Abstract
OBJECTIVE
People with type 1 diabetes are at high risk of premature atherosclerosis. Existing evidence suggests that impaired vitamin D metabolism may contribute to the development of atherosclerosis. We tested associations of circulating vitamin D metabolite concentrations with subclinical atherosclerosis among 1,193 participants with type 1 diabetes in the DCCT/EDIC study.
RESEARCH DESIGN AND METHODS
We measured plasma concentrations of 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D, and 24,25-dihydroxyvitamin D by mass spectrometry at the end of the DCCT. In a staggered cross-sectional design, we tested associations with coronary artery calcium (CAC), measured by computed tomography a median of 10 years later, and with common and internal carotid intima-media thickness (IMT), measured by B-mode ultrasonography on two occasions a median of 4 years later and a median of 10 years later. We hypothesized that lower concentrations of each vitamin D metabolite would be associated with increased risk of CAC and greater carotid IMT.
RESULTS
At the time metabolites were measured, mean age was 32.4 years and mean duration of diabetes was 7.5 years. The prevalence and severity of CAC tended to be lower—not higher—with lower concentrations of each vitamin D metabolite. For instance, in a fully adjusted multinomial logistic model, a 25 nmol/L lower 25-hydroxyvitamin D was associated with a 0.8-fold decrease in the odds of having higher CAC (95% CI 0.68–0.96, P = 0.01). No vitamin D metabolite was associated with either common or internal mean IMT.
CONCLUSIONS
We did not find evidence linking impaired vitamin D metabolism with increased subclinical atherosclerosis in type 1 diabetes.
Premature atherosclerosis is a cause of substantial morbidity and mortality in type 1 diabetes (1). Treatment to control glycemia, plasma lipids, and blood pressure can reduce the risk of developing cardiovascular disease (2–4). However, identification of additional therapeutic targets is desirable to further reduce risk.
Existing evidence suggests that impaired vitamin D metabolism may promote atherosclerosis (5). In animal experimental models, insufficient 1,25-dihydroxyvitamin D [1,25(OH)2D, the active vitamin D hormone] stimulates the renin-angiotensin system, which promotes vascular damage through hemodynamic and metabolic effects and unfavorably modulates immune cell function in the arterial wall (5–8). Human studies suggest that these actions may be clinically relevant. Low circulating concentrations of 25-hydroxyvitamin D [25(OH)D, reflecting total vitamin D intake from cutaneous synthesis and oral consumption] have been associated with increased risks of coronary artery calcium (CAC), cardiovascular disease events, and death in populations with and without diabetes (9–16). Also, one study showed that low circulating levels of 1,25(OH)2D are associated with increased risk of CAC (17). In addition, genetic variation in CYP24A1, the main enzyme responsible for catabolism of 25(OH)D to 24,25-dihydroxyvitamin D3 [24,25(OH)2D3] and of 1,25(OH)2D to 1,24,25-trihydroxyvitamin D, has been associated with risk of CAC (18).
We measured serum concentrations of 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D3 among 1,193 participants in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. We hypothesized that low concentrations of each of these metabolites, reflecting decreased vitamin D intake, activity, and turnover, respectively, would be associated with increased risk of subclinical cardiovascular disease, manifest as a greater prevalence and severity of CAC and greater carotid intima-media thickness (IMT).
RESEARCH DESIGN AND METHODS
The DCCT enrolled 1,441 persons with type 1 diabetes from 1983 to 1989 to determine the effects of intensive diabetes therapy on long-term complications of diabetes. Participants were randomly assigned to intensive diabetes therapy aimed at lowering glucose concentrations as close as safely possible to the normal range or to conventional therapy aimed at preventing symptoms of hyperglycemia and hypoglycemia. In 1994, after completion of the DCCT, 1,375 participants (96% of the surviving cohort) agreed to participate in the EDIC study. During EDIC, diabetes therapy and glycemic control as measured by A1C became similar in the two original DCCT treatment groups, and yearly follow-up has continued through the present time. The current study includes all participants with available frozen plasma collected at or near the end of the DCCT, excluding seven participants who were pregnant at the time of plasma collection (N = 1,193; 83% of randomized DCCT participants) (19).
