Abstract
OBJECTIVE
To compare clinical profile of long-term survivors and nonsurvivors with type 2 diabetes (T2DM).
RESEARCH DESIGN AND METHODS
After conducting a retrospective survey of >200,000 case records, we identified T2DM survivors (>40 years of duration) and age at diagnosis and sex-matched T2DM nonsurvivors. Prevalence of complications and causes of death were analyzed. Retinopathy was diagnosed by retinal photography. Microalbuminuria and macroalbuminuria, peripheral vascular disease based on ankle-brachial index <0.9, coronary artery disease based on history of myocardial infarction or coronary revascularization, and neuropathy based on vibration perception threshold >20 V were compared in both groups.
RESULTS
The mean duration of diabetes of survivors (n = 238) was 43.7 ± 3.9 years, and that of the nonsurvivors (n = 307), at time of death, was 22.4 ± 11.0 years (P < 0.001). Nonsurvivors had significantly higher systolic and diastolic blood pressures, plasma glucose, HbA1c, serum cholesterol, LDL cholesterol, and triglycerides and lower HDL cholesterol compared with long-term survivors (P < 0.001 for all parameters except systolic blood pressure, which was P = 0.027). Myocardial infarction (46.4%) and renal failure (16.6%) were the most common causes of death. Prevalence of most complications was higher among survivors because of longer duration and older age, as follows, for survivors versus nonsurvivors: retinopathy, 76 vs. 62%; microalbuminuria, 39.1 vs. 27.3%; macroalbuminuria, 8.4 vs. 23.7%; neuropathy, 86.5 vs. 63.5%; peripheral vascular disease, 23.1 vs. 11.4%; and coronary artery disease, 44.5 vs. 40.7%.
CONCLUSIONS
Long-term survivors with T2DM had better glycemic and blood pressure control and more favorable lipid profiles.
Type 2 diabetes mellitus (T2DM) has become a global problem that is associated with increased morbidity and mortality attributable to microvascular and macrovascular complications (1). With 62 million diabetic patients, India has the world’s second largest diabetes population (2) and >90% have T2DM (3). According to the International Diabetes Federation, this number is expected to surpass 100 million by the year 2030 (4). Asian Indians are particularly susceptible to diabetes because of increased insulin resistance and central adiposity (5–8). Refined grain intake and decreased physical activity also are known to increase the risk of diabetes in this ethnic group (9,10). There are also unique genes among Asian Indians that may increase susceptibility to diabetes (11–14). Thus, Asian Indians are known to have development of T2DM at a younger age and at a lower BMI compared with Caucasians (15).
With increasing awareness of diabetes and improved therapy, reports of patients surviving for long periods with diabetes have begun to emerge. However, virtually all reports until now have been on type 1 diabetes (T1DM). The Golden Years Cohort provided a description of patients in the United Kingdom who survived with T1DM for at least 50 years (16). Patients in that cohort had higher HDL cholesterol levels and a possible genetic predisposition contributing to long-term survival. The more recent Joslin 50-year Medalist Study of T1DM patients with ≥50 years of duration showed that close to 40% of long-term T1DM patients remained free of diabetes-related complications (17). It was reported that worse glycemic control, longer diabetes duration, hypertension, and hyperlipidemia were associated with increased risk of development of vascular complications in these T1DM individuals (17).
Although these studies have reported on the survival of T1DM patients, similar studies in T2DM patients are limited, perhaps because T2DM usually has a much older age of onset and, hence, long-term survival of ≥40 years is less likely. Because Asian Indians tend to have development of T2DM earlier, we were able to collect a series of T2DM survivors with >40 years of duration of diabetes. In this report, we compare the clinical profile of a group of long-term survivors with T2DM and an age at diagnosis and gender-matched group of nonsurvivors with T2DM examined at a tertiary diabetes center in southern India. We also describe the causes of death among the nonsurvivors and the prevalence of complications among both the groups.
RESEARCH DESIGN AND METHODS
The electronic database at Dr. Mohan’s Diabetes Specialities Center in Chennai City (formerly Madras), in southern India, called Diabetes Electronic Medical Records, was established at the time of inception of the center in 1991 and has data of >200,000 diabetic patients registered between 1991 and 2011. A preliminary analysis of the Diabetes Electronic Medical Records was published recently (18). We have a unique identifying number so that any given patient can have only one record with multiple follow-up visits documented in that record. Because ours is a tertiary diabetes center, we get patients from different parts of the country. Because it is a private center, our patients mostly belong to the middle or upper socioeconomic sections of society; however, through our charitable free clinics, several poor patients also attend the clinic. The period of follow-up of the patients varies; some have long periods of follow-up and others have much shorter periods. Many have died or have been lost to follow-up. Accurate classification of patients is performed wherever possible using a battery of clinical and biochemical investigations including C-peptide and GAD antibodies.
Approximately 94% of our patients are Hindus, 3% are Muslims, 2% are Christians, and 1% practice other religions. Most have middle or upper socioeconomic status. We usually prescribe a diet with complex carbohydrates (60%), protein (15%), and fat (25%). Dietary adherence is checked at every visit by a dietitian. However, it is difficult to ensure dietary compliance and physical activity over prolonged periods of time.
T2DM is diagnosed based on absence of ketosis, good β-cell reserve as shown by fasting C-peptide assay (≥0.6 pmol/mL), absence of pancreatic calculi (on abdominal radiograph), and response to oral hypoglycemic agents for >2 years.
