rates of 16%-21% for single-agent therapy and 24% for bevacizumab in combination with cyclophosphamide in the single phase II study of this regimen [6,7,11]. Response rates in both prospective and retrospective studies of bevacizumab and cyclophosphamide have varied from 10% to 54% [12-14].
This retrospective study was performed to determine the efficacy, PFS, and, OS in heavily pre-treated patients with recurrent ovarian, fallopian tube and primary peritoneal carcinoma treated with intravenous bevacizumab and metronomic oral cyclophosphamide.
MATERIALS AND METHODS
Following approval by the Northwestern University Institutional Review Board, all patients who received intravenous bevacizumab in combination with oral cyclophosphamide between January 2006 and December 2010 were identified by retrospectively searching pharmacy records. Medical records were then reviewed for all patients identified by pharmacy records. All patients who received bevacizumab in combination with cyclophosphamide for treatment of recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer were included. There were no patients from this cohort who were specifically excluded, although treatment was at attending physician discretion and thus patients felt to be at a high risk of bowel perforation may not have been included. Demographic variables were abstracted from the medical record including age, gravity, parity, and personal or family history of breast or ovarian cancer. Disease-specific variables abstracted included International Federation of Gynecology and Obstetrics (FIGO) stage, histologic subtype, prior treatment regimens, CA-125 values, response to treatment, cytoreduction at initial surgery, time to disease progression, and time to death. Performance status and treatment side effects were recorded following each cycle of treatment and this data was abstracted. Reason for discontinuation of treatment was also recorded including specific side effects leading to treatment discontinuation.
All patients were treated with initial surgical debulking followed by standard chemotherapy with a platinum and taxane. No patients received neoadjuvant chemotherapy. All patients were either primarily or secondarily platinum-resistant having recurred within 6 months of initial platinum-based chemotherapy or having recurred again after secondary platinum therapy. Patients were treated with intravenous bevacizumab 10 mg/kg every 14 out of 28 days and oral cyclophosphamide 50 mg daily for at least one treatment cycle. A single treatment cycle was defined as 28 days. Response to treatment was determined by change in disease status according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria. If imaging was not available, response was determined by CA-125 levels using modified Rustin criteria. All RECIST measurements were performed by radiologists. Response using CA-125 levels defined a complete response (CR) to be a normalization of CA-125 for at least 4 weeks, partial response (PR) to be a decrease in CA-125 level of greater than 50%, and progressive disease (PD) to be an increase in CA-125 of at least 25%. SD was defined as a response that did not meet these criteria. Responders were defined as those patients who had either a CR or PR. Non-responders were defined as those patients who had either SD or PD.
Overall PFS was calculated from the date of initial surgery until date of progression by RECIST or modified Rustin criteria. PFS from bevacizumab and cyclophosphamide (PFS-BC) was calculated from the date of first receipt of drug to date of progression by modified Rustin or RECIST criteria. OS was calculated from the date of diagnosis until date of death or date of last known status. OS from bevacizumab and cyclophosphamide (OS-BC) was calculated from date of first receipt of drug to date of death or date of last known status.
Patient demographics, treatment history, and tumor characteristics were used to stratify patients to determine associations between these variables and response to treatment. Student's t-test was used to compare normally distributed continuous variables, Wilcoxon rank-sum test was used for variables not normally distributed, chi-squared analysis was used to compare categorical variables, and Fisher exact analysis was used for comparison of categorical variables where outcomes had a cell frequency of less than five. Kaplan-Meier curves were generated using survival and progression data. SAS (SAS Institute, Cary, NC, USA) was used for all statistical analysis.
RESULTS
Sixty-six patients were treated with intravenous bevacizumab in combination with metronomic oral cyclophosphamide from January 2006 to December 2010. Demographic and disease characteristics of these patients are reported in Table 1. Median patient age at diagnosis was 53 years (range, 36 to 90 years). Fifty-five patients (83%) underwent optimal cytoreduction during primary surgery, and 18 (27.3%) of those surgeries involved bowel resection (Table 2). All patients were treated with initial platinum and taxane chemotherapy and 40 patients (60.6%) were initially platinum sensitive. At the time of treatment with bevacizumab and cyclophosphamide, all patients were either primarily or secondarily platinum resistant. Responders and non-responders were similar with respect to demographic and disease characteristics (Table 1) as well as treatment history (Table 2). Only history of treatment with intra-peritoneal chemotherapy significantly differed between the two groups with 32.1% of responders having received intra-peritoneal chemotherapy compared with 7.9% of the non-responders (p=0.021).