Circulating vitamin D metabolites
Concentrations of 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D were measured in plasma samples that were obtained at or soon before the end of the DCCT (19). The median calendar year of collection was 1992 (range 1988–1993). Vitamin D metabolites are known to be stable during long-term storage. Plasma samples were stored continuously at −70°C until measurements were performed using high-performance liquid chromatography–tandem mass spectrometry. These methods quantify 25(OH)D2 and 25(OH)D3 [summed to evaluate total 25(OH)D], 1,25(OH)2D2 and 1,25(OH)2D3 [summed to evaluate total 1,25(OH)2D], and 24,25(OH)2D3 [referred to throughout as 24,25(OH)2D] (20–22). The lower limits of detection for 25(OH)D2, 25(OH)D3, 1,25(OH)2D2, 1,25(OH)2D3, and 24,25(OH)2D3 were 2.5 nmol/L (965 samples below), 2.5 nmol/L (0 samples below), 12.5 pmol/L (973 samples below), 12.5 pmol/L (0 samples below), and 1.25 nmol/L (13 samples below), respectively. Measurements that were below the lower limits of detection were truncated to the limit. Interassay imprecision was 6.63% at 70.2 nmol/L for 25(OH)D3, 5.27% at 74.5 nmol/L for 25(OH)D2, 9.6% at 86.3 pmol/L for 1,25(OH)2D2, 12.2% at 94.6 pmol/L for 1,25(OH)2D3, and 10.5% at 7.9 nmol/L for 24,25(OH)2D3. The calibration for the measurement of 25(OH)D was verified using Standard Reference Material 972 from National Institute of Standards and Technology with accuracy of 91–95% for 25(OH)D3 and 100–116% for 25(OH)D2.
Measures of subclinical cardiovascular disease
Computed tomography (CT) was performed by certified technicians between November 2000 and March 2003, 11–20 years after enrollment into the DCCT and 8–15 years after collection of blood for vitamin D metabolite measurement (median 10 years), in 1,205 (86%) of the surviving 1,404 participants with specific patient consent. CT was performed in 19 scanning sites using a C-150 cardiac-gated electron beam CT scanner (n = 9; Imatron, San Francisco, CA), a Lightspeed (n = 7; General Electric Medical Systems, Waukesha, WI), or a Volume Zoom (Siemens, Erlanger, Germany) multidetector CT system; a Lightspeed Marconi MX-8000 (GE); or a Somatom 4 (Siemens) (n = 3). All participants were scanned twice over calibration phantoms of known physical calcium concentration. Scans were read centrally at the Harbor-UCLA Research and Education Institute (University of California, Los Angeles, Torrance, CA) to identify and quantify coronary artery calcium (CAC), calibrated according to the readings of the phantom using the method of Agatston et al. (23) The average score from the two scans was used in the analysis. Readers were masked to subject identity and prior treatment assignment.
Carotid IMT was measured by B-mode ultrasonography in 1994 and again in 2000/2001 (24). IMT was measured in both the common and internal carotid arteries. A single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries and three longitudinal views in different imaging planes of each internal carotid artery were obtained. The internal carotid artery was defined as including both the carotid bulb and the 10-mm segment distal to the tip of the flow divider that separates the internal from the external carotid artery. Studies were performed by certified technicians at the clinical centers, recorded on videotapes, and read in a central unit (Tufts University) by a single reader (24).
Covariates
Covariates were ascertained concurrently with measurement of vitamin D metabolites. Demographic characteristics and smoking were ascertained by questionnaire. Solar irradiation of each DCCT site was quantified as the mean annual ultraviolet index in 1992, as published online by the National Weather Service (ftp://ftp.cpc.ncep.noaa.gov/long/uv/cities, accessed October 2011). A1C was measured using high-performance liquid chromatography (25). Glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease–Epidemiology (CKD-EPI) equation on the basis of serum creatinine concentration, age, sex, and race (26). In order to increase precision, baseline albumin excretion rate (AER) for this study was calculated as the geometric mean of the AER measured concurrently with vitamin D metabolites and the AER preceding vitamin D measurement, usually within 1 year. Plasma concentrations of parathyroid hormone and fibroblast growth factor-23 were measured concurrently with vitamin D metabolites in a subset of 300 participants using immunoassay and ELISA, respectively (19). Data on vitamin D supplement use were not available.
Statistical methods
Associations of circulating vitamin D metabolites with CAC were assessed with a binomial regression model to estimate the relative risk for presence (vs. absence) of CAC. A study of the Multi-Ethnic Study of Atherosclerosis (MESA) developed the CAC categories: 0, 0–100, 100–300, and >300 (27). Proportional odds models were estimated using these established categories as the outcome. Coefficients from the polytomous model are interpreted as the odds ratio for having higher CAC associated with a unit difference in the exposure (28). This model has the advantage of using all of the available data, while the interpretation of the coefficients remains meaningful. In addition, Tobit regression models were estimated to the combined binary and continuous CAC outcomes (29). Nonzero CAC scores were transformed using the natural logarithm because the distribution is highly skewed. The Tobit regression coefficient represents the log ratio of the geometric mean CAC score per unit increase in the covariate, assuming some true measurable calcification for all subjects, including those with undetectable levels.