From our database, we identified 238 patients with T2DM who had survived with >40 years of documented duration of diabetes (survivors). We then obtained data on 307 T2DM subjects who were matched for age at onset of T2DM and for gender with the survivor cohort but who had died of various causes before 40 years of duration (nonsurvivors) to compare the clinical profile of the survivors and nonsurvivors. Medical records of both groups of patients were reviewed and the biochemical and clinical data were recorded. Patient demographics, including age at last visit, age at diagnosis, and duration of diabetes among the survivors, and the age and duration of diabetes at time of death among nonsurvivors, were noted along with family and smoking history and the details of medications. For the nonsurvivors, the cause of death was ascertained by examining medical records, death certificates, discharge summaries from hospitals, and, wherever possible, a verbal autopsy.
Hypertension was diagnosed based on drug treatment for hypertension or if blood pressure was >140/90 mmHg (19). Height and weight measured from the most recent clinic visit were obtained to calculate BMI in kg/m2. Based on criteria of the World Health Organization Asia Pacific guidelines (20), patients of either gender were classified as overweight if they had BMI between 23 kg/m2 and 24.9 kg/m2 and as obese if they had BMI ≥25 kg/m2.
Biochemical profile, including fasting plasma glucose, lipids, and liver function tests, was obtained from the medical records. Because this is a retrospective study going back >20 years, the methods used for biochemical parameters varied slightly over the years. Until 1998, plasma glucose estimations were performed by glucose oxidase method, serum cholesterol was measured by cholesterol oxidase phenol plus aminophenazone method, triglycerides were measured by glycerol-3 phosphate oxidase phenol plus aminophenazone method, and serum creatinine was measured by modified kinetic method of Jaffe using CIBA Corning Express plus Auto analyzer (Corning). Since 1999, plasma glucose estimations were performed by hexokinase method. Total cholesterol, triglycerides, HDL cholesterol, and serum creatinine measurements were performed by the same methods, but on a Hitachi 912 Autoanalyser (Mannheim, Germany) using the same commercial kits. Since 1993, glycated hemoglobin (HbA1c) has been estimated by high-performance liquid chromatography method using the Variant machine (Bio-Rad, Hercules, CA). Our center participates in external quality-control programs and is certified by the College of American Pathologists as well as the National Accreditation Board for Testing and Calibration of Laboratories of the government of India. The intra-assay and interassay coefficients of variation for the biochemical assays ranged between 3.1 and 7.6%. Institutional Ethics Committee approval was obtained, and written informed consent to use their anonymized medical data was obtained from all study subjects.
Hypercholesterolemia was diagnosed if total cholesterol was ≥5.18 mmol/L (200 mg/dL) and hypertriglyceridemia was diagnosed if triglyceride levels were ≥1.69 mmol/L (150 mg/dL) or if the subject was using drug treatment for dyslipidemia. High LDL cholesterol was diagnosed if LDL cholesterol was ≥2.6 mmol/L (100 mg/dL), and low HDL cholesterol was diagnosed if the value was <1.0 mmol/L (40 mg/dL) in males and <1.3 mmol/L (50 mg/dL) in females (21).
Assessments of diabetes complications, including retinopathy, microalbuminuria, macroalbuminuria, neuropathy, peripheral vascular disease (PVD), and coronary artery disease (CAD), were performed as described.
Retinopathy
A comprehensive ocular examination was performed and visual acuity was recorded using an illuminated Snellen chart. A detailed retinal (fundus) examination was performed by direct and indirect ophthalmoscope by a retinal specialist trained in grading of retinal lesions. Retinal (fundus) photography was performed using four-field stereo color retinal photography (model FF 450 plus camera; Carl Zeiss, Jena, Switzerland) whenever possible. An Early Treatment Diabetic Retinopathy Study grading system that has been modified and standardized in other population-based studies was used for the diagnosis of diabetic retinopathy (22,23).
Nephropathy
Urinary albumin concentration was measured in a fasting urine sample using a immunoturbidometric assay (Hitachi 902 autoanalyzer; Roche Diagnostics). Microalbuminuria was diagnosed if the albumin excretion was between 30 and 299 g/mg creatinine. Macroalbuminuria was diagnosed if albumin excretion was ≥300 g/mg creatinine (24).The Modification of Diet in Renal Disease study equation is as follows: glomerular filtration rate = 186 × (serum creatinine) −1.154 × age − 0.203 × (0.742 if female) × (1.210 if African American [not applicable to our population]); it was used to calculate the estimated glomerular filtration rate (25).
Neuropathy
Neuropathy was assessed using a biothesiometer. Vibratory perception threshold of the great toes was measured in a standardized manner by a single observer, and neuropathy was diagnosed if the mean vibratory perception threshold was ≥20 V (26).
PVD
PVD was diagnosed by Doppler measurement of ankle-brachial index. Blood pressure recordings were made of the brachial pulses in the upper limb. Similar recordings were made of the dorsalis pedis and posterior tibial pulses in the lower limb by inflating the cuff proximal to the ankle, and the mean of these two readings was taken as the ankle pressure. Ankle-brachial index <0.9 was used for the diagnosis of PVD (27).
Coronary artery disease
Coronary artery disease (CAD) was diagnosed based on a history of documented myocardial infarction, coronary revascularization, electrocardiogram changes, or, if using drug treatment, drug treatment for CAD (aspirin plus nitrates).