Table 1.
Demographic characteristics
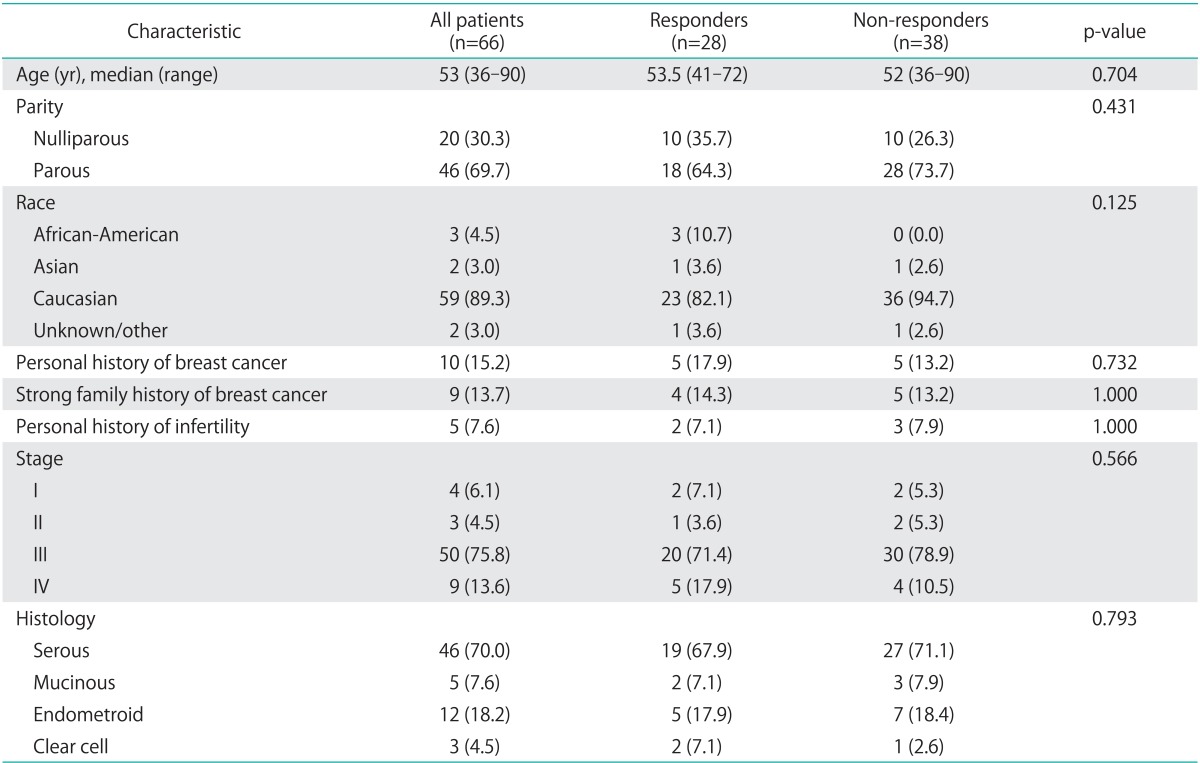
Values are presented as number (%). Demographic characteristics are presented for all patients. Non-responders are compared to responders for all demographic characteristics.
Table 2.