Associations of vitamin D metabolites with IMT were estimated using linear regression. Internal carotid IMT was log transformed prior to analysis, yielding estimates reflecting relative differences in internal carotid IMT. Generalized estimating equations were used to determine valid inference while using both the measurements from 1994 and the measurements from 2000/2001 for each subject simultaneously (30). Due to observed differences in IMT between 1994 and 2000/2001, the models were adjusted for year of measurement. No interactions by year were observed.
Plasma 25(OH)D concentrations were examined in clinically relevant categories: ≥75 nmol/L (30 ng/mL), 50–75 nmol/L (20–30 ng/mL), and < 50 nmol/L (20 ng/mL). Because no such categories exist for 24,25(OH)2D and 1,25(OH)2D, these metabolites were examined in quartiles. Adjusted coefficients and corresponding 95% CIs were generated for each category of vitamin D metabolite, and P values were generated evaluating each vitamin D metabolite as a continuous variable.
Models were adjusted for variables (measured at the time of plasma collection) that could confound associations of circulating vitamin D metabolites with study outcomes: age (continuous), sex, race (white vs. nonwhite), duration of diabetes (categories), DCCT treatment assignment, year of measurement (IMT only), solar irradiation of DCCT/EDIC site (continuous), BMI (continuous), AER (continuous), and estimated GFR (continuous). P values <0.05 were considered statistically significant. All analyses were performed using R, version 2.15 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
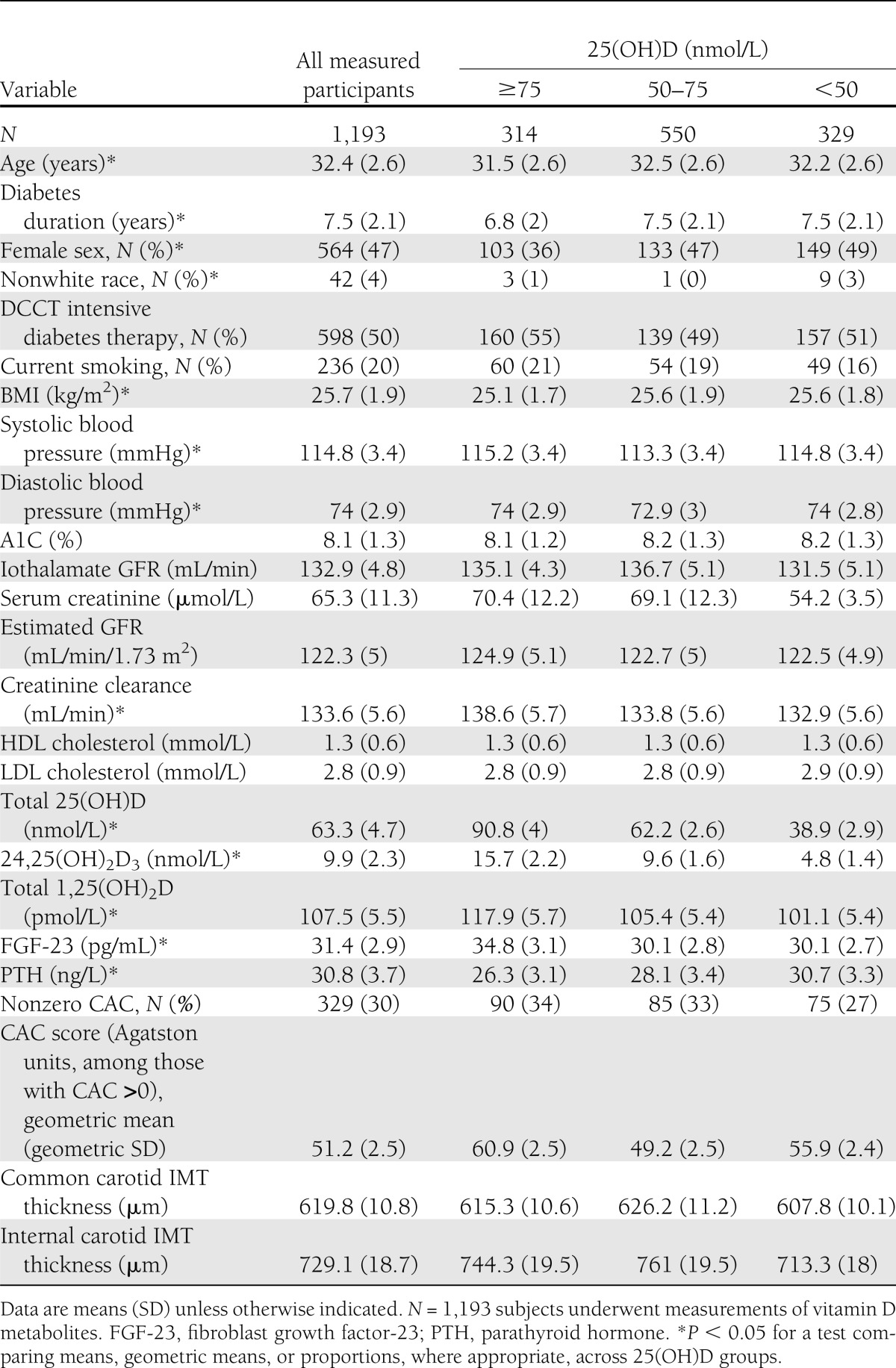