Statistical analysis
SPSS for Windows version 15.0 was used for data analysis. Descriptive analysis was used for continuous variables and χ2 test was used for categorical variables. Multiple logistic regression analyses were performed using survivors and nonsurvivors as dependent variables and HbA1c, diastolic blood pressure, total serum cholesterol, serum triglycerides, and LDL cholesterol as independent variables. Kaplan-Meier curves were plotted for cumulative survival in relation to the duration of diabetes among the nonsurvivor group.
RESULTS
Clinical characteristics
All 238 T2DM survivors had duration of diabetes >40 years, whereas 21 (8.8%) had survived at least 50 years with diabetes.
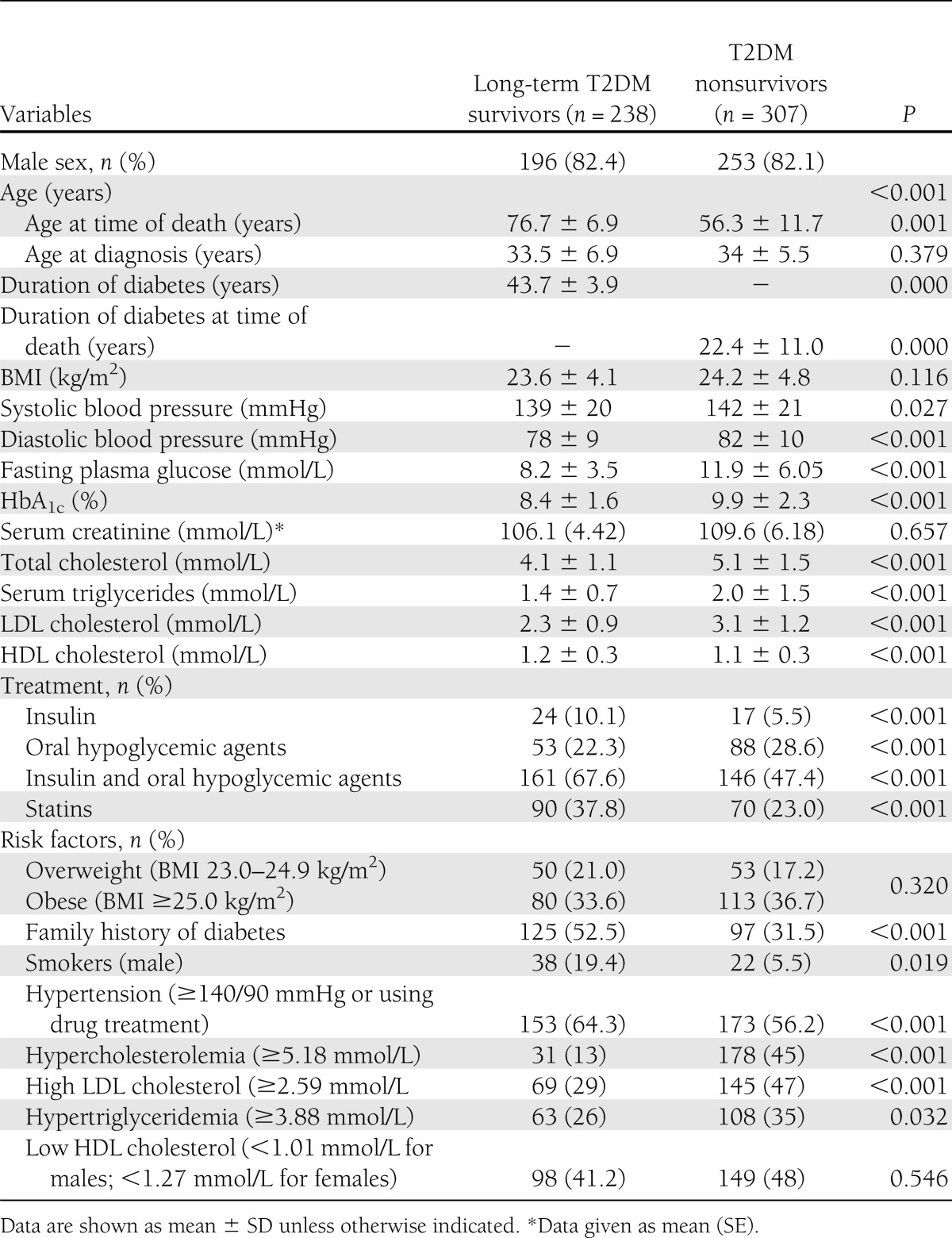
Table 1 shows the clinical and biochemical parameters of the 238 survivors and 307 nonsurvivors with T2DM. The mean ages at diagnosis of the survivors and nonsurvivors were 33.5 ± 6.9 and 34 ± 5.5 years, respectively (P = 0.379). The mean age of the survivors was 76.7 ± 6.9 years, whereas the mean age at the time of death of the nonsurvivors was 56.3 ± 11.7 years (P < 0.001).The mean duration of diabetes of survivors was 43.7 ± 3.9 years, and that of the nonsurvivors, at time of death, was 22.4 ± 11.0 years (P < 0.001). The nonsurvivors had significantly higher systolic and diastolic blood pressures, blood glucose, HbA1c, serum cholesterol, LDL cholesterol, and triglycerides and lower HDL cholesterol compared with long-term survivors (P < 0.001 for all parameters except systolic blood pressure, which was P = 0.027).
Table 1.
Clinical and biochemical characteristics of T2DM survivors and nonsurvivors
Fifty-three percent of survivors reported a family history of diabetes in a parent compared with 31.5% of the nonsurvivors (P < 0.001). The details of the drug treatment in the two groups are shown in Table 1, and it shows higher use of insulin (P < 0.001) and statins (P < 0.001) among the survivors.