Comparison between responders and non-responders to bevacizumab and cyclophosphamide (BC)
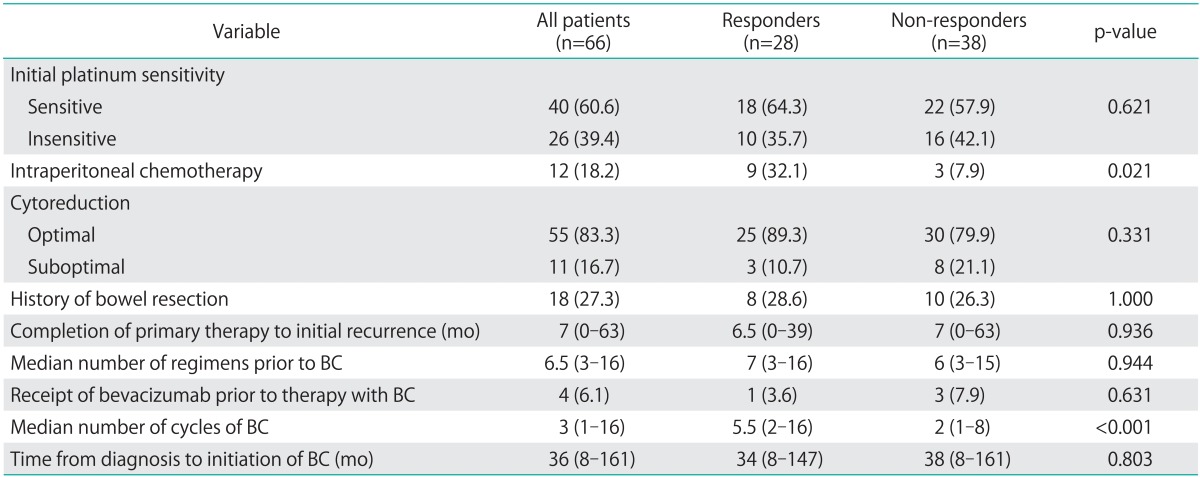
Treatment history is reported for all patients. Responders and non-responders are compared. Values are presented as number (%) for categorical data and median (range) for continuous data.
Median time from diagnosis to beginning treatment with bevacizumab and cyclophosphamide was 36 months (range, 8 to 161 months). Median number of prior chemotherapy treatments was 6.5 (range, 3 to 16). Only four patients had received treatment with bevacizumab prior to therapy with combination bevacizumab and cyclophosphamide. One patient received single agent therapy and the other three received bevacizumab in combination with docetaxel, paclitaxel, or liposomal doxorubicin. No patients received other anti-angiogenics prior to treatment with bevacizumab and cyclophosphamide. A total of 258 cycles of bevacizumab and cyclophophamide were administered with a median number of cycles per patient of 3 (range, 1 to 16). Patients were treated with bevacizumab and cyclophosphamide until PD or side effects required discontinuing treatment.
Eight patients (12.1%) had side effects that required discontinuation of bevacizumab and cyclophosphamide after a median of 2 cycles (range, 1 to 6 cycles). By National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grading, two patients had grade 4 hypertension with headache requiring presentation to the emergency room. One patient had grade 2 hypertension requiring initiation of a medication. One had grade 2 fatigue and one had grade 2 myalgias. Two patients had nephrotic range proteinuria. There was one bowel perforation (1.5%). There were no treatment related deaths. The single bowel perforation occurred in a patient who had recevied two cycles of bevacizumab and docetaxel followed by three cycles of bevacizumab and cyclophosphamide. She presented with a five-day history of abdominal pain, no flatus, nausea, and vomiting. CT scan revealed a bowel perforation, intraoperative findings were notable for carcinomatosis and a bowel perforation in the terminal ileum with 600 mL of purulent ascites. She underwent an ileostomy with resection of 40 cm of small bowel. She recovered well from the surgery and went on to survive another four years.
All patients were evaluated for response. A CR was noted in 7 patients (10.6%) and a PR was seen in 21 patients (31.8%), for an overall response rate of 42.4%. Fifteen patients (22.7%) had stable disease and 23 patients (34.8%) had PD. Those with a CR had a median disease-free interval of 11 months (range, 4 to 14 months). In multivariate analysis, no demographic or disease characteristics were predictive of response, including number of prior chemotherapy regimens or initial platinum sensitivity.
All patients had monthly CA-125 levels drawn and thus all patients were evaluated by Rustin criteria. Thirty-seven patients (56%) had imaging performed, which confirmed by RECIST criteria, either response or progression. Of the 28 responders, 18 were confirmed by RECIST criteria, including six of the seven patients with a CR. Of the 38 non-responders, 19 were confirmed by RECIST criteria. For patients in which imaging was performed after the CA-125 levels had started to increase, the date of recurrence was recorded as the earlier date.