Of the 1,193 subjects included in this study, 47% were women and 96% were white (Table 1). At the time of biomarker measurement (at or near the end of the DCCT), mean age was 32.4 years, mean duration of diabetes was 7.5 years, 20% of the subjects smoked, mean systolic blood pressure was 114.8 mmHg, and mean LDL cholesterol was 2.8 mmol/L. Lower plasma 25(OH)D concentration was associated with greater age, longer duration of diabetes, female sex, nonwhite race, and higher BMI (Table 1). Similar associations were observed for 24,25(OH)2D3 (Supplementary Table 1), which was highly correlated with 25(OH)D (Kendall τ = 0.7). Lower plasma 1,25(OH)2D was associated with longer duration of diabetes and white race (Supplementary Table 2).
Table 1.
Description of DCCT/EDIC study population at the time of biomarker measurement, except for CAC/IMT measures, which occurred at year 6
Coronary artery calcium
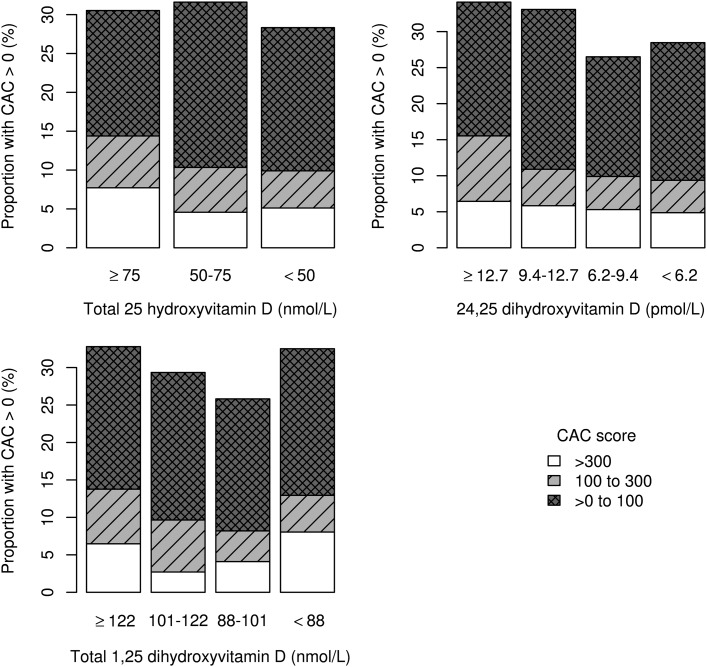
Both plasma 25(OH)D concentration and CAC were measured in 1,081 subjects; 329 subjects had nonzero CAC scores (30%), and among those subjects the mean and median Agatston scores were 215.5 and 48.8, respectively, reflecting the highly skewed distribution of CAC. The prevalence of CAC tended to be lower—not higher—with lower concentrations of each vitamin D metabolite, though none of these trends were statistically significant (Supplementary Table 3). When CAC in multiple categories was examined as the outcome, lower circulating levels of all three vitamin D metabolites were associated with lower mean CAC (Table 2). Similar associations were observed using Tobit regression (Supplementary Table 4). There was a suggestion of a U-shaped relationship between plasma 1,25(OH)2D and CAC (Fig. 1). In a separate post hoc model not reported in the tables, we used the middle two quartiles as the reference category in a polytomous regression model. Compared with the middle two categories, the lowest quartile was associated with 1.2 times the odds of higher mean CAC (95% CI 0.8–1.7) and the highest quartile was associated with 1.7 times the odds (1.2–1.7). Associations of the vitamin D metabolites with CAC did not differ by sex or DCCT treatment assignment (Supplementary Table 5).