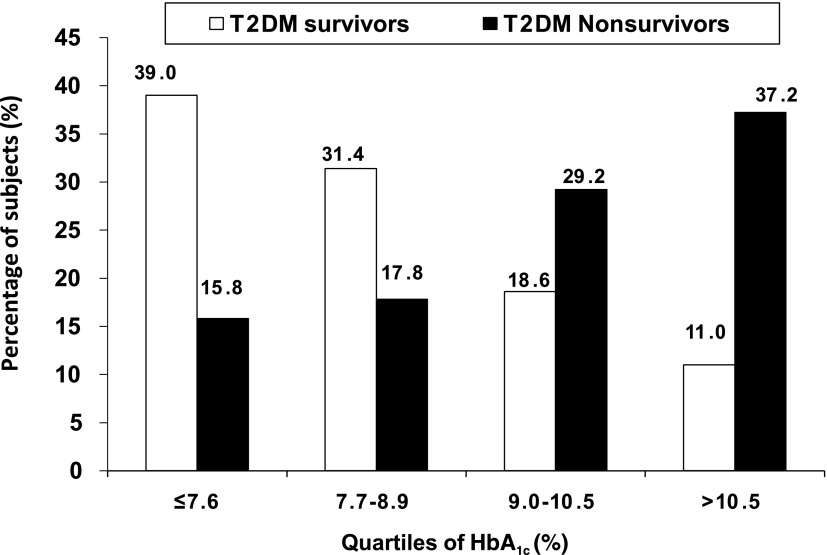
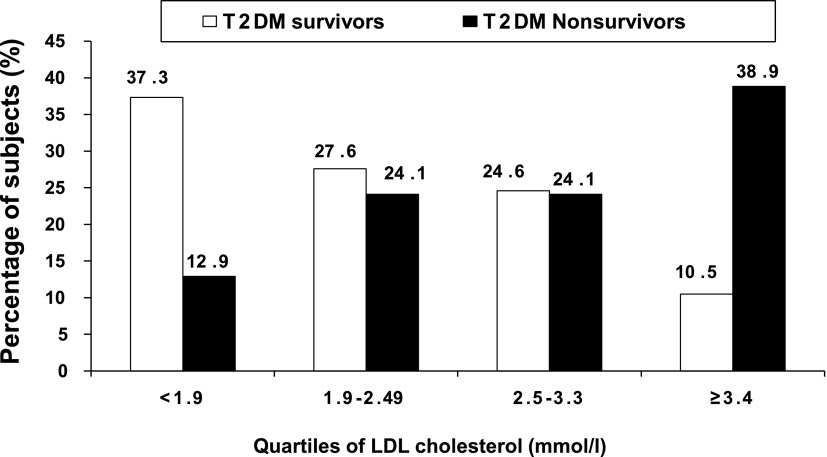
The distribution of survivors and nonsurvivors according to quartiles of HbA1c is presented in Fig. 1. There was a significant increase in percentage of the nonsurvivors in the higher quartiles of HbA1c compared with the survivors (trend χ2 = 70.5; P < 0.001). The LDL cholesterol distributions are presented in Fig. 2, and this again shows that more nonsurvivors were in the higher quartiles of LDL cholesterol (trend χ2 = 59.6; P < 0.001).
Figure 1.
Distribution of T2DM survivors and nonsurvivors according to quartiles of HbA1c.
Figure 2.
Distribution of T2DM survivors and T2DM nonsurvivors according to quartiles of LDL cholesterol.
Table 2 shows the prevalence of complications among the survivors and nonsurvivors. Retinopathy screening could be performed in 171 of 238 survivors (71.8%), whereas among the remaining 67 individuals it could not be performed because of either cataracts (n = 43) or refusals (n = 24). Out of 171 subjects screened, 130 subjects had retinopathy (76%), of whom 125 had nonproliferative diabetic retinopathy and 5 had proliferative diabetic retinopathy. Ten (5.8%) subjects had significant reduction in visual acuity. However, 41 subjects (24%) remained free of retinopathy. Among survivors, the HbA1c of those who had retinopathy was 8.6% compared with 7.9% in those without retinopathy. Among the nonsurvivors, 62% (n = 150/242 screened) had retinopathy, of whom 131 (87.3%) had nonproliferative diabetic retinopathy and 19 (12.7%), had proliferative diabetic retinopathy. Twenty-one subjects (6.8%) had significant reduction in visual acuity.
Table 2.