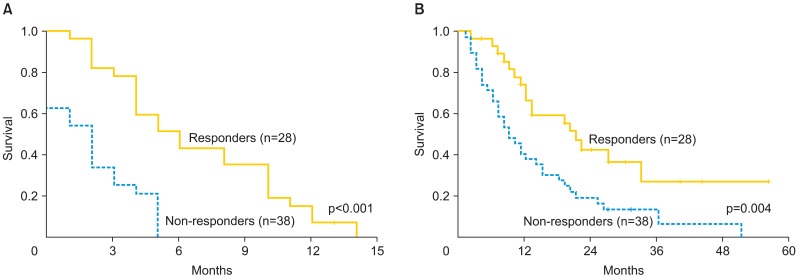
Median PFS-BC for all patients was 3 months (range, 0 to 14 months). PFS-BC for responders was 5 months (range, 2 to 14 months) (Fig. 1A). Median OS-BC was 12 months; responders had an improved survival of 20 months (range, 2 to 56 months) compared with 9 months (range, 2 to 51 months) for non-responders (p=0.004) (Fig. 1B). Median OS from diagnosis to last known status also differed between responders and non-responders with responders experiencing an OS of 57 months (range, 15 to 174 months) compared with 51.5 months (range, 16 to 197 months) for non-responders (p=0.004) (Table 3).
Fig. 1.
Progression-free (A) and overall survival (B) from the initiation of bevacizumab and cylophosphamide between responders and non-responders.
Table 3.
Survival comparison between responders and non-responders to bevacizumab and cyclophosphamide (BC)
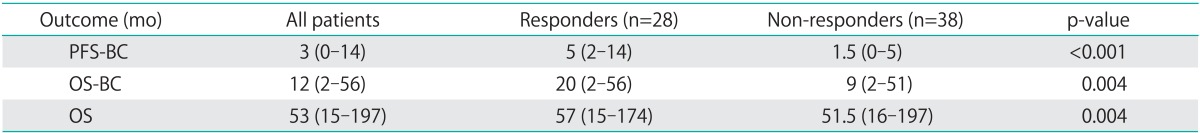
Values are presented as median (range).
OS, time from diagnosis to last known status; OS-BC, time from initiation of bevacizumab/cyclophosphamide to last known status; PFS-BC, time from initiation of bevacizumab/cyclophosphamide to disease progression; OS, overall survival; PFS, progression-free survival.
DISCUSSION
The results of our study indicate that the combination of metronomic oral cyclophosphamide and intravenous bevacizumab has activity against recurrent ovarian cancer in heavily pre-treated patients. In addition, overall and PFS was improved in the cohort of patients that responded to this treatment as compared to non-responders. Current second-line chemotherapy for recurrent platinum-resistant disease includes liposomal doxorubicin, topotecan, taxanes, etoposide, and gemcitabine with response rates of 10%-20% [2]. Our response rate of 42.4% with 65.1% deriving clinical benefit (CR, PR, SD) is superior to these accepted second-line agents and our reported progression free survival was comparable as well.
The activity of bevacizumab in ovarian cancer is well documented. A phase II study of single-agent bevacizumab in 62 patients with recurrent ovarian cancer showed a 21% response rate [6]. An additional paper focusing on heavily pretreated patients with three prior regimens reported a 16% response rate, but the study was terminated early secondary to increased gastrointestinal perforations (11.4%) [7].
Data from bevacizumab in combination with cytotoxic agents has been even more promising than single-agent therapy. Two retrospective studies of bevacizumab in combination with a variety of cytotoxic agents in pretreated patients with a median of 4.5 and 7 prior therapies revealed response rates of 21% and 35%, respectively [15]. All of these were PRs. The combination of bevacizumab and metronomic oral cyclophosphamide, specifically, has shown even higher response rates of 40.5% [14] and 53.5% [12] in two retrospective series with approximately 10% in each series demonstrating a CR which is comparable to the results of our study. Table 4 displays the results of the currently published studies examining the use of bevacizumab and cyclophosphamide for recurrent ovarian cancer. This high response rate for patients with recurrent ovarian cancer is hypothesized to be due to the anti-angeogenic effects of cyclophosphamide which when given in metronomic dosing directly inhibits endothelial cell proliferation.
Table 4.