Table 2.
Associations of circulating vitamin D metabolites measured at the end of the DCCT with CAC measured during the EDIC study among 1,081 DCCT/EDIC participants
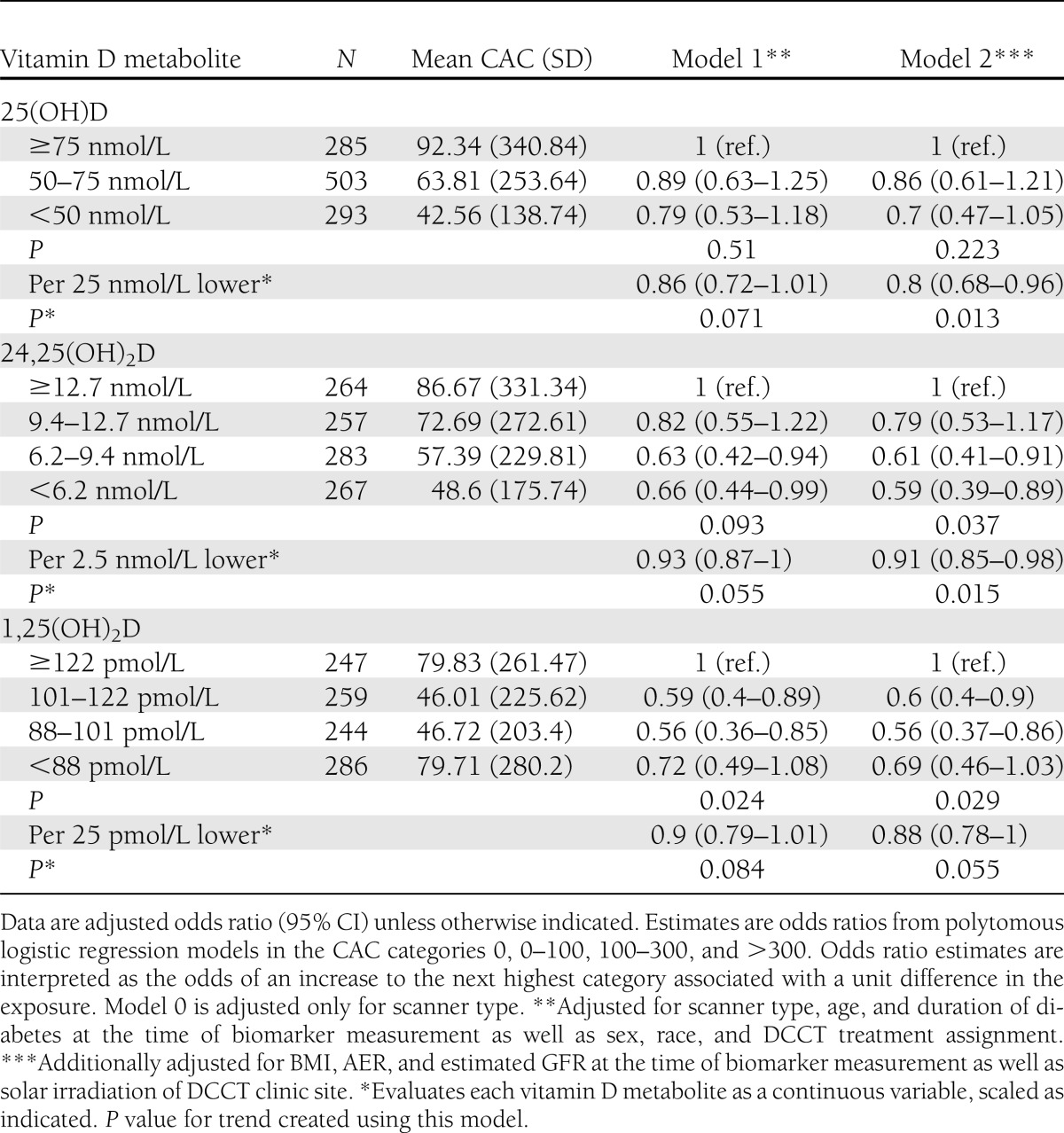
Figure 1.
Distributions of CAC by categories of circulating vitamin D metabolites among 1,081 DCCT/EDIC participants.