Prevalence of complications among the T2DM of survivors and nonsurvivors
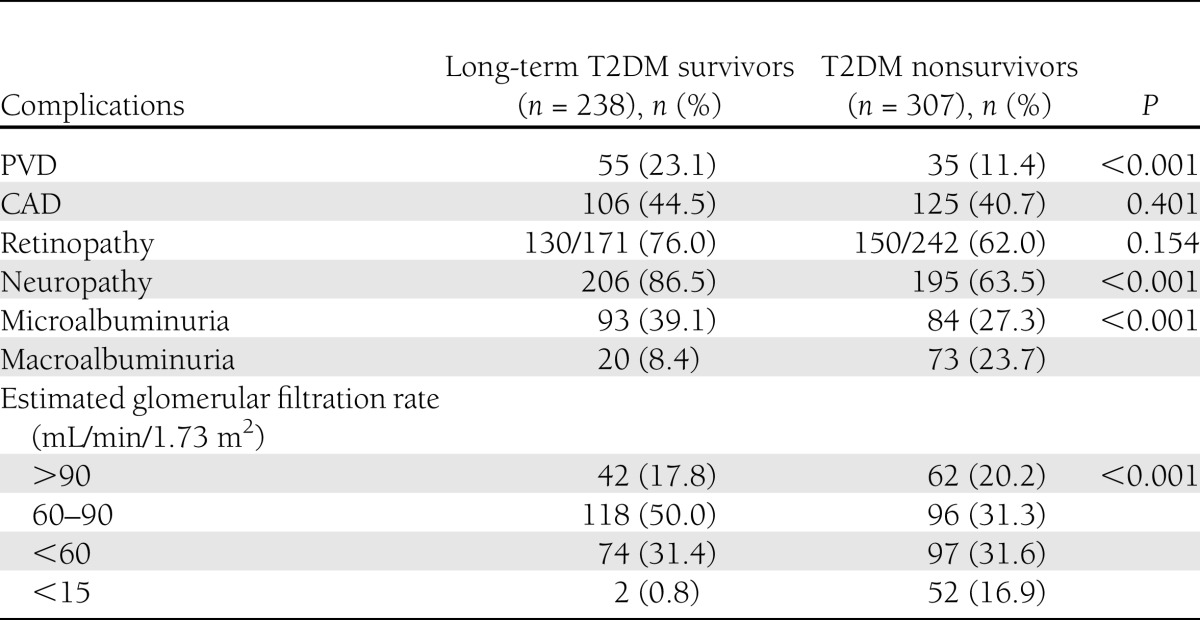
The prevalence of renal failure, defined as estimated glomerular filtration rate <15, was higher among nonsurvivors compared with survivors (16.9 vs. 0.8%; P < 0.001). Neuropathy was seen in 86.5% of survivors and in 63.5% of nonsurvivors. The prevalence of PVD among survivors and among nonsurvivors was 23.1 and 11.4%, respectively. CAD was present in 44.5% of survivors and 40.7% of nonsurvivors.
Table 3 presents the logistic regression of various independent protective factors for survivors compared with nonsurvivors, taken as reference. Serum cholesterol (odds ratio [OR] 0.182 [95% CI 0.116–0.284]; P < 0.001), LDL cholesterol (0.265 [0.180–0.391]; P < 0.001), serum triglycerides (0.471 [0.320–0.692]; P < 0.001), HbA1c (0.658 [0.592–0.732]; P < 0.001), and diastolic blood pressure (0.957 [0.937–0.976]; P < 0.001) were the protective factors among the survivors.
Table 3.
Logistic regression for long-term T2DM survivors compared with T2DM nonsurvivors
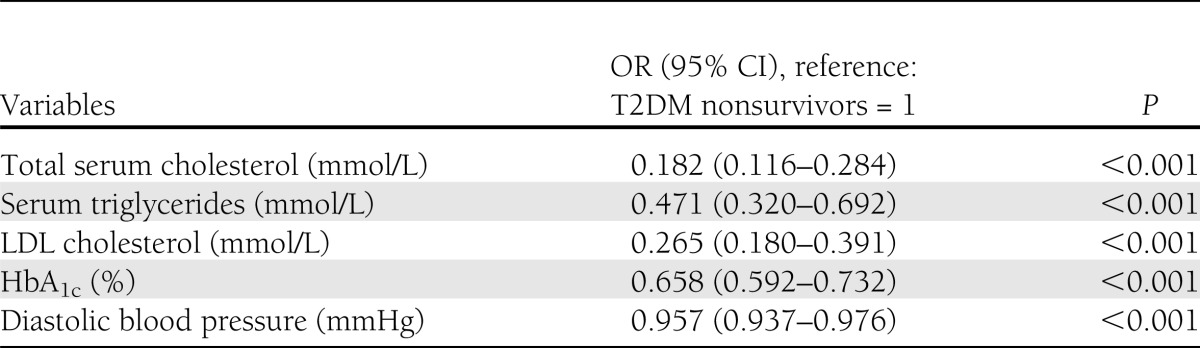
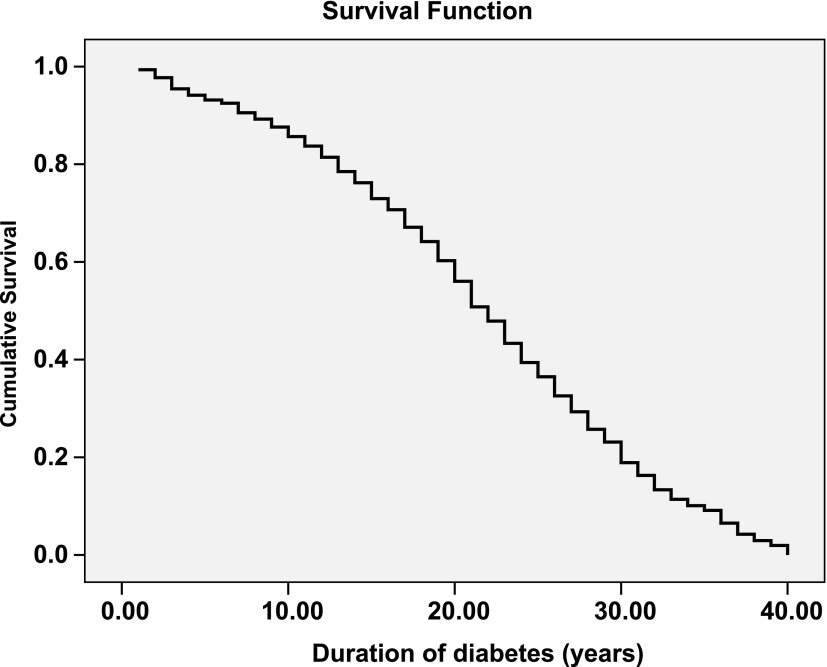
Fig. 3 shows the cumulative survival for the nonsurvivors with respect to duration of diabetes and shows that within 10 years 14.3% had died, by 20 years 43.6% had died, by 30 years 81.1% had died, and by 40 years 100% of the nonsurvivors had died.