Relevant studies of bevacizumab and cyclophophamide for recurrent ovarian cancer
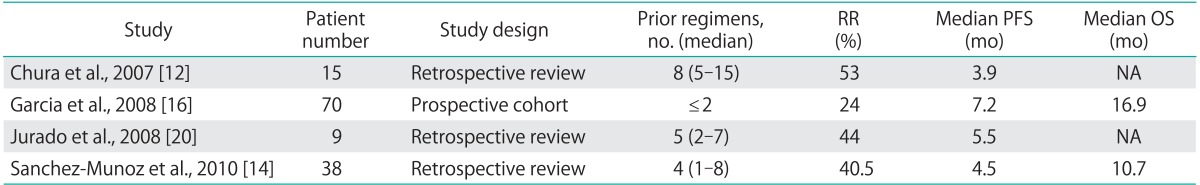
NA, not available; OS, overall survival; PFS, progression-free survival; RR, response rate.
The single prospective phase II study of bevacizumab and cyclophosphamide in patients with only two prior regimens demonstrated a response rate of 24% (all PRs) and a PFS of 7.2 months [16]. Our results are consistent with these prior studies; our observed response rate was 42.4% with a PFS of 5 months for responders. Additionally, 7 of our patients obtained a CR including one very heavily pre-treated patient with 14 prior regimens who obtained a 12-month PFS. Given the demonstrated benefit of the addition of cyclophosphamide to bevacizumab, the low cost of adding cyclophosphamide to this regimen, and no observed increased toxicity as compared to becacizumab alone, this regimen could be utilized in place of single-agent bevacizumab.
Of note, in our study, patients with a history of treatment with IP chemotherapy were more likely to be responders than those patients who had not received intraperitoneal chemotherapy. This finding has not been reported previously and the cause of this observation is unclear. It could be due to differences in performance status between patients who received treatment with IP chemotherapy or that the tumor biology or recurrence patterns differ between patients who have received IP chemotherapy and those who have not. Additionally, patients who received IP chemotherapy may have had less intra-abdominal carcinomatosis and therefore continued on bevacizumab longer as providers were less concerned about the risk of bowel perforation in these patients. Further studies, possibly incorporating tumor response assays, are needed to elucidate the significance of this finding.
Our treatment regimen of intravenous bevacizumab 10 mg/kg every 14 out of 28 days and oral cyclophosphamide 50 mg daily was very well tolerated with only 12% of patients encountering a side effect that required discontinuing treatment. Of note, we observed only one bowel perforation (1.5%) in a cohort of heavily pretreated patients of whom 27% had a bowel resection during their initial surgery. This treatment regimen is the same one used in the only phase II study of the combination of bevacizumab and cyclophosphamide, Garcia et al. [16], in which a similar percentage of patients, 2.8%, had a bowel perforation. Interestingly, a phase II study of single agent bevacizumab at a dose of 15 mg/kg every 21 days had a higher perforation rate of 11.4% [7]. It may be that while treating patients with recurrent ovarian cancer who are at a high risk of bowel perforation, using a lower dose of bevacizumab at more frequent intervals may lower the perforation rate without altering the response rate.
Limitations of this study include all of those inherent in a retrospective review, including an inability to demonstrate cause and effect associated with treatment. For example, although we showed an increased overall surivival in those patients who responded to bevacizumab and cyclophosphamide we could not attribute this increased survival directly to the treatment given inherent differences between the group of patients who responded and those that did not. Both responders and non-responders received numerous other chemotherapeutic agents making it difficult to attribute the observed survival benefit directly to bevacizumab and cyclophosphamide. Additionally, as patients in this study were receiving bevacizumab and cyclophosphamide off a study protocol they did not receive regular imaging to monitor PD. We used CA-125 levels to evaluate for disease response when imaging was not available to evaluate disease by RECIST criteria. CA-125 levels and disease status by RECIST criteria have been shown to correlate well [17-19]. However, CA-125 levels may show a CR (be in normal range) when disease persists on imaging, thus the use of CA-125 levels may have resulted in an over estimation of our CRs [12]. In contrast, approximately 10% of patients show an earlier recurrence date when using CA-125 levels when compared with RECIST criteria [19] which would lead to an underestimation of length of our PFS. Additionally, use of CA-125 levels may have led to an underestimation of our PFS as many patients progressed by CA-125 criteria in 0 or 1 month intervals from starting the drug. This progression would not have been noted until the next imaging study was performed in a trial using only RECIST criteria. When both imaging and CA-125 were available and were discrepant, we used whichever indicated recurrence sooner and therefore our data may actually underestimate the drug's benefit.