IMT
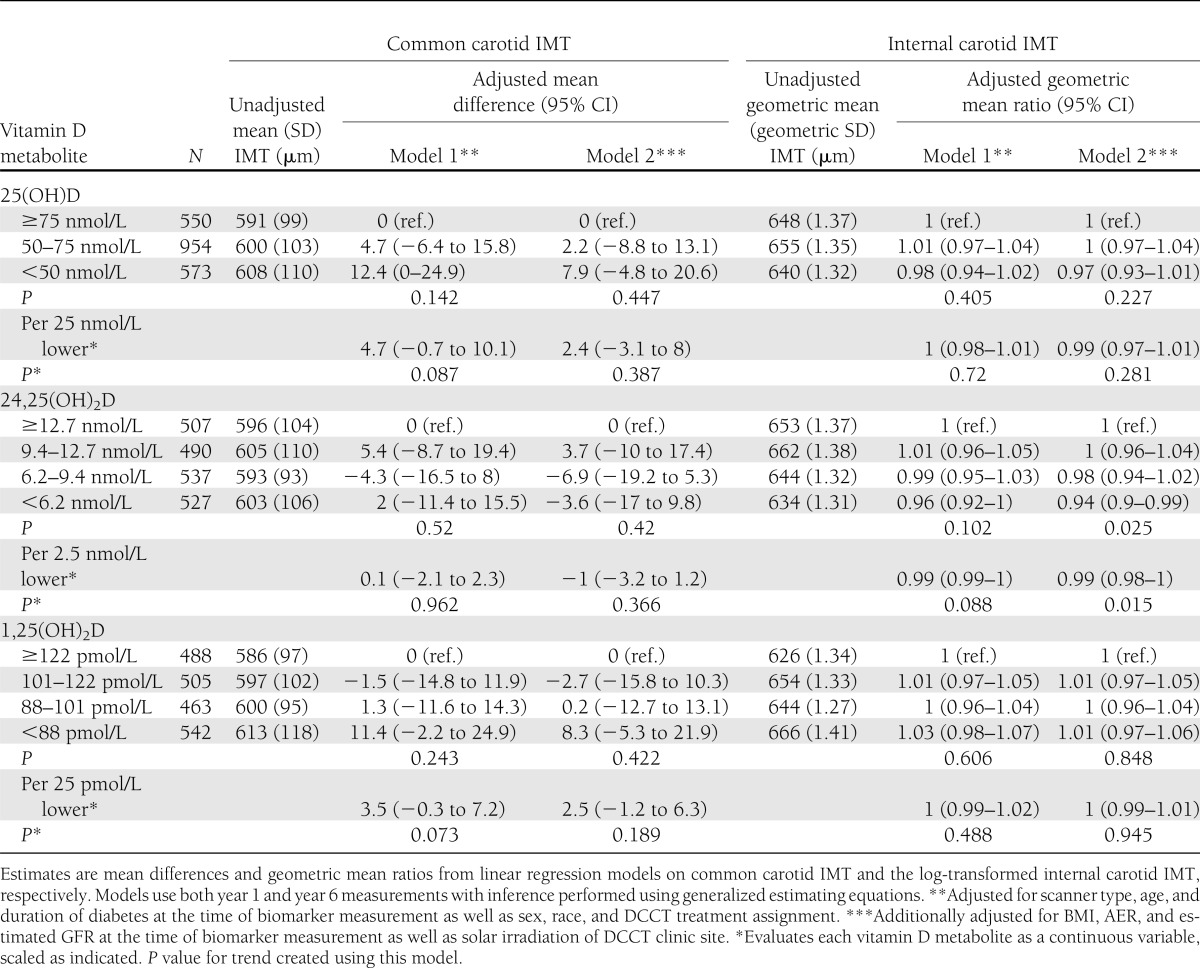
Both plasma 25(OH) concentrations and carotid IMT were measured among 1,086 participants in 1994 and 991 participants in 2000/2001. The distribution of common IMT was unimodal and approximately symmetric, while the distribution of internal IMT was skewed to the right. The mean (SD) common carotid IMT in 1994 was 581 (86) μm and in 2000/2001 was 620 (117) μm. The mean internal carotid IMT in 1994 was 642 (221) μm and in 2000/2001 was 729 (349) μm. There was a suggestion that the lowest concentrations of 25(OH)D and 1,25(OH)2D were associated with modestly greater common carotid IMT, but none of the three vitamin D metabolites were significantly associated with either common or internal mean IMT (Table 3). No plasma vitamin D metabolite was associated with mean IMT measured in 1994 only (evaluated using linear regression) or with change in IMT from 1994 to 2000/2001 (evaluated using generalized estimating equations including time–by–vitamin D metabolite interactions). Associations of the vitamin D metabolites with IMT did not differ by sex or DCCT treatment assignment (Supplementary Table 5).