Figure 3.
Survival curve for the T2DM nonsurvivors.
Analysis of causes of death among nonsurvivors showed that myocardial infarction (46.4%) and renal insufficiency (16.6%) were the leading causes of death, followed by respiratory diseases (9.7%), septicemia (7.8%), cancer (5.8%), hepatic failure (5.2%), stroke (3.2%), and others such as accident or unnatural causes (4.9%).
CONCLUSIONS
There have been several studies describing the long-term survival of T1DM patients (16,17). Here, we compare the clinical profile of a group of long-term survivors with T2DM and a matched group of nonsurvivors selected from the electronic database of a tertiary diabetes center in south India. The study shows that the long-term survivors had better control of glycemia and a better lipid profile, with lower total cholesterol, LDL cholesterol, and higher HDL cholesterol, and they also had lower systolic and diastolic blood pressures. All of these factors could have contributed to their longer survival.
Obesity was present in 37% of the nonsurvivors compared with 33.6% of long-term survivors, which is significantly lower than the prevalence of 80–90% commonly reported in European T2DM patients (28). The mean BMI for all T2DM patients at our center is 25.8 ± 4.2 kg/m2 (18), whereas it was 23.6 ± 4.1 kg/m2 among the survivors. Thus, overall, the survivors were leaner than the average T2DM patients attending our center.
Diabetes affects 10–25% of elderly people (older than 65 years) worldwide, with particularly high rates in populations such as Pima Indians, Mexican-Americans, South Asians, and, more specifically, Indians. Elderly persons with diabetes mellitus, being a dependent population, impose considerable economic, social, and health burdens (29). In urban India, the prevalence of diabetes in those older than 65 years of age was 28.1% according to the Chennai Urban Rural Epidemiology Study (CURES) (8). In the national India Council of Medial Research–India Diabetes Study (ICMR-INDIAB) study, 168 of 1,087 (15.5%) had diabetes at an age older than 65 years (2).
According to India’s 2011 Census, the mean life expectancy in India is 67.3 years for males and 69.6 years for females (http://www.censusindia.gov.in/2011-prov-results/data_files/india/Final_PPT_2011_chapter8.pdf). Thus, this group of T2DM survivors has lived much longer than the average Indian despite the long duration of T2DM. The Chennai Urban Population Study (30) reported that the overall deaths were higher among diabetic subjects (11.9%) compared with nondiabetic subjects (3.3%). In that study, among nondiabetic subjects a large proportion of deaths were attributable to gastrointestinal (12.1%), respiratory (9.1%), lifestyle-related (6.1%), and unnatural causes (18.2%); however, among those with diabetes, cardiovascular disease contributed to 52.9% of deaths, renal disease contributed to 23.5% of deaths, and infections and age-related causes contributed to the remaining deaths (30). In the current study, myocardial infarction and renal disease were the most common causes of death, whereas infective and other causes, including cancer, contributed the rest. Zhang et al. (31) have reported that patients with T2DM have an increased risk of development of cancer. Thus, it appears reasonable to conclude that if T2DM patients do not die of CAD or nephropathy within the first 20–30 years of T2DM, then they tend to live longer and die of other causes, including cancer.
The mean HbA1c of 8.4% of T2DM survivors is above the recommended target of ≤7%, and only 48 patients (20.3%) had HbA1c ≤7% among the long-term survivors. The recent American Diabetes Association and European Association for the Study of Diabetes guidelines, however, recommend “less stringent” control in some groups of patients (32), and the patients in this series would fit into that category because they were older and had long duration of diabetes. Moreover, the HbA1c levels were considerably better than in the nonsurvivor group (mean HbA1c, 9.9%; P < 0.001).
Among the long-term survivors, 37.8% were using statins compared with 23% of the nonsurvivors. The more favorable lipid profiles and the use of statin treatment may be one of the factors contributing to the long-term survival of the survivors. In previous studies, statin therapy has been associated with a 19–55% reduction in cardiovascular disease events in patients with diabetes (33). Statins also are known to reduce the rate of progression of microvascular complications, including diabetic retinopathy, nephropathy, and neuropathy (34–36).
Comparison of the complication rates between survivors and nonsurvivors is not meaningful because the survivors were much older than the nonsurvivors (76.7 ± 6.9 vs. 56.3 ± 11.7 years) and the mean duration of diabetes was also much greater (43.7 ± 3.9 vs. 22.4 ± 11 years at time of death of the nonsurvivors). However, data on the prevalence of complications among survivors are of interest because this has not been reported in T2DM with such a long duration of diabetes.
Retinopathy was present in 76% of the survivors in this study. A previous study reported a prevalence of 71.9% among all T2DM patients with duration of at least 15 years (37). The CURES reports the prevalence of diabetic retinopathy to be 20.8% among known T2DM patients with a mean duration of diabetes of 5.9 ± 5.3 years (23). Thus, the increased prevalence of retinopathy in our study can be attributed to the longer duration of diabetes. However, it is of interest that approximately one-quarter of T2DM patients did not have development of retinopathy even after 40 years duration of diabetes, pointing to the role of protective factors (possibly genetic factors) in some T2DM subjects.