In this study, use of the combination of intravenous bevacizumab and oral metronomic cyclophosphamide in heavily treated platinum-resistant ovarian cancer patients resulted in a higher total response rate than other accepted second line agents and was well tolerated by patients who had received numerous prior chemotherapeutic regimens. This combination of chemotherapy agents significantly increased both PFS and OS in responders. The combination of bevacizumab and cyclophosphamide should be considered for use in patients with recurrent ovarian cancer due to its impressive response rate, favorable side-effect profile, and tolerability, which is important in a heavily pretreated group of patients where treating providers must always consider the patient's quality of life.
Footnotes
Presented as a poster at the Annual Meeting of the American Society of Clinical Oncology, June 1st-5th, 2012 in Chicago, IL, USA.
Dr. Julian Schink served on the Genentech Advisory Board in 2011. Otherwise, the authors have no conflicts of interest to disclose.
References
- 1.National Cancer Institute. SEER stat fact sheets: ovary [Internet] Bethesda, MD: National Cancer Institute; [cited 2013 Feb 14]. Available from: http://seer.cancer.gov/statfacts/html/ovary.html. [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Abulafia O, Triest WE, Sherer DM. Angiogenesis in primary and metastatic epithelial ovarian carcinoma. Am J Obstet Gynecol. 1997;177:541–547. doi: 10.1016/s0002-9378(97)70143-1. [DOI] [PubMed] [Google Scholar]
- 4.Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett. 1997;121:169–175. doi: 10.1016/s0304-3835(97)00350-9. [DOI] [PubMed] [Google Scholar]
- 5.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80:98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 7.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 9.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. [PubMed] [Google Scholar]
- 10.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol. 2007;25:2902–2908. doi: 10.1200/JCO.2007.12.1509. [DOI] [PubMed] [Google Scholar]
- 12.Chura JC, Van Iseghem K, Downs LS, Jr, Carson LF, Judson PL. Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol Oncol. 2007;107:326–330. doi: 10.1016/j.ygyno.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Matulonis UA, Pereira L, Liu J, Lee H, Lee J, Whalen C, et al. Sequential bevacizumab and oral cyclophosphamide for recurrent ovarian cancer. Gynecol Oncol. 2012;126:41–46. doi: 10.1016/j.ygyno.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Munoz A, Mendiola C, Perez-Ruiz E, Rodriguez-Sanchez CA, Jurado JM, Alonso-Carrion L, et al. Bevacizumab plus low-dose metronomic oral cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Oncology. 2010;79:98–104. doi: 10.1159/000320602. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, Moroney JW, Levenback CF, Fu S, Jaishuen A, Kavanagh JJ. What is the benefit of bevacizumab combined with chemotherapy in patients with recurrent ovarian, fallopian tube or primary peritoneal malignancies? J Chemother. 2009;21:566–572. doi: 10.1179/joc.2009.21.5.566. [DOI] [PubMed] [Google Scholar]
- 16.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 17.Alexandre J, Brown C, Coeffic D, Raban N, Pfisterer J, Maenpaa J, et al. CA-125 can be part of the tumour evaluation criteria in ovarian cancer trials: experience of the GCIG CALYPSO trial. Br J Cancer. 2012;106:633–637. doi: 10.1038/bjc.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog TJ, Vermorken JB, Pujade-Lauraine E, Provencher DM, Jagiello-Gruszfeld A, Kong B, et al. Correlation between CA-125 serum level and response by RECIST in a phase III recurrent ovarian cancer study. Gynecol Oncol. 2011;122:350–355. doi: 10.1016/j.ygyno.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Randall LM, Sill MW, Burger RA, Monk BJ, Buening B, Sorosky JI. Predictive value of serum CA-125 levels in patients with persistent or recurrent epithelial ovarian cancer or peritoneal cancer treated with bevacizumab on a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2012;124:563–568. doi: 10.1016/j.ygyno.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–586. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]