Table 3.
Associations of circulating vitamin D metabolites measured at the end of the DCCT with internal carotid IMT measured during the EDIC study among 991 DCCT/EDIC participants
CONCLUSIONS
In this study of 1,193 people with type 1 diabetes, we did not observe the hypothesized associations of lower plasma vitamin D metabolite concentrations with increased risks of subclinical cardiovascular disease, as measured by CAC or carotid IMT. To our surprise, we observed trends toward associations of lower concentrations of vitamin D metabolites with reduced CAC prevalence and severity, while risk estimates for IMT were close to null. Our findings contrast with those of previous studies that have shown associations of low serum concentrations of 25(OH)D and 1,25(OH)2D with increased risk of CAC and cardiovascular mortality.
A number of prior studies have reported associations of low circulating 25(OH)D or 1,25(OH)2D concentrations with increased risk of coronary heart disease. Among 374 participants in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study, circulating 25(OH)D <50 nmol/L was associated with a 3.3-fold increased odds of prevalent CAC compared with 25(OH)D ≥75 nmol/L in minimally adjusted analyses (15). Among 200 Steno Clinic patients with type 2 diabetes, plasma 25(OH)D <12.5 nmol/L (N = 19) was associated with increased risk of Agatston coronary calcium score ≥400 compared with 25(OH)D ≥12.5 nmol/L (16). Among 1,370 mostly nondiabetic participants in MESA, each 25 nmol/L decrement in 25(OH)D was associated with a 23% increased risk of developing CAC over 3 years’ median follow-up (10). Lower 25(OH)D concentration has also been associated with increased risks of cardiovascular disease events in the general population (30,31) and with increased mortality risk in types 1 and 2 diabetes (12,14), each presumably reflecting in part an increased tendency to develop coronary heart disease. Lower circulating 1,25(OH)2D concentrations were associated with increased risk of prevalent CAC among a population referred for evaluation of hyperlipidemia and with a trend toward increased risk of incident CAC in MESA (10,17).
Differences in our results compared with prior studies may reflect differences in study population, study design, or covariate adjustment. Regarding study population, associations of vitamin D metabolite concentrations with atherosclerosis could conceivably vary by age, sex, or diabetes status, which differ substantially in the studies noted above. Our study is most similar to the CACTI study, since both evaluated participants with type 1 diabetes, evaluated 25(OH)D using similar assays and categories, and measured CAC at similar ages. However, compared with CACTI, mean plasma 25(OH)D concentration (63.2 vs. 88.1 nmol/L) and the prevalence of CAC (30 vs. 45.5%) were each lower in our study. There were also differences in study design and covariate adjustment. Specifically, we measured CAC a median of 10 years after circulating vitamin D metabolites and thus tested associations of vitamin D metabolites with a combination of prevalent and incident CAC in a staggered cross-sectional analysis. The timing of the vitamin D and CAC measurements is temporally appropriate and clinically meaningful. Coronary atherosclerosis develops over many years, and in type 1 diabetes one might expect impaired vitamin D metabolism assessed in the early 30s to manifest as subclinical atherosclerosis in the early 40s. Covariate adjustment exposed trends toward direct correlations of vitamin D metabolite concentrations with CAC prevalence and severity in our study—opposite our hypothesis. Similarly, covariate adjustment attenuated associations of 25(OH)D concentration with CAC in the CACTI and MESA studies and rendered associations statistically insignificant in CACTI. The Steno CAC study did not include adjustment for BMI, which is an important potential confounder. This highlights the susceptibility of 25(OH)D analyses to confounding and suggests that residual confounding may have affected some prior studies.
In view of conflicting prior reports, the association of higher circulating vitamin D metabolite concentrations with greater CAC was surprising and should be viewed with caution. This result was only significant after multivariable adjustment, and we are unaware of a convincing biologic explanation. Several studies have reported U-shaped associations of circulating 25(OH)D concentration with risk of adverse health outcomes (32). Similarly, in a mouse model of chronic kidney disease, 1,25(OH)2D3 administration protected against aortic calcification at low doses but promoted aortic calcification at high doses (33). These studies suggest that excess vitamin D may be harmful, perhaps by promoting extraosseous calcification. However, in our population, circulating vitamin D metabolite concentrations did not reach into a range high enough to expect such effects.