Microabluminuria was present in 39.1% of patients, which is greater than the reported prevalence of 19% among south Asian T2DM patients in the United Kingdom (38) and in our earlier population-based study, in which the prevalence was 26.9% (23). The prevalence of macroalbuminuria in the current study was 8.4%, which was similar to the prevalence of 8.0% in a north Indian clinic report (39). Only one patient among the survivors required dialysis in this study. Thus, progression to end-stage renal disease appears to be uncommon once T2DM subjects survive for >30 years. Significantly, prevalence of renal disease was higher among the nonsurvivors.
The most common complication among survivors was neuropathy, which was reported in 86.5% of our patients. Neuropathy was also the most common complication among T1DM patients in the Joslin 50-year Medialist Study, but the prevalence was 60.6% (17). However, the Joslin study used different criteria for diagnosis of neuropathy. Age, long duration, and poor control could contribute to the higher prevalence of neuropathy in our study. Moreover, T2DM long-term survivors tend to be significantly older than T1DM survivors as well as the nonsurvivors in this study, which also might explain the higher prevalence of neuropathy noted in this study among the survivors.
PVD was diagnosed in 23.1% of the survivors, which is significantly higher than the 7.8% reported in the Chennai Urban Population Study (CUPS) (22) and 8.6–9.8% reported in earlier clinic-based studies (40,41). However, it should be noted that the mean age of patients in CUPS was 46 ± 15 years compared with the 76.7 ± 6.9 years in this study. The prevalence of PVD is usually low in our T2DM population, probably because of younger age at onset of T2DM. It is known that in older T2DM population, the prevalence is much higher (22). Thus, the higher prevalence of PVD in this study may be attributed to the older age of our survivors.
CAD was diagnosed in 45% of patients. This is significantly higher than the 21.4% prevalence reported in our population-based study, CUPS (42), which is, again, likely a reflection of the older age of our present cohort. Although the overall prevalence of CAD was not different between survivors and nonsurvivors, it is significant that the majority of the nonsurvivors died of CAD, suggesting that the severity of CAD was likely greater among nonsurvivors. Use of statins and worse glucose and lipid profile and higher blood pressure may be some of the factors contributing to the mortality of the nonsurvivors.
Among the survivors, one patient (0.4%) had all of the five complications, namely, retinopathy, nephropathy, neuropathy, PVD, and CAD. Four complications were found in 69 (29%) patients, 3 complications were found in 49 (20.6%), 2 complications were found in 71 (29.8%), 1 complication was found in 38 (16%), and 4.2% (n = 10) of patients did not have any of diabetes-related complications. Among the nonsurvivors, 25(8.1%) had all the 5 complications, 4 complications were found in 97 (31.6%), 3 complications were found in 85 (27.7%), 2 complications were found in 68 (22.1%), and 1 complication was found in 32 (10.4%).
One of the limitations is that because this was a retrospective study, we were not able to present longitudinal data for lipids and A1C or complications for all subjects, but we have provided whatever data were available. Second, some of the laboratory methods had changed, although it is unlikely that this could have affected the results because of constant laboratory standardization and participation in international laboratory quality-control programs. Third, because autopsies are not usually performed in India, the causes of death were ascertained by perusal of medical records and, whenever possible, by verbal autopsy. Hence, the actual cause of death could have been different, although the similarity to the causes of death noted in our earlier population-based study is reassuring.
In summary, we report on the clinical profile of survivors and nonsurvivors with T2DM. The survivors had more favorable glycemic control and lipid profiles and lower blood pressures and greater use of statins, which probably contributed to their longer survival. Further studies, including genetic analysis, possibly could help to identify the factors responsible for long-term survival and the relative protection from end-stage complications of this group of long-term T2DM survivors.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
V.M. conceived the study and revised all drafts of the article. C.S.S.R. and A.A. performed the corrections in consecutive drafts and provided the input for statistical analysis of the data. S.D. wrote the first draft of the article. R.M.A., B.P., and R.U. gave valuable comments and suggestions for the writing of the article. C.S.S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff of Dr. Mohan’s Diabetes Specialities Centre and the Madras Diabetes Research Foundation, Chennai, India, for their help with this study.
References
- 1.Zargar AH, Wani AI, Masoodi SR, Laway BA, Bashir MI. Mortality in diabetes mellitus—data from a developing region of the world. Diabetes Res Clin Pract 1999;43:67–74 [DOI] [PubMed] [Google Scholar]
- 2.Anjana RM, Pradeepa R, Deepa M, et al. ICMR–INDIAB Collaborative Study Group Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia 2011;54:3022–3027 [DOI] [PubMed] [Google Scholar]
- 3.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 2007;125:217–230 [PubMed] [Google Scholar]
- 4.International Diabetes Federation IDF Diabetes Atlas, 5th ed. Brussels, Belgium, International Diabetes Federation, 2011 [Google Scholar]
- 5.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 1999;84:137–144 [DOI] [PubMed] [Google Scholar]
- 6.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991;337:382–386 [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran A, Snehalatha C, Kapur A, et al. Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001;44:1094–1101 [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Deepa M, Deepa R, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India—the Chennai Urban Rural Epidemiology Study (CURES-17). Diabetologia 2006;49:1175–1178 [DOI] [PubMed] [Google Scholar]
- 9.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br J Nutr 2009;102:1498–1506 [DOI] [PubMed] [Google Scholar]
- 10.Mohan V, Gokulakrishnan K, Deepa R, Shanthirani CS, Datta M. Association of physical inactivity with components of metabolic syndrome and coronary artery disease—the Chennai Urban Population Study (CUPS no. 15). Diabet Med 2005;22:1206–1211 [DOI] [PubMed] [Google Scholar]
- 11.Abate N, Chandalia M, Satija P, et al. ENPP1/PC-1 K121Q polymorphism and genetic susceptibility to type 2 diabetes. Diabetes 2005;54:1207–1213 [DOI] [PubMed] [Google Scholar]
- 12.Vimaleswaran KS, Radha V, Ghosh S, et al. Peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene polymorphisms and their relationship to Type 2 diabetes in Asian Indians. Diabet Med 2005;22:1516–1521 [DOI] [PubMed] [Google Scholar]
- 13.Kooner JS, Saleheen D, Sim X, et al. DIAGRAM. MuTHER Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 2011;43:984–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radha V, Mohan V. Genetic predisposition to type 2 diabetes among Asian Indians. Indian J Med Res 2007;125:259–274 [PubMed] [Google Scholar]
- 15.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab 2001;86:5366–5371 [DOI] [PubMed] [Google Scholar]
- 16.Bain SC, Gill GV, Dyer PH, et al. Characteristics of Type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 17.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradeepa R, Prabu AV, Jebarani S, Subhashini S, Mohan V. Use of a large diabetes electronic medical record system in India: clinical and research applications. J Diabetes Sci Tech 2011;5:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) The Asia Pacific Perspective. Redefining Obesity and Its Treatment. International Association for the Study of Obesity and International Obesity Task Force. Melbourne, International Diabetes Institute, 2000 [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 22.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98(Suppl):786–806 [PubMed] [Google Scholar]
- 23.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci 2005;46:2328–2333 [DOI] [PubMed] [Google Scholar]
- 24.Unnikrishnan RI, Rema M, Pradeepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 2007;30:2019–2024 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 26.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55). Diabet Med 2008;25:407–412 [DOI] [PubMed] [Google Scholar]
- 27.Premalatha G, Shanthirani S, Deepa R, Markovitz J, Mohan V, The Chennai Urban Population Study (CUPS) Prevalence and risk factors of peripheral vascular disease in a selected South Indian population: the Chennai Urban Population Study. Diabetes Care 2000;23:1295–1300 [DOI] [PubMed] [Google Scholar]
- 28.Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JPH, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J 2006;82:280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24:1936-1940. [DOI] [PubMed]
- 30.Mohan V, Shanthirani CS, Deepa M, Deepa R, Unnikrishnan RI, Datta M. Mortality rates due to diabetes in a selected urban south Indian population—the Chennai Urban Population Study [CUPS—16]. J Assoc Physicians India 2006;54:113–117 [PubMed] [Google Scholar]
- 31.Zhang PH, Chen ZW, Lv D, et al. Increased risk of cancer in patients with type 2 diabetes mellitus: a retrospective cohort study in China. BMC Public Health 2012;12:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 33.Buse J. Statin treatment in diabetes mellitus. Clin Diabetes 2003;21:168–172 [Google Scholar]
- 34.Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract 2002;56:1–11 [DOI] [PubMed] [Google Scholar]
- 35.Elisaf M, Mikhailidis DP. Statins and renal function. Angiology 2002;53:493–502 [DOI] [PubMed] [Google Scholar]
- 36.Fried LF, Forrest KY, Ellis D, Chang Y, Silvers N, Orchard TJ. Lipid modulation in insulin-dependent diabetes mellitus: effect on microvascular outcomes. J Diabetes Complications 2001;15:113–119 [DOI] [PubMed] [Google Scholar]
- 37.Amutha A, Datta M, Unnikrishnan IR, et al. Clinical profile of diabetes in the young seen between 1992 and 2009 at a specialist diabetes centre in south India. Prim Care Diabetes 2011;5:223–229 [DOI] [PubMed] [Google Scholar]
- 38.Raymond NT, Paul O’Hare J, Bellary S, Kumar S, Jones A, Barnett AH, UKADS Study Group Comparative risk of microalbuminuria and proteinuria in UK residents of south Asian and white European ethnic background with type 2 diabetes: a report from UKADS. Curr Med Res Opin 2011;27(Suppl. 3):47–55 [DOI] [PubMed] [Google Scholar]
- 39.Bhansali A, Chattopadhyay A, Dash RJ. Mortality in diabetes: a retrospective analysis from a tertiary care hospital in North India. Diabetes Res Clin Practice 2003;60;119-124. [DOI] [PubMed]
- 40.Tekin N, Baskan M, Yesilkayali T, Karabay O. Prevalence of peripheral arterial disease and related risk factors in Turkish elders. BMC Fam Pract 2011;12:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng CH, Tseng FH, Chong CK, et al. Angiotensin-converting enzyme genotype and peripheral arterial disease in diabetic patients. Exp Diabetes Res 2012;2012:1-7 [DOI] [PMC free article] [PubMed]
- 42.Mohan V, Deepa R, Rani SS, Premalatha G, Chennai Urban Population Study (CUPS No.5) Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban Population Study (CUPS No. 5). J Am Coll Cardiol 2001;38:682–687 [DOI] [PubMed] [Google Scholar]