Current data evaluating the association of vitamin D with carotid atherosclerosis are limited. Inverse cross-sectional correlations of circulating 25(OH)D concentrations with carotid IMT have been demonstrated in several studies (9,11,13), while negative findings have also been reported (34,35). Positive studies have shown stronger associations with IMT in the internal carotid artery, while the negative studies only measured IMT in the common carotid artery. In this study, we measured IMT in both the internal and common carotid artery—each on two occasions. However, no association of any vitamin D metabolite was observed with IMT measured at either site.
Strengths of this study include the assessment of a large type 1 diabetic population, use of novel and specific mass spectrometry assays for three complementary circulating vitamin D metabolites, use of two established measurements of subclinical CVD, and a combination of detailed baseline covariate data and a relatively homogenous study population that minimizes the risk of confounding. Limitations include the availability of vitamin D metabolite measurements at only one point in time, availability of CAC measures at only one point in time, and the observational nature of the EDIC study, which allows for unmeasured confounding and precludes inferences about causality. 25(OH)D concentrations have been observed to be relatively stable within individuals over time (36), and our study was conducted during a period in which vitamin D supplementation was not common. However, unmeasured changes in vitamin D metabolite concentrations over time could still lead to misclassification and bias results toward the null. In conclusion, we did not find evidence linking impaired vitamin D metabolism with increased risk of subclinical CVD in type 1 diabetes.
Acknowledgments
This study was supported by grants RC4DK090766, R01DK087726, and R01KD088762 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The DCCT/EDIC study is supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the NIDDK; National Eye Institute; National Institute of Neurological Disorders and Stroke; and the General Clinical Research Centers Program and by funding from the Clinical and Translational Science Awards Program; National Center for Research Resources; and Genentech through a Cooperative Research and Development Agreement with NIDDK. Free or discounted supplies or equipment were contributed by Abbott; Animas; Aventis; Bayer; Becton, Dickinson, and Company; CanAm; Eli Lilly; LifeScan; Medtronic; MiniMed; Omron; OmniPod; Roche; and Sanofi. No other potential conflicts of interest relevant to this article were reported.
M.C.S. analyzed the data and wrote the manuscript. J.D.B., J.M.L., and M.W.S. contributed to the discussion and edited the manuscript. P.A.C., M.E.M., and B.Z. edited the manuscript. A.N.H. measured the data and edited the manuscript. I.H.d.B. conceived the study, contributed to the discussion, and edited the manuscript. M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank W. Sun and J.-Y. Backlund of The George Washington University for their help preparing the data.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2020/-/DC1.
References
- 1.Costacou T, Edmundowicz D, Prince C, Conway B, Orchard TJ. Progression of coronary artery calcium in type 1 diabetes mellitus. Am J Cardiol 2007;100:1543–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 3.Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS investigators Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JYC, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int 2006;69:33–43 [DOI] [PubMed] [Google Scholar]
- 6.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda M, Yamashita T, Sasaki N, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol 2010;30:2495–2503 [DOI] [PubMed] [Google Scholar]
- 8.Szeto FL, Reardon CA, Yoon D, et al. Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol 2012;26:1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597 [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 2009;20:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis JP, von Mühlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis 2009;207:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care 2010;33:2238–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi AI, Lo JC, Mulligan K, et al. Association of vitamin D insufficiency with carotid intima-media thickness in HIV-infected persons. Clin Infect Dis 2011;52:941–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care 2011;34:1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young KA, Snell-Bergeon JK, Naik RG, et al. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care 2011;34:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joergensen C, Reinhard H, Schmedes A, et al. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care 2012;35:168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 1997;96:1755–1760 [DOI] [PubMed] [Google Scholar]
- 18.Shen H, Bielak LF, Ferguson JF, et al. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol 2010;30:2648–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer IH, Sachs MC, Cleary PA, et al. Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Circulating vitamin D metabolites and kidney disease in type 1 diabetes. J Clin Endocrinol Metab 2012;97:4780–4788DOI: 10.1210/jc.2012-2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 2012;82:693–700DOI: 10.1038/ki.2012.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 2011;57:1279–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1639–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832 [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345 [DOI] [PubMed] [Google Scholar]
- 28.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol 1997;26:1323–1333 [DOI] [PubMed] [Google Scholar]
- 29.Tobin J. Estimation of relationships for limited dependent variables. Econometrica 1958;26:24–36 [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 31.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol 2008;19:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim S, Shin H, Kim MJ, et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab 2012;97:169–178 [DOI] [PubMed] [Google Scholar]
- 36.Michos ED, Streeten EA, Ryan KA, et al. Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or C-reactive protein in the old order amish. Calcif Tissue Int 2009;84:